Background: Neuregulin-4 is a selective ligand for the ErbB4 receptor tyrosine kinase.
Results: In colon epithelial cells, inflammatory cytokine-induced apoptosis is blocked by neuregulin-4, but proliferation and migration are not affected.
Conclusion: ErbB4 activation by neuregulin-4 is a specific anti-apoptotic signal.
Significance: Neuregulin-4/ErbB4 signaling might be therapeutically useful for diseases involving excessive epithelial apoptosis.
Keywords: Apoptosis, Cell Signaling, Colitis, Growth Factors, Receptor Tyrosine Kinase
Abstract
Expression of the ErbB4 tyrosine kinase is elevated in colonic epithelial cells during inflammatory bowel disease, whereas ErbB4 overexpression in cultured colonocytes blocks TNF-induced apoptosis in a ligand-dependent manner. Together, these observations suggest that ErbB4 induction may be a protective response. However, the effects of ErbB4 signaling in the colonic epithelium in vivo are not known. Furthermore, previous work on ErbB4 used ligands shared with other receptors, raising the question of whether the observed responses are explicitly due to ErbB4. In this study, we used the ErbB4-specific ligand neuregulin-4 (NRG4) to activate ErbB4 and define its role in colonocyte biology. NRG4 treatment, either in cultured cells or in mice, blocked colonic epithelial apoptosis induced by TNF and IFN-γ. It was also protective in a murine experimental colitis model. NRG4 stimulated phosphorylation of ErbB4 but not other ErbB receptors, indicating that this is a specific response. Furthermore, in contrast to related ligands, NRG4 enhanced cell survival but not proliferation or migration, and stimulated phosphorylation of the anti-apoptotic mediator Akt but not ERK MAPK. Pharmacological inhibition of PI3K/Akt signaling reversed the anti-apoptotic effects of NRG4, confirming the role of this cascade in NRG4-induced cell survival. With regard to the potential clinical importance of this pathway, NRG4 expression was decreased in human inflammatory bowel disease samples and mouse models of colitis, suggesting that activation of ErbB4 is altered in disease. Thus, exogenous NRG4 may be beneficial for disorders in which epithelial apoptosis is part of the pathology.
Introduction
ErbB4 is the least well understood member of the family of receptor tyrosine kinases that also includes EGF receptor (EGFR2/ErbB1), ErbB2/HER2, and ErbB3 (1). ErbBs recognize and are activated by a suite of ligands, including heparin-binding EGF-like growth factor (HB-EGF), betacellulin, and the heregulin/neuregulin family (2). Ligand binding is associated with receptor dimerization, increased tyrosine kinase activity, and autophosphorylation on C-terminal tyrosine residues, which then provide docking sites for downstream effectors (3). Different ligands show distinct specificities and affinities for different ErbB receptors and stimulate diverse dimerization patterns, signaling, and cellular responses (4, 5).
ErbB4 has several features that distinguish it from other tyrosine kinases, making it a unique target both in terms of signaling and a potential role in human disease. It can bind both heregulin/neuregulin growth factors and a subset of EGF family factors (6), but at least one peptide ligand, NRG4, is exclusive to ErbB4 and does not bind ErbB1 to −3 (7). Furthermore, ErbB4 associates with a divergent and more restricted suite of Src homology 2 domain-containing targets than EGFR, ErbB2, or ErbB3 (8). Thus, selective ErbB4 activation with NRG4 may elicit different cellular outcomes than stimulation with other EGF-like or heregulin family molecules.
Crohn's disease and ulcerative colitis, collectively known as inflammatory bowel disease (IBD), together affect more than 1.4 million American patients (9). The causes and cures of IBD remain elusive, but it is clear that a general feature of the pathology of these disorders is elevated apoptosis in the intestinal epithelium (10, 11), driven by inflammatory cytokines, such as TNF and IFN-γ. Thus, identifying signal transduction pathways that protect colon epithelial cells from cytokine- or injury-induced apoptosis will lead to new methods to control disease flares.
ErbB4 is induced in colonic epithelial cells by inflammatory cytokines and is present at elevated levels in the inflamed colonic mucosa of IBD patients (12). This appears to be a compensatory protective response rather than a pathological process because ectopic ErbB4 overexpression protects cultured mouse colon epithelial cells from cytokine-induced apoptosis in a ligand-dependent manner (12–14). However, these studies, like most investigation of ErbB4 function, used shared ErbB ligands heregulin (HRG)-1β or HB-EGF, raising the question of signal specificity. Furthermore, the in vivo biology of this receptor in the colon has not been investigated. In this study, we addressed these issues by using the ErbB4-specific ligand NRG4, to test (a) the cellular response to selective ErbB4 activation and (b) the effects of ErbB4 activation in vivo.
EXPERIMENTAL PROCEDURES
Cell Culture
Conditionally immortalized, non-transformed young adult mouse colon (YAMC) epithelial cells were provided by Dr. Robert Whitehead (15). These cells express low levels of endogenous ErbB4; YAMC-B4 cells expressing a human ErbB4 construct were generated as described previously (12). Cell pools showing no autocrine ErbB4 activation were selected for use and maintained under permissive conditions (33 °C in RPMI 1640 with 5% FBS, 5 units/ml mouse interferon-γ (Peprotech, Rocky Hill, NJ), 100 units/ml penicillin and streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenous acid (BD Biosciences)) and then shifted to nonpermissive conditions (RPMI 1640 containing 0.5% FBS, streptomycin and penicillin without IFN-γ, insulin, transferrin, or selenous acid at 37 °C) overnight before use in experiments.
Antibodies, Cytokines, Growth Factors, and Inhibitors
Antibodies were purchased from the following companies: monoclonal anti-actin (Sigma); polyclonal anti-phospho-Tyr-1284 ErbB4, phospho-Tyr-1068 EGFR, phospho-Tyr-1248 ErbB2, phospho-Tyr-1289 ErbB3, EGFR, ErbB2, ErbB3, phospho-Ser-473 Akt, cleaved caspase-3, cleaved poly(ADP-ribose) polymerase, phospho-p38, and phospho-ERK (Cell Signaling, Danvers, MA); polyclonal anti-ErbB4 (c-18) and goat polyclonal anti-COX-2, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); anti-Ki67 (Dako Corp., Carpinteria, CA); IRDye anti-mouse, anti-rabbit, and anti-goat (LI-COR Corp., Lincoln, NE); AlexaFluor-555-conjugated anti-rabbit (Invitrogen). Recombinant murine TNF (also known as TNF-α), IFN-γ, and EGF were from Peprotech. HRG-1β and anti-caspase antibody were from R&D Systems (Minneapolis, MN). Recombinant NRG4 (A1/A2 ectodomain sequence (16)) was synthesized by Genscript Corp. (Piscataway, NJ). LY294002 and wortmannin (PI3K inhibitors) were from Cell Signaling and Enzo Life Sciences (Farmingdale, NY), respectively.
