Abstract
The self-fertilizing mangrove killifish, Kryptolebias marmoratus, is an upcoming model species for a range of biological disciplines. To further establish this model in the field of developmental biology, we examined several techniques for embryonic manipulation and for imaging that can be used in an array of experimental designs. These methodological approaches can be divided into two categories: handling of embryos with and without their chorionic membrane. Embryos still enclosed in their chorion can be manipulated using an agarose bed or a methyl cellulose system, holding them in place and allowing their rotation to more specific angles and positions. Using these methods, we demonstrate microinjection of embryos and monitoring of fluorescent yolk syncytial nuclei (YSN) using both stereo and compound microscopes. For higher magnification imaging using compound microscopes as well as time-lapse analyses, embryos were dechorionated and embedded in low-melting-point agarose. To demonstrate this embedding technique, we further examined fluorescent YSN and also analyzed the yolk surface of K. marmoratus embryos. The latter was observed to provide an excellent imaging platform for study of the behavior and morphology of cells during embryonic development, for various types of cells. Our data demonstrate that K. marmoratus is an excellent model species for research in developmental biology, as methodological approaches for the manipulation and imaging of embryos are efficient and readily available.
Introduction
Found in tropical mangrove forests of the Americas, the amphibious mangrove killifish, Kryptolebias marmoratus, has the potential of becoming a strong comparative model for a range of biological disciplines. This capacity for fame lies, partly, in its ability to self-fertilize, a process known to occur in invertebrate animals (Jarne and Aude 2006), but until the early 1960s was unknown for vertebrates (Harrington 1961). Indeed, most individuals of K. marmoratus are hermaphroditic and possess an ovotestis (Sakakura et al. 2006), giving them the potential to produce homozygous progeny (Kallman and Harrington 1964). Mangrove killifish also possess an androdioecious reproductive system, meaning that populations are composed of males and hermaphrodites (Tatarenkov et al. 2007). Thus, there remains an opportunity for outcrossing (be it in the wild, or in the laboratory), providing the basis for the formation of new clonal lines (Mackiewicz et al. 2006). There are currently 21 established and genetically verified clonal lines (Tatarenkov et al. 2010), of which the Hon9 line is used in the present study.
Primary males are rare in most of the wild populations (Turner et al. 2006) and can be produced by incubating embryos during late stages of development at temperatures no higher than 20°C (Harrington 1967, 1968). Secondary males may arise from hermaphrodites that lose their female reproductive function late in their life cycle, but also can be achieved by exposing fish to high temperatures and shortened photoperiod (Harrington 1967, 1971). In addition, primary males can also be produced efficiently in the laboratory by treating embryos with 17α-methyltestosterone (Kanamori et al. 2006).
On top of their self-fertilizing ability, mangrove killifish are also extremely resilient fish. They are capable of surviving extreme and rapid changes in salinity and over a broad range of temperatures (King et al. 1989; Taylor et al. 1995). They are considered amphibious and can breathe air through a network of cutaneous capillaries and blood vessels in their fins (Grizzle and Thiyagarajah 1987). Mangrove killifish are easily maintained and kept in the laboratory, and great interest has been shown in their ecology physiology, and genetics. As previously mentioned, their unique ability to self-fertilize offers researchers the ability to work with “isogenic” (genetically identical) individuals. Highly inbred strains of an organism give rise to a specific phenotype, which confers particular advantages for research. Inbreeding for specific characteristics is a common practice in research, using the mammalian model Mus musculus (Beck et al. 2000). The self-fertilizing mangrove killifish allows researchers to more readily work with homozygous individuals, without the strenuous procedures involved in inbreeding and maintenance of animals such as M. musculus. Being an oviparous fish, K. marmoratus offers all the advantages of popular models such as zebrafish or medaka, with the additional benefit of unique genetics. Thus, it is important to fully establish the mangrove killifish as a model species. In an effort to build the embryological knowledge and methods available to researchers, in this article, we focus on the creation of tools for the manipulation of K. marmoratus’ embryos.