Animals
All animal use was approved and monitored by the Children's Hospital Los Angeles Institutional Animal Care and Use Committee. Experiments used 6–8-week-old male C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME). Cytokines, growth factors, and inhibitors were given by intraperitoneal injection. 24 h after injections, mice were sacrificed, and colons were collected either for fixation and embedding or for epithelial lysates. For the latter, colons were incubated in Cell Recovery Solution (BD Biosciences) for 1 h at 4 °C; epithelial crypts were released by vigorous shaking and collected by low speed centrifugation, similar to previous reports (17). Collected epithelial tissue was lysed and processed similar to cultured cells as described below. Purity of the epithelial isolates was assessed by microscopic inspection at isolation and Western blot analysis for E-cadherin and smooth muscle actin (not shown); the epithelial isolates consisted primarily of intact released crypts that were E-cadherin-positive and smooth muscle actin-negative.
For acute colitis studies, mice were given 3% (w/v) dextran sulfate sodium (DSS) in drinking water for 7 days with daily intraperitoneal injections of either PBS or NRG4 (100 μg/kg). Animals were weighed daily throughout the experiment and then sacrificed on day 7. Colons were removed, cleaned, rolled, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned for histological evaluation. Histological damage was scored using a protocol similar to that of Dieleman et al. (18), in which five categories (amount of inflammation, depth of inflammation, percentage of crypts involved by inflammation, crypt damage, and percentage of crypts involved by crypt damage) are scored 0–3 by a pathologist blind to experimental conditions. Total scores are the sum of these five categories.
For chronic murine colitis samples, IL-10−/− mice on C57Bl/6 background were maintained unchallenged until 32–36 weeks of age, at which point knock-out mice but not wild-type animals have extensive inflammatory cytokine-driven colitis (19). Homogenates of flash-frozen whole colons or isolated epithelial cells were prepared.
Immunofluorescence and Histochemical Analysis
Tissues and cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Immunostaining was performed using standard techniques on 4–6 μm paraffin-embedded sections or fixed YAMC-B4 cells, using the manufacturers' suggestions for antibody dilutions. For immunofluorescence, slides were mounted with Vectashield medium containing DAPI (Vector Laboratories, Burlingame, CA). For immunohistochemistry, diaminobenzidine substrate was from Sigma, and sections were counterstained with methyl green (Dako).
Cell Lysates, Immunoprecipitation, and Western Blot Analysis
Cell lysates were extracted in modified radioimmune precipitation assay buffer as described previously (20), cleared by centrifugation, and boiled in Laemmli sample buffer. For immunoprecipitation experiments, cleared radioimmune precipitation assay lysates were sequentially incubated with 2 μg of anti-ErbB4 and then protein A/G-agarose beads (Santa Cruz Biotechnology, Inc.); immunocomplexes were collected, washed, and eluted by boiling in Laemmli buffer.
Samples were separated on SDS-polyacrylamide gels (6–10% as appropriate), blotted on PVDF membranes, and subjected to quantitative immunoblot using the LI-COR Odyssey infrared detection system. Equal loading was monitored by immunoblots for actin and at least one additional protein.
Apoptosis Assays
Apoptosis was stimulated in cell culture by a 6-h exposure to a cytokine mixture containing TNF (100 ng/ml) plus IFN-γ (150 units/ml), with or without recombinant NRG4 (100 ng/ml). In some experiments, PI3K inhibitor LY294002 (5 μm) or wortmannin (10 nm) was also used. Cells were fixed in 4% paraformaldehyde and immunostained for cleaved caspase-3.
For apoptosis analysis in vivo, mice were injected intraperitoneally with PBS/vehicle, TNF (250 μg/kg) plus IFN-γ (250 units/g), NRG4 (100 μg/kg), or NRG4 plus TNF and IFN-γ. In some experiments, LY294002 (50 mg/kg) or wortmannin (1 mg/kg) was included. After 24 h, mice were euthanized, and colons were removed and either fixed or used to make mucosal homogenates. Apoptosis was detected by in situ oligonucleotide ligation (ISOL; EMD Millipore, Billerica, MA) staining for DNA fragmentation in epithelial cells on sections of paraffin-embedded tissue and by cleaved caspase-3 Western blot on homogenates.
Proliferation Assays
The proliferative index in fixed colon specimens was assessed by immunohistochemical stain for Ki67. In vitro, cells were exposed to vehicle or growth factors for 24 h and then labeled with 5-ethynyl-2-deoxyuridine (EdU) for 2 h and fixed. Nuclei were marked with DAPI, and incorporated EdU was detected using a Click-iT EdU kit (Invitrogen).
Cell Migration/Restitution
Cells grown on fibronectin-coated plates were subjected to multiple circular wounds with a rotating silicone probe as described previously (21). Cultures were photographed immediately and 8 h after wounding; percentage closure was determined by measuring wounds in ImageJ (National Institutes of Health, Bethesda, MD).
RNA Isolation and RT-PCR Detection of ErbB Ligands
Total RNA from flash-frozen whole mouse colon or isolated epithelial cells was purified with RNeasy columns (Qiagen, Valencia, CA), including on-column DNase treatment. cDNA was synthesized from 1 μg of RNA with iScript (Bio-Rad), amplified by standard PCR techniques using previously described primers (22), and visualized by gel electrophoresis.
Real-time Quantitative PCR (qPCR) Analysis of NRG4 in IBD
NRG4 and HRG-1β expression levels were determined in human IBD using TaqMan gene expression assays (Invitrogen) on TissueScan Crohn's/colitis qPCR arrays (OriGene Technologies (Rockville MD); these specimens from resected human disease were fully deidentified before receipt, and the study was reviewed by the Children's Hospital Los Angeles Institutional Review Board and determined to not qualify as “human subject” research per §46.102(f)(2)). Relative mRNA levels were calculated using the 2−ΔΔCT method with β-actin as the reference; validity of the reference was confirmed by comparison with a second reference gene (β-glucuronidase).
Statistics and Replicates
All data are representative of at least three independent experiments. Statistical analyses were performed with Prism software (GraphPad Inc., La Jolla, CA). Statistical significance was assessed by analysis of variance with Tukey's post-test or Kruskal-Wallis test, as appropriate. Error bars indicate S.E. values.
RESULTS
NRG4 Blocks Inflammatory Cytokine-induced Colonocyte Apoptosis both in Vitro and in Vivo
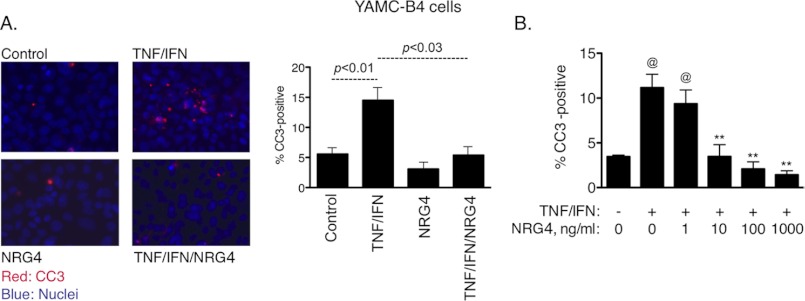
Multiple ErbB family ligands are expressed in the colon (Table 1). Of these, only NRG4 is thought to be specific for ErbB4 versus other family members (7); thus, we used this ligand to define the role of ErbB4 activation in colonic epithelial biology. When cultured mouse colonic epithelial cells expressing ErbB4 (YAMC-B4 cells) were given 100 ng/ml NRG4, cells were protected from apoptosis induced by 6-h exposure to a cytokine mixture containing TNF (100 ng/ml) and IFN-γ (150 units/ml), as measured by immunofluorescence analysis for cleaved caspase-3 (Fig. 1A; 2.7-fold decrease with cytokines plus NRG4 versus cytokines alone, p < 0.03). Similar results were obtained by TUNEL assay (not shown). Apoptosis inhibition by NRG4 was dose-dependent, with significant effects at 10 ng/ml and above (Fig. 1B). To confirm that this result was specifically due to ErbB4 stimulation, we exposed YAMC-B4 cells to NRG4 for 10 min and determined phosphorylation of ErbB family members by Western blot analysis of cell lysates. Although the positive controls EGF and HRG-1β stimulated phosphorylation of multiple ErbBs (Fig. 2A), NRG4 activated only ErbB4, confirming reports from other systems that NRG4 is an ErbB4-specific ligand. Furthermore, whereas HRG-1β promoted association between ErbB4 and other ErbB receptors as shown by immunoprecipitation analysis, NRG4 did not (Fig. 2B).
TABLE 1.
ErbB ligands expressed in mouse colon
RNA was prepared from flash-frozen whole mouse colon or isolated colon epithelium (n = 4 each) and then subjected to RT-PCR analysis for presence of the indicated ligands (receptor binding specificities indicated in parentheses).
| Ligand | Whole colon | Isolated epithelial cells |
|---|---|---|
| EGF (EGFR) | + | − |
| HB-EGF (EGFR, ErbB4) | + | + |
| Betacellulin (EGFR, ErbB4) | + | − |
| NRG4 (ErbB4) | + | − |
| HRG-1β (ErbB3, ErbB4) | + | − |
| TGF-α (EGFR) | + | + |
FIGURE 1.
NRG4 blocks cytokine-induced apoptosis in cultured colon epithelial cells. A, YAMC-B4 cells were exposed to TNF (100 ng/ml) plus IFN-γ (150 units/ml), with or without NRG4 (100 ng/ml). After 6 h, cells were fixed, and apoptosis was assessed by immunofluorescence analysis for cleaved caspase-3. The graph represents results from four independent experiments; error bars indicate S.E. values. Statistical significance was assessed by analysis of variance with Tukey's post-test. B, cells were treated with TNF plus IFN, with 0–1000 ng/ml NRG4, and apoptosis rates were determined. @, p < 0.01 versus control; **, p < 0.01 versus TNF + IFN. The graph represents results from three independent experiments.
FIGURE 2.
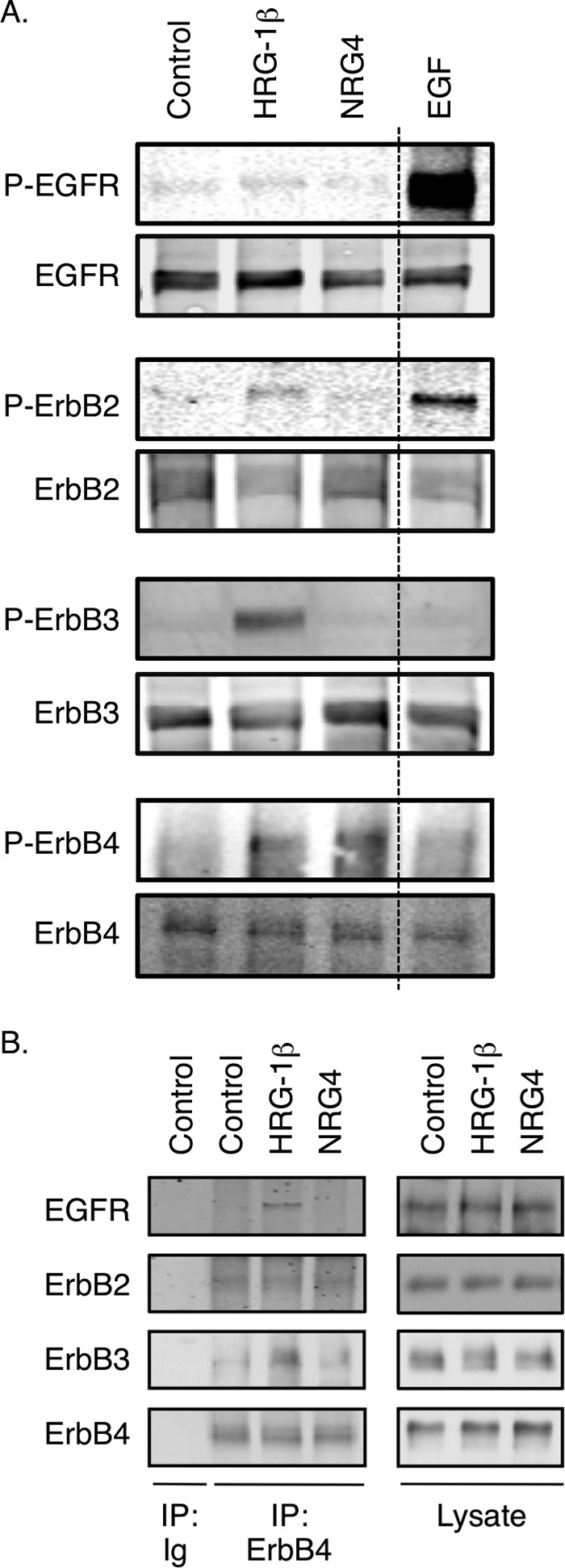
ErbB4, but not ErbB1 to −3, is stimulated by NRG4 in colonocytes. YAMC-B4 cells were exposed to HRG-1β (100 ng/ml), NRG4 (100 ng/ml), or EGF (10 ng/ml) for 10 min. A, phosphorylation of EGFR, ErbB2, ErbB3, and ErbB4 was determined by Western blot analysis using phospho-specific antibodies. The dashed line indicates excision of a superfluous lane from the blot. B, ErbB4 was immunoprecipitated (IP), and immunocomplexes were subjected to Western blot analysis for EGFR, ErbB2, and ErbB3 to detect receptor co-association/dimerization. Blots are representative of results from at least three independent experiments.