We recently published a staging series for the mangrove killifish, providing detailed micrographs of the various morphological features associated with each stage of development from fertilization to hatching (Mourabit et al. 2011). Building on this literature, we present additional information and procedures for the manipulation and imaging of K. marmoratus embryos in experimental research. We have established key protocols for the analyses of embryos under light and fluorescent microscopy, for both stereomicroscopes and compound microscopes. These involve handling techniques, using embryos still surrounded by their chorion (or eggshell), as well as dechorionated embryos. In addition, we provide detailed instructions for the microinjection of DNA or RNA, further introducing molecular techniques into embryological research on K. marmoratus. As an example for imaging studies, cells and other structures on the embryonic yolk’s surface were analyzed in detail. We report that the surface of the yolk of K. marmoratus provides an excellent imaging platform for the study of cell behavior during embryonic development for several types of cells, such as melanophores and endothelial cells.
Materials and methods
Experimental animals
Mangrove killifish of the Hon9 clonal lineage were obtained from an existing stock at the University of Exeter (UK). Hermaphrodites were kept individually in 1.5 L plastic containers (25°C, 14‰ salinity, 12:12 h light:dark photoperiod), and were fed daily on Artemia nauplii. Brackish water was made using demineralized water and marine salts (Tropic Marin, Germany). Eggs were collected from either spawning mops or aquaria filter pads placed in the tanks (both providing a substrate for oviposition). All embryos were reared under controlled conditions (25°C, 14‰ salinity). Embryos still encased in their chorion were simply incubated in plastic petri dishes, and dechorionated embryos (more fragile and susceptible to injuries) were kept in dishes lined with a 1% agarose bed (using 14‰ brackish water) (Sigma A9539).
Experimental protocols
Imaging
Low-magnification micrographs were taken using a Nikon Digital Sight DS-U2 camera mounted on a Nikon SMZ1500 microscope, and an Olympus XC10 camera mounted on an Olympus SZX16 microscope. High-magnification micrographs of multiple cell layers were taken using a Zeiss AxioCam MRm camera mounted on a Zeiss Axio Observer Z1 microscope (using ×10 and ×20 dry-objective lenses and a ×40 oil-dipping lens). Time-lapse analyses of melanophores on the yolk were performed using a Leica DFC480 camera mounted on a Leica DMI 4000B microscope (using a ×20 dry-objective lens).
Imaging techniques for embryos within their chorion
Agarose
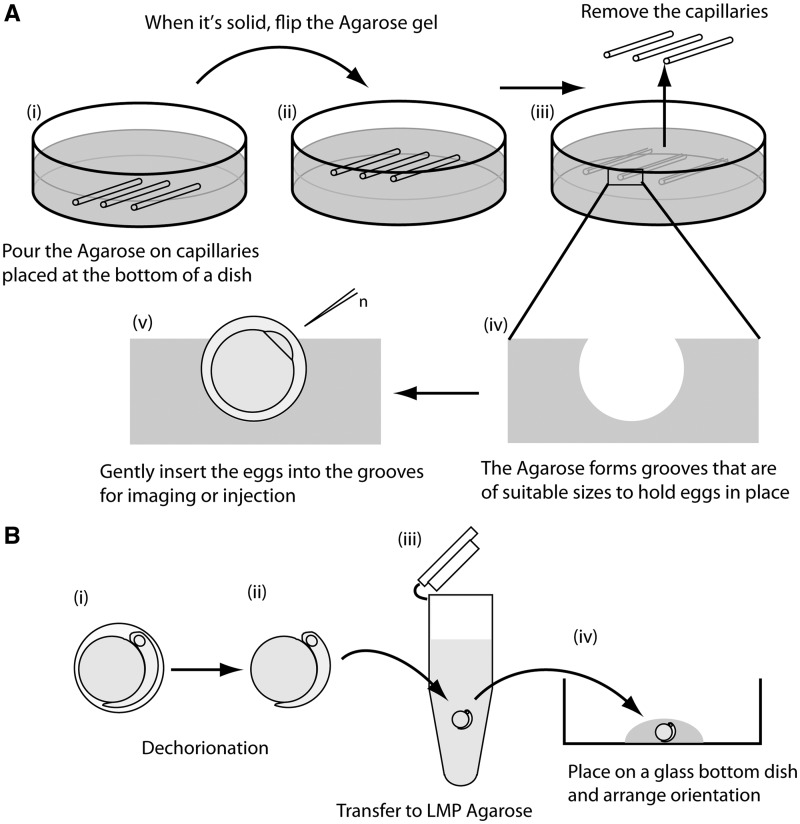
Agarose beds were prepared following the method described by Mourabit et al. (2011). Glass capillaries (1.2 mm outer diameter, Harvard Apparatus) were cut into smaller pieces (e.g., 3 cm length) and placed in a 5 cm plastic petri dish. Agarose (1% with 14‰ brackish water (Sigma A9539) was poured in the dish and left to set (Fig. 1A [i]). The solid gel was then flipped and placed back in the dish using a small spatula (Fig. 1A [ii]). The capillaries were removed and the dish was filled with 14‰ brackish water (Fig. 1A [iii]). The embryos were then gently inserted into the grooves with blunt forceps and used for imaging and for experiments on microinjection (Fig. 1A [iv and v]).