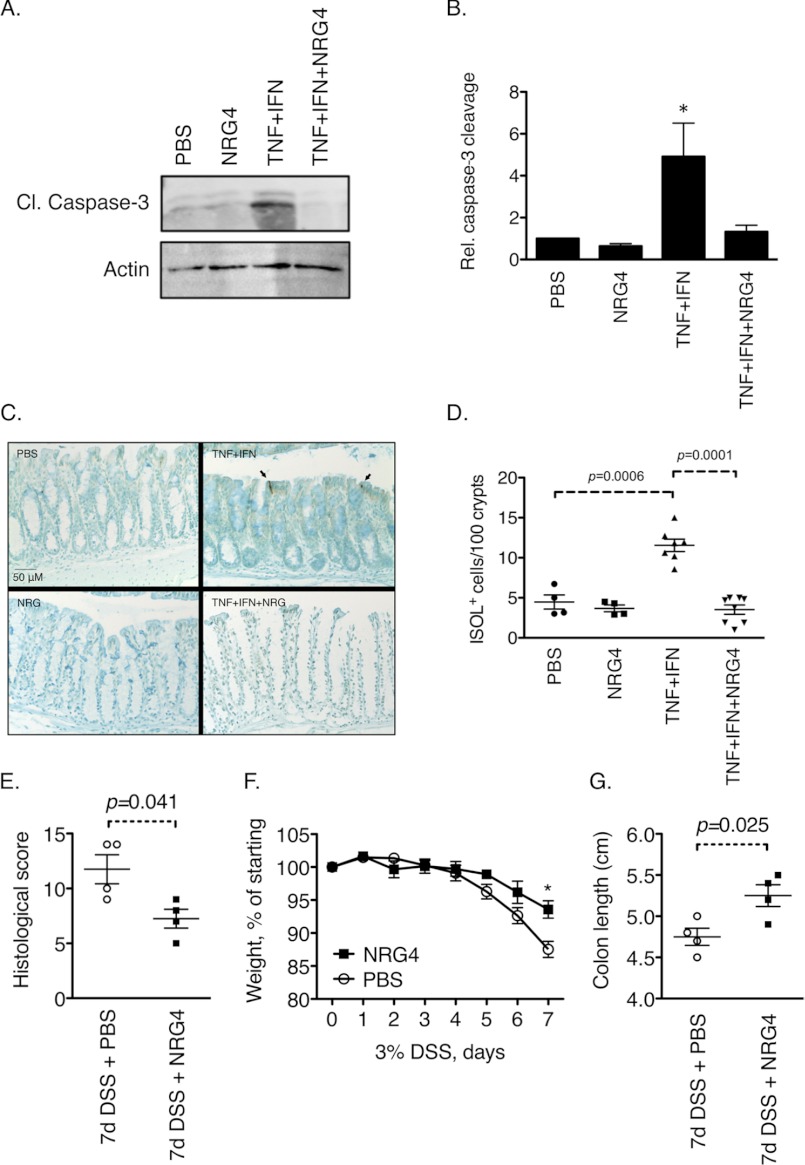
To translate these results into an in vivo setting, we injected 6–8-week-old C57Bl/6 mice intraperitoneally with 250 μg/kg TNF plus 250 units/g IFN-γ, with or without NRG4 (100 μg/kg). 24 h after injections, colons were harvested. In agreement with the cell culture data, Western blot analysis for cleaved caspase-3 on isolated epithelial cells determined that NRG4 blocked cytokine-induced apoptosis in vivo (Fig. 3, A and B; 3.6-fold decrease, p = 0.012). Similar results were obtained with Western blot analysis for cleaved poly(ADP-ribose) polymerase (data not shown). ISOL staining on sections of fixed, paraffin-embedded colons also showed a significant reduction in epithelial apoptosis with NRG4 (Fig. 3, C and D; 3.1-fold decrease, p < 0.0001). Together, our data show that NRG4 activation of ErbB4 in colon epithelial cells protects them from cytokine-induced apoptosis both in cell culture and in vivo.
FIGURE 3.
NRG4 blocks cytokine-induced apoptosis in vivo and improves experimental colitis. A–D, mice were injected intraperitoneally with TNF (250 μg/kg) plus IFN-γ (250 units/g), with or without NRG4 (100 μg/kg). After 24 h, colons were excised. Apoptosis was assessed by Western blot (A and B) for cleaved caspase-3 on epithelial isolates (graph in B is a quantification of blots from four mice per condition) and ISOL stain (C and D) (images in C show representative labeled cells) on sections of fixed, paraffin-embedded tissue. E–G, mice were given 3% DSS for 7 days and injected daily with either PBS vehicle or NRG4. Disease parameters, including histopathological scoring on fixed, rolled colons (E), weight loss over the course of the 7-day DSS exposure (F), and colon shortening (G), were determined. Error bars, S.E.
NRG4 Reduces the Severity of DSS Colitis
Because increased levels of colonic epithelial apoptosis may be involved in IBD (10, 11), we asked whether NRG4 affects the course of acute experimental colitis using the well characterized DSS model (23). 8-week-old C57Bl/6 mice were given 3% DSS in drinking water for 7 days to induce colitis, with either PBS or NRG4 (100 μg/kg) daily by injection. Severity of disease was determined by histological scores (18), weight loss, and colon shortening. All three parameters were significantly improved by NRG4 administration (Fig. 3, E–G). No inflammation/damage, weight change, or altered colon length were observed in mice given PBS or NRG4 without DSS (data not shown). These results suggest that NRG4 promotion of cell survival may translate to protection from colitis.
Colonocyte Proliferation and Migration Are Not Affected by NRG4
Most ErbB ligands stimulate multiple cellular processes, including proliferation and migration, in colon epithelial cells (24). To determine if NRG4 is similarly broad in its cellular effects, we subjected YAMC-B4 cells to proliferation assays in the presence of NRG4. After 24-h stimulation with NRG4, cellular uptake of the modified nucleoside EdU was not different from control, whereas in contrast, EGF stimulated proliferation by 31.5% over control (Fig. 4A). In vivo, the number of Ki67-positive nuclei per crypt in PBS- versus NRG4-injected mice was not altered 24 h postinjection (Fig. 4B).
FIGURE 4.
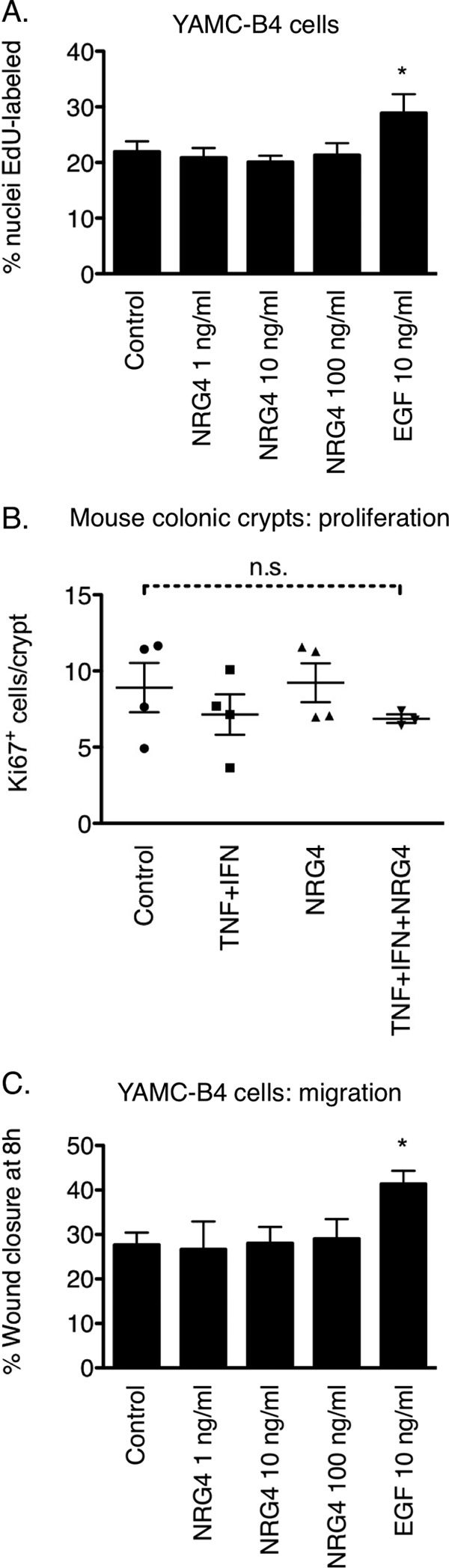
NRG4 does not stimulate colonocyte proliferation or migration. A, YAMC-B4 cells were given NRG4 or EGF (10 ng/ml, positive control for cell stimulation) for 24 h and then labeled with EdU to determine proliferative index. The graph depicts results from three independent experiments. *, p < 0.01 versus all other columns. B, fixed colon sections from PBS- or NRG4-injected mice were immunostained for the proliferative marker Ki67, and the number of labeled cells/crypt was counted. Data points are average cells/crypt in individual mice. n.s., not significant. C, YAMC-B4 cells were subjected to an 8-h migration/restitution assay in the presence of NRG4 or EGF. *, p < 0.01 versus all other columns. Error bars, S.E.
To test the potential effect of NRG4-ErbB4 signaling on cell motility, we used a modified scratch assay for restitution/migration (21). Over an 8-h period, NRG4 had no effect on the rate at which YAMC-B4 cells moved into the denuded area of a culture plate (Fig. 4C), whereas in contrast, EGF caused a 54% increase in migration over control. Together, these results indicate that, unlike many other growth factors, NRG4 selectively promotes colon epithelial cell survival without affecting cell proliferation or migration.
NRG4-ErbB4 Signaling Stimulates Akt Activation
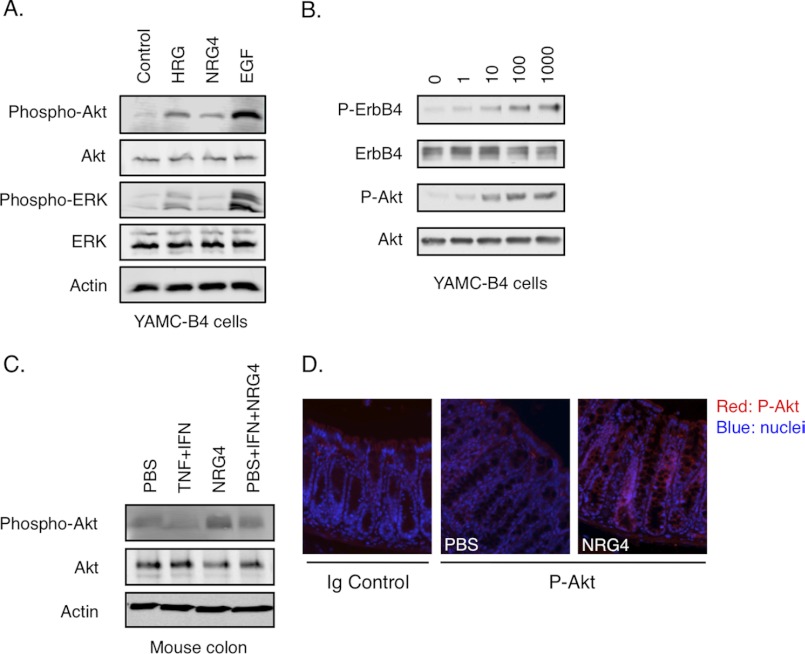
To identify signaling pathways that could be involved in NRG4-stimulated cell survival, we exposed YAMC-B4 cells to NRG4, HRG-1β, or EGF and prepared whole cell lysates for Western blot analysis. NRG4 exposure (100 ng/ml, 10 min) resulted in phosphorylation of Akt but not ERK MAPK (Fig. 5A) in a dose-dependent manner (Fig. 5B), in contrast to HRG-1β and EGF, which activated both cascades. Furthermore, COX-2 expression, which is induced by either HRG-1β or EGF after 6-h exposure and is required for cell survival induced by ErbB4-EGFR dimers (14), was not induced by NRG4 (data not shown). Thus, NRG4 promotes cell survival through pathways at least partly distinct from those activated by other ligands. Similar to the cell culture data, Western blot analysis of colon epithelial cells isolated from mice injected with NRG4 (with or without TNF plus IFN-γ) showed increased Akt phosphorylation versus controls (Fig. 5C); increased Akt phosphorylation was also detectable by immunofluorescence analysis on fixed colons from NRG4-injected mice (Fig. 5D).
FIGURE 5.
Akt phosphorylation is stimulated by NRG4 in vitro and in vivo. A, YAMC-B4 cells were stimulated with NRG4 for 10 min, and whole cell lysates were prepared. B, cells were treated with 0–1000 ng/ml NRG4. C, mice were injected with NRG4 with or without TNF + IFN-γ; after 24 h, epithelial homogenates were prepared. Expression and phosphorylation of the indicated molecules were determined by Western blot analysis. D, Akt phosphorylation in fixed, paraffin-embedded colonic tissue was assessed by immunofluorescence analysis. Data are representative of at least three independent experiments or mice per condition. P-Akt, phospho-Akt.
PI3K/Akt Signaling Is Necessary for NRG4-induced Cell Survival in Vitro and in Vivo
To test the role of PI3K/Akt signaling in NRG4-stimulated YAMC-B4 cell survival, we added the PI3K inhibitor LY294002 (5 μm) or wortmannin (10 nm) to cultures incubated with TNF plus IFN-γ or TNF plus IFN-γ and NRG4. Immunofluorescence analysis for cleaved caspase-3 showed that the PI3K inhibitors reversed the protective effect of NRG4 in YAMC-B4 cells (Fig. 6A). In vivo, injection of either LY294002 (50 μg/kg) or wortmannin (1 mg/kg) reversed the NRG4 blockade of cytokine-induced colon epithelial apoptosis as measured by ISOL stain (Fig. 6B).
FIGURE 6.
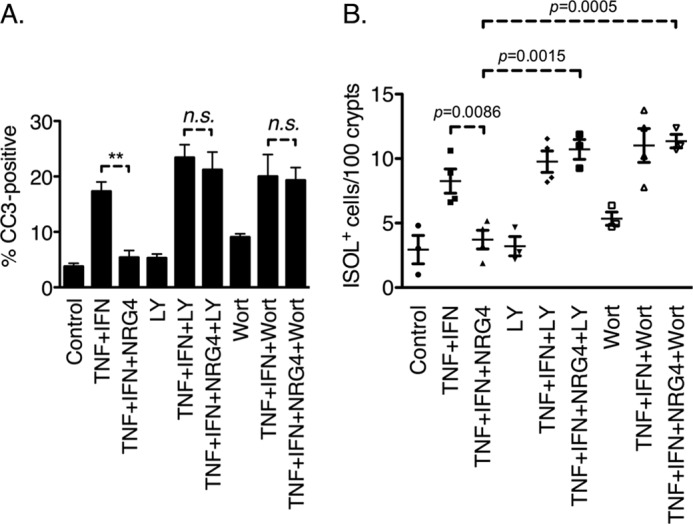
PI3K inhibition blocks NRG4 anti-apoptotic effects. A, YAMC-B4 cells were exposed to TNF + IFN-γ, with or without NRG4 and/or inhibitors to PI3K (LY294002 (LY) or wortmannin (Wort)). After 6 h, cells were fixed, and apoptosis was assessed by immunofluorescent staining for cleaved caspase-3. Data are representative of four independent experiments. n.s., not significant. B, mice were injected with TNF + IFN-γ, with or without NRG4 and/or PI3K inhibitors. After 24 h, colons were fixed, and apoptosis was quantified by ISOL stain.