Fig. 1.
(A) Holding K. marmoratus embryos in agarose grooves. Glass capillaries (outer diameter 1.2 mm) were cut into small pieces and placed in a 5 cm plastic petri dish. Agarose (1%) was poured into the dish and left to set (i). When solid, the gel was flipped to place the capillaries upward (ii). Capillaries were removed using fine tweezers (iii) molding grooves in the agarose that are of suitable sizes to hold eggs in place (iv). After filling the dish with 14‰ brackish water, eggs were gently inserted in the grooves using blunt foreceps (v). For injection, the animal pole was positioned so that the blastomere faced the injection needle (n). (B) Embedding dechorionated embryos in LMP agarose. Embryos were dechorionated (i and ii) and, using a large mouthed glass pipette, transferred to LMP agarose (at body temperature) (iii), and then immediately transferred to a glass-bottomed culture dish (iv). Before the agarose solidified, positions and angles of the embryos were adjusted by moving them gently with a glass capillary whose tip had been melted to form a smooth round edge.
Methyl cellulose
Embryos were placed in methyl cellulose (2% in deionized water) (Sigma M0387) in a glass-bottomed culture dish (35 mm petri dish with a 14 mm glass base) (MatTek Corporation). The viscous nature of methyl cellulose keeps the embryo in place within the camera frame, and allows rotation of the egg for more specific imaging.
Imaging techniques for dechorionated embryos
In order to embed embryos in agarose, they were first enzymatically dechorionated (Mourabit et al. 2011). Note that dechorionated embryos are fragile, and their yolk can easily break if caught on an uneven surface, or if the embryo comes in contact with air or bubbles. It is important to handle these embryos with care and use a glass pipette to move them. Standard glass pipettes must be broken to obtain a bigger diameter of tip, and so the rim must be heated to polish the glass. After dechorionation (Fig. 1B [i and ii]), embryos were transferred to an eppendorf tube containing 1 mL of 0.7% low melting point (LMP) agarose (Fig. 1B [iii]) (Sigma A9414). Prior to transfer, the liquid LMP agarose (prepared at 60–70°C) must be cooled down to approximately body temperature. Once in LMP agarose, the embryos were immediately transferred to a glass-bottomed culture dish (Fig. 1B [iv]); MatTek Corporation). Positions and angles of the embryos were adjusted by moving them gently with a glass capillary (Harvard Apparatus) whose tip had been melted to form a smooth, rounded edge.