NRG4 Expression Is Reduced in Crohn's Disease and Ulcerative Colitis
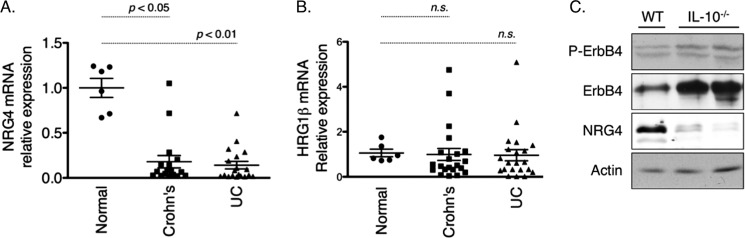
To investigate the potential clinical relevance of a colon epithelial cell survival pathway driven by NRG4, we studied the ligand's expression in human inflammatory bowel disease. qPCR analysis of specimens from patients with active Crohn's disease and ulcerative colitis showed 5.6-fold (p < 0.05) and 7.1-fold (p < 0.01) reductions, respectively, in NRG4 expression versus uninflamed controls (Fig. 7A). In contrast, we detected no change in expression of the shared ErbB3/ErbB4 ligand HRG-1β (Fig. 7B), suggesting a selective loss of the specific ErbB4 ligand only. Among the UC and CD groups, no correlation between gender, age, or location of the lesion with expression of either ligand was observed (data not shown). In the IL-10−/− mouse model of chronic colitis (19), NRG4 protein levels were reduced in colitic animals as determined by Western blot analysis of colonic homogenates (Fig. 7C). Furthermore, whereas ErbB4 expression was elevated in the inflamed colons, similar to what we previously reported for human IBD or murine DSS colitis (12), ErbB4 phosphorylation was not increased (Fig. 7C). Densitometric analysis showed a relative 43 ± 6.9% decrease of the normalized phosphorylated/total ErbB4 ratio in IL-10−/− mice. Thus, loss of the NRG4 ligand is associated with failure to activate ErbB4 despite up-regulation of the receptor.
FIGURE 7.
NRG4 levels are decreased in inflammatory bowel disease. A and B, qPCR analysis for NRG4 (A) and HRG-1β (B) gene expression was performed on TissueScan Crohn's/colitis qPCR arrays. Relative mRNA levels were calculated using the 2−ΔΔCT method with β-actin as the reference. C, colonic homogenates from wild type controls (WT) or IL-10−/− mice were subjected to Western blot analysis for ErbB4, phospho-ErbB4 (P-ErbB4), and NRG4. Results shown are representative of four mice per genotype. UC, ulcerative colitis.
DISCUSSION
Previous investigation into the role of the ErbB4 receptor tyrosine kinase in epithelial tissues has largely relied on results using ligands, such as HB-EGF or HRG-1β (14, 25), which also activate other ErbB family members (e.g. see Fig. 2). In this study, we used NRG4 to specifically activate ErbB4 in colonocytes. We find that ErbB4 activation in the absence of detectable phosphorylation of EGFR, ErbB2, or ErbB3 results in an increase in anti-apoptotic signaling both in vitro (Fig. 1) and in vivo (Fig. 3), with no change in cell proliferation or migration (Fig. 4). NRG4-induced suppression of apoptosis was dependent on the PI3K/Akt pathway (Figs. 5 and 6). Thus, selective activation of ErbB4 with NRG4 appears to be a specific cell survival stimulus.
Our data show phosphorylation of ErbB4, but not other ErbBs, in response to NRG4. Taken together with previous observations that NRG4 does not directly bind other receptors (7), this suggests that ErbB4 homodimers are the effective mediator of its signaling. It is formally possible that asymmetric heterodimers (26, 27) play a role in NRG4-induced effects. However, although there is some base-line association between ErbB4 and other family members in mouse colonocytes, we have not been able to detect NRG4-driven changes in this association (Fig. 2B). Thus, in the absence of their phosphorylation or increased ErbB4 association, it seems unlikely that ErbB1 to −3 play a significant role in downstream signaling in response to NRG4.
In agreement with our results, an anti-apoptotic role for ErbB4 signaling has been demonstrated in several systems, including PC12 cells (28), colorectal cancer cells (29), and breast cancer cells (30). However, it should be noted that this receptor's relationship to cell survival is complex. ErbB4-induced survival responses in 3T3 cells are isoform-dependent (31), and under certain circumstances, overexpression of constitutively active ErbB4 or ErbB4 ICD fragments can actually trigger apoptosis of mammary epithelial cells (32, 33). Although these disparate findings may in part be explained by differential expression of alternatively spliced ErbB4 forms (34), in colon epithelial cells, the cell survival effects of ErbB4 expression do not appear to be isoform-dependent (12). The available dimerization partners in a given cell, specific ligands present in the tissue microenvironment, and overall ErbB4 expression/activation status are additional factors that may be important in tuning a cell's response. Defining the ways in which cellular context regulates ErbB4 signaling will be important to define the biology and potential therapeutic uses of this receptor and its ligands.
In this context, it is interesting that NRG4, unlike other ErbB ligands, promotes survival but not proliferation or migration of colon epithelial cells. In proteomic peptide-binding studies, ErbB4 associates with a more restricted suite of Src homology 2 domain-containing targets than EGFR, ErbB2, or ErbB3 (8). Furthermore, in the current report, we show that specific ErbB4 activation elicits only a subset of the downstream signaling, including activation of Akt, but not ERK MAPK, compared with other ErbB ligands, including HRG-1β (Fig. 5 and Ref. 12), HB-EGF (35), and TGF-α (36). These observations are consistent with our data showing that NRG4 is selectively a survival factor, thus positioning ErbB4 as the only family member that can promote cell survival without affecting proliferation or migration. Interestingly, some pathways (e.g. COX-2) required for the cell survival response to HRG-1β are apparently not necessary in the case of NRG4; whether the absence of proliferative signaling (e.g. ERK) with NRG4 narrows the requirement for cell protection from apoptosis or, in contrast, an overlapping but distinct set of alternative pathways are activated by the different ligands, is an area of ongoing study in our laboratory.
Specificity for cell survival but not cell division is in agreement with the apparent lower oncogenic potential reported in the literature for ErbB4 versus other receptor tyrosine kinases. Although increased levels or activity of EGFR, ErbB2, or ErbB3 are in general associated with increased tumor growth, the role of ErbB4 is less certain. It is overexpressed in endometrial (37) and non-small cell lung cancers (38), whereas transitional cell carcinoma of the bladder (39, 40) and prostate cancer (41, 42) show either no correlation between ErbB4 levels and tumor behavior or an association between expression and good prognosis. Studies on breast cancer have yielded a contradictory literature, with different papers suggesting that ErbB4 expression is associated with either poor (43, 44) or favorable (45, 46) outcome. These apparently inconsistent findings may be in part explained by our results if ErbB4 signaling per se does not necessarily promote cell proliferation. ErbB4 activation by HRG-1β, which stimulates multiple receptors, does activate cancer-associated pathways, such as COX-2, but this response is dependent on partnering with EGFR (14). It may be that ErbB4 only promotes tumorigenesis in partnership with other, more frankly oncogenic, ErbBs, as in for example ErbB2/4 heterodimerization observed in late stage colorectal cancers (47) or the results of Lee et al. (29) identifying ErbB3/4 dimers as tumor promoters.
The ability to block cytokine-stimulated colonocyte apoptosis, combined with the decreased risk for proliferative disorders compared with other growth factors, makes NRG4 an attractive potential therapy for conditions, such as IBD, that involve elevated apoptosis in the epithelium of the small intestine or colon (10, 11). Because ErbB4 has a number of biochemical features (including being the sole receptor for NRG4) that distinguish it from other ErbB family members (48), it is a unique and specific signaling target. In this regard, the observations that (a) NRG4 is deficient in both human IBD and murine colitis (Fig. 7A) and (b) although ErbB4 expression is elevated in the IL-10−/− murine colitis model, it is not phosphorylated/activated (Fig. 7C), raise the possibility that NRG4 down-regulation may lead to deficient epithelial cell survival signaling despite ErbB4 up-regulation. Consistent with this possibility, recent work from Feng and Teitelbaum (49) showed loss of NRG4 in a model of total parenteral nutrition, which is associated with increased inflammatory cytokines and decreased Akt phosphorylation (50). A dysregulated NRG4/ErbB4 balance and an altered ratio between NRG4 and other ErbB ligands may be important features of IBD that can be addressed with exogenous ligand. Ongoing work in our laboratory is aimed at further defining NRG4 regulation in IBD, including possible clinical parameters affecting ligand expression that were not revealed by the sample set available for the current study.
In summary, our data show that selective activation of the ErbB4 receptor tyrosine kinase with its specific ligand NRG4 is a survival signal in colon epithelial cells. This pathway activates PI3K/Akt signaling and blocks inflammatory cytokine-induced apoptosis without affecting cell proliferation or migration. However, this pathway is deficient in IBD due to a loss of the ligand. Our observations underscore the unique properties of ErbB4 compared with other ErbB family members and suggest that selective ErbB4 activation represents a divergent branch of receptor tyrosine kinase signaling with potential therapeutic use in injury or inflammatory diseases.
Acknowledgments
We thank Drs. David Warburton, Michael Stallcup, Andre Ouellette, and D. Brent Polk for helpful discussions during preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants K01DK077956 and R03DK090295. This work was also supported by a Senior Research Award from the Crohn's and Colitis Foundation of America and a Research Career Development Award from the Saban Research Institute.
- EGFR
- EGF receptor
- IBD
- inflammatory bowel disease
- NRG4
- neuregulin-4
- HB-EGF
- heparin-binding EGF-like growth factor
- HRG
- heregulin
- YAMC
- young adult mouse colon
- ISOL
- in situ oligonucleotide ligation
- EdU
- 5-ethynyl-2-deoxyuridine
- qPCR
- real-time quantitative PCR
- DSS
- dextran sulfate sodium.
REFERENCES
- 1. Wieduwilt M. J., Moasser M. M. (2008) The epidermal growth factor receptor family. Biology driving targeted therapeutics. Cell Mol. Life Sci. 65, 1566–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson K. J., Gilmore J. L., Foley J., Lemmon M. A., Riese D. J., 2nd. (2009) Functional selectivity of EGF family peptide growth factors. Implications for cancer. Pharmacol. Ther. 122, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bublil E. M., Yarden Y. (2007) The EGF receptor family. Spearheading a merger of signaling and therapeutics. Curr. Opin. Cell Biol. 19, 124–134 [DOI] [PubMed] [Google Scholar]
- 4. Saito T., Okada S., Ohshima K., Yamada E., Sato M., Uehara Y., Shimizu H., Pessin J. E., Mori M. (2004) Differential activation of epidermal growth factor (EGF) receptor downstream signaling pathways by betacellulin and EGF. Endocrinology 145, 4232–4243 [DOI] [PubMed] [Google Scholar]
- 5. Sweeney C., Lai C., Riese D. J., 2nd, Diamonti A. J., Cantley L. C., Carraway K. L., 3rd (2000) Ligand discrimination in signaling through an ErbB4 receptor homodimer. J. Biol. Chem. 275, 19803–19807 [DOI] [PubMed] [Google Scholar]
- 6. Jones J. T., Akita R. W., Sliwkowski M. X. (1999) Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 447, 227–231 [DOI] [PubMed] [Google Scholar]
- 7. Harari D., Tzahar E., Romano J., Shelly M., Pierce J. H., Andrews G. C., Yarden Y. (1999) Neuregulin-4. A novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene 18, 2681–2689 [DOI] [PubMed] [Google Scholar]
- 8. Kaushansky A., Gordus A., Budnik B. A., Lane W. S., Rush J., MacBeath G. (2008) System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem. Biol. 15, 808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strober W., Fuss I., Mannon P. (2007) The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 117, 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qiu W., Wu B., Wang X., Buchanan M. E., Regueiro M. D., Hartman D. J., Schoen R. E., Yu J., Zhang L. (2011) PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J. Clin. Invest. 121, 1722–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Sabatino A., Ciccocioppo R., Luinetti O., Ricevuti L., Morera R., Cifone M. G., Solcia E., Corazza G. R. (2003) Increased enterocyte apoptosis in inflamed areas of Crohn's disease. Dis. Colon Rectum 46, 1498–1507 [DOI] [PubMed] [Google Scholar]
- 12. Frey M. R., Edelblum K. L., Mullane M. T., Liang D., Polk D. B. (2009) The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology 136, 217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hilliard V. C., Frey M. R., Dempsey P. J., Peek R. M., Jr., Polk D. B. (2011) TNF-α converting enzyme-mediated ErbB4 transactivation by TNF promotes colonic epithelial cell survival. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G338–G346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frey M. R., Hilliard V. C., Mullane M. T., Polk D. B. (2010) ErbB4 promotes cyclooxygenase-2 expression and cell survival in colon epithelial cells. Lab. Invest. 90, 1415–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitehead R. H., VanEeden P. E., Noble M. D., Ataliotis P., Jat P. S. (1993) Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 90, 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayes N. V., Newsam R. J., Baines A. J., Gullick W. J. (2008) Characterization of the cell membrane-associated products of the Neuregulin 4 gene. Oncogene 27, 715–720 [DOI] [PubMed] [Google Scholar]
- 17. Whitehead R. H., Robinson P. S. (2009) Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G455–G460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dieleman L. A., Palmen M. J., Akol H., Bloemena E., Peña A. S., Meuwissen S. G., Van Rees E. P. (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 [DOI] [PubMed] [Google Scholar]
- 20. Frey M. R., Dise R. S., Edelblum K. L., Polk D. B. (2006) p38 kinase regulates epidermal growth factor receptor down-regulation and cellular migration. EMBO J. 25, 5683–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corredor J., Yan F., Shen C. C., Tong W., John S. K., Wilson G., Whitehead R., Polk D. B. (2003) Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am. J. Physiol. Cell Physiol. 284, C953–C961 [DOI] [PubMed] [Google Scholar]
- 22. Huotari M. A., Miettinen P. J., Palgi J., Koivisto T., Ustinov J., Harari D., Yarden Y., Otonkoski T. (2002) ErbB signaling regulates lineage determination of developing pancreatic islet cells in embryonic organ culture. Endocrinology 143, 4437–4446 [DOI] [PubMed] [Google Scholar]
- 23. Egger B., Büchler M. W., Lakshmanan J., Moore P., Eysselein V. E. (2000) Mice harboring a defective epidermal growth factor receptor (waved-2) have an increased susceptibility to acute dextran sulfate-induced colitis. Scand. J. Gastroenterol 35, 1181–1187 [DOI] [PubMed] [Google Scholar]
- 24. Frey M. R., Golovin A., Polk D. B. (2004) Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J. Biol. Chem. 279, 44513–44521 [DOI] [PubMed] [Google Scholar]
- 25. Ni C. Y., Murphy M. P., Golde T. E., Carpenter G. (2001) γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294, 2179–2181 [DOI] [PubMed] [Google Scholar]
- 26. Monsey J., Shen W., Schlesinger P., Bose R. (2010) Her4 and Her2/neu tyrosine kinase domains dimerize and activate in a reconstituted in vitro system. J. Biol. Chem. 285, 7035–7044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macdonald-Obermann J. L., Piwnica-Worms D., Pike L. J. (2012) Mechanics of EGF receptor/ErbB2 kinase activation revealed by luciferase fragment complementation imaging. Proc. Natl. Acad. Sci. U.S.A. 109, 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erlich S., Goldshmit Y., Lupowitz Z., Pinkas-Kramarski R. (2001) ErbB-4 activation inhibits apoptosis in PC12 cells. Neuroscience 107, 353–362 [DOI] [PubMed] [Google Scholar]
- 29. Lee D., Yu M., Lee E., Kim H., Yang Y., Kim K., Pannicia C., Kurie J. M., Threadgill D. W. (2009) Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J. Clin. Invest. 119, 2702–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Määttä J. A., Sundvall M., Junttila T. T., Peri L., Laine V. J., Isola J., Egeblad M., Elenius K. (2006) Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol. Biol. Cell 17, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kainulainen V., Sundvall M., Määttä J. A., Santiestevan E., Klagsbrun M., Elenius K. (2000) A natural ErbB4 isoform that does not activate phosphoinositide 3-kinase mediates proliferation but not survival or chemotaxis. J. Biol. Chem. 275, 8641–8649 [DOI] [PubMed] [Google Scholar]
- 32. Naresh A., Long W., Vidal G. A., Wimley W. C., Marrero L., Sartor C. I., Tovey S., Cooke T. G., Bartlett J. M., Jones F. E. (2006) The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 66, 6412–6420 [DOI] [PubMed] [Google Scholar]
- 33. Vidal G. A., Clark D. E., Marrero L., Jones F. E. (2007) A constitutively active ERBB4/HER4 allele with enhanced transcriptional coactivation and cell-killing activities. Oncogene 26, 462–466 [DOI] [PubMed] [Google Scholar]
- 34. Veikkolainen V., Vaparanta K., Halkilahti K., Iljin K., Sundvall M., Elenius K. (2011) Function of ERBB4 is determined by alternative splicing. Cell Cycle 10, 2647–2657 [DOI] [PubMed] [Google Scholar]
- 35. Bongers G., Muniz L. R., Pacer M. E., Iuga A. C., Thirunarayanan N., Slinger E., Smit M. J., Reddy E. P., Mayer L., Furtado G. C., Harpaz N., Lira S. A. (2012) A role for the epidermal growth factor receptor signaling in development of intestinal serrated polyps in mice and humans. Gastroenterology 143, 730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCole D. F., Keely S. J., Coffey R. J., Barrett K. E. (2002) Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-α. J. Biol. Chem. 277, 42603–42612 [DOI] [PubMed] [Google Scholar]
- 37. Srinivasan R., Benton E., McCormick F., Thomas H., Gullick W. J. (1999) Expression of the c-erbB-3/HER-3 and c-erbB-4/HER-4 growth factor receptors and their ligands, neuregulin-1 α, neuregulin-1 β, and betacellulin, in normal endometrium and endometrial cancer. Clin. Cancer Res. 5, 2877–2883 [PubMed] [Google Scholar]
- 38. Starr A., Greif J., Vexler A., Ashkenazy-Voghera M., Gladesh V., Rubin C., Kerber G., Marmor S., Lev-Ari S., Inbar M., Yarden Y., Ben-Yosef R. (2006) ErbB4 increases the proliferation potential of human lung cancer cells and its blockage can be used as a target for anti-cancer therapy. Int. J. Cancer 119, 269–274 [DOI] [PubMed] [Google Scholar]
- 39. Memon A. A., Sorensen B. S., Melgard P., Fokdal L., Thykjaer T., Nexo E. (2004) Expression of HER3, HER4 and their ligand heregulin-4 is associated with better survival in bladder cancer patients. Br. J. Cancer 91, 2034–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Røtterud R., Nesland J. M., Berner A., Fosså S. D. (2005) Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 95, 1344–1350 [DOI] [PubMed] [Google Scholar]
- 41. Edwards J., Traynor P., Munro A. F., Pirret C. F., Dunne B., Bartlett J. M. (2006) The role of HER1-HER4 and EGFRvIII in hormone-refractory prostate cancer. Clin. Cancer Res. 12, 123–130 [DOI] [PubMed] [Google Scholar]
- 42. Robinson D., He F., Pretlow T., Kung H. J. (1996) A tyrosine kinase profile of prostate carcinoma. Proc. Natl. Acad. Sci. U.S.A. 93, 5958–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Srinivasan R., Gillett C. E., Barnes D. M., Gullick W. J. (2000) Nuclear expression of the c-erbB-4/HER-4 growth factor receptor in invasive breast cancers. Cancer Res. 60, 1483–1487 [PubMed] [Google Scholar]
- 44. Junttila T. T., Sundvall M., Lundin M., Lundin J., Tanner M., Härkönen P., Joensuu H., Isola J., Elenius K. (2005) Cleavable ErbB4 isoform in estrogen receptor-regulated growth of breast cancer cells. Cancer Res. 65, 1384–1393 [DOI] [PubMed] [Google Scholar]
- 45. Tovey S. M., Witton C. J., Bartlett J. M., Stanton P. D., Reeves J. R., Cooke T. G. (2004) Outcome and human epidermal growth factor receptor (HER) 1–4 status in invasive breast carcinomas with proliferation indices evaluated by bromodeoxyuridine labelling. Breast Cancer Res. 6, R246–R251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Witton C. J., Reeves J. R., Going J. J., Cooke T. G., Bartlett J. M. (2003) Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J. Pathol. 200, 290–297 [DOI] [PubMed] [Google Scholar]
- 47. Lee J. C., Wang S. T., Chow N. H., Yang H. B. (2002) Investigation of the prognostic value of coexpressed erbB family members for the survival of colorectal cancer patients after curative surgery. Eur. J. Cancer 38, 1065–1071 [DOI] [PubMed] [Google Scholar]
- 48. Carpenter G. (2003) ErbB-4. Mechanism of action and biology. Exp. Cell Res. 284, 66–77 [DOI] [PubMed] [Google Scholar]
- 49. Feng Y., Teitelbaum D. H. (2012) Epidermal growth factor/TNF-α transactivation modulates epithelial cell proliferation and apoptosis in a mouse model of parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G236–G249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feng Y., Ralls M. W., Xiao W., Miyasaka E., Herman R. S., Teitelbaum D. H. (2012) Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Ann. N.Y. Acad. Sci. 1258, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]