Microinjection
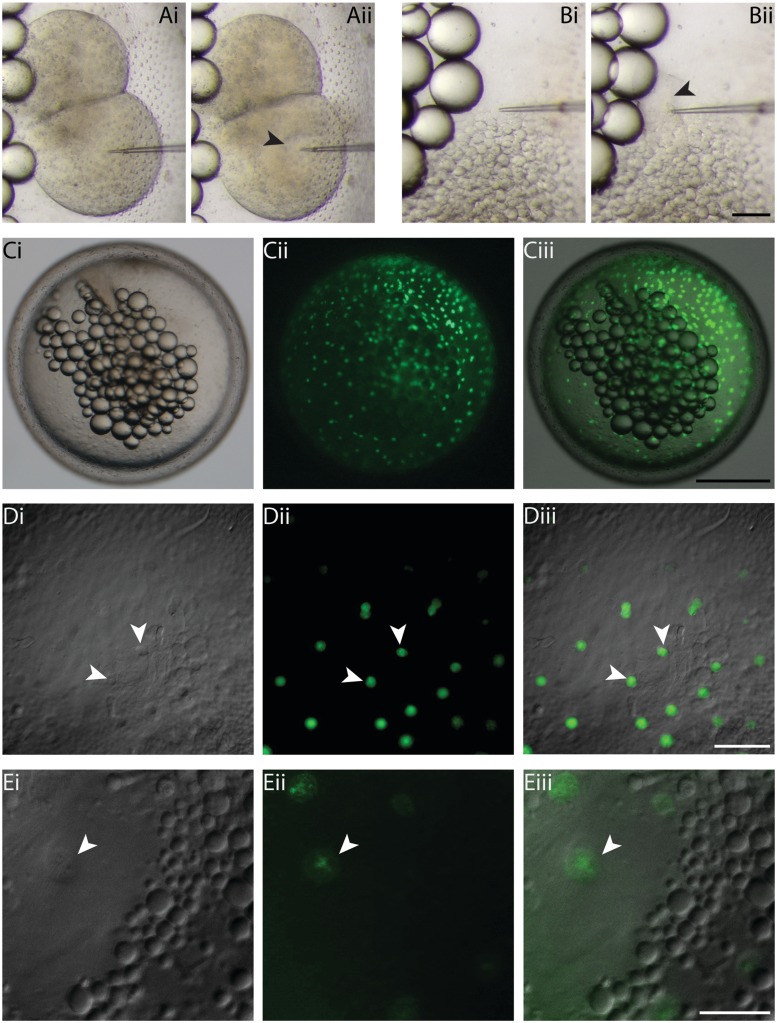
Despite their hard chorion, DNA, RNA, or chemicals can easily be microinjected into K. marmoratus embryos. To demonstrate this, embryos were injected at the two-cell and late-blastula stages (Fig. 2A and B) following the procedure described by Mourabit et al. (2011). Embryos must be placed in an agarose groove (see above), with the target location facing the microneedle (Fig. 1A [v]). Injecting at an angle is difficult as the microneedle can bend and break. It is useful to keep a pair of forceps handy in order to pull back the embryo if the needle gets stuck. For staining of the yolk syncytial layer (YSL), sytox green (Invitrogen, 0.5 mM) was injected at the late-blastula stage at the interface between the yolk and the blastoderm. Fluorescence of YSL nuclei was assessed 10–15 min after injections, and photographs were taken at Day 1 (Fig. 2C and D) and day 3 (Fig. 2E) postfertilization.
Fig. 2.
Microinjection technique and sytox green staining of YSN in K. marmoratus embryos. Using the agarose groove method, embryos can easily be microinjected in precise locations, as shown here with a two-cell (A [i and ii]) and late-blastula (B [i and ii]) injection. Despite the dark orange color of blastomeres, injected material can be tracked without the use of dyes (A [ii]; black arrowhead). To monitor fluorescence of YSN, sytox green (0.5 mM) was injected in the YSL at the late-blastula stage of development (B [ii]; black arrowhead). Fluorescence of YSN was examined in embryos still surrounded by their chorion, using a stereomicroscope (C [i–iii]), and at high magnification on an inverted compound microscope (×10 dry-objective lens) (D [i–iii]; white arrowheads). D (i) = DIC, D (ii) = fluorescence, D (iii) = overlay. To observe YSN at higher magnifications, the embryos were dechorionated and embedded in LMP agarose (×20 dry-objective lens) (E [i–iii]; white arrowheads). E (i) = DIC, E (ii) = fluorescence, E (iii) = overlay. Scale bars and associated images: 100 µm for A and B; 500 µm for C; 100 µm for D; and 50 µm for E.
Results and discussion
In order to establish the mangrove killifish as a model system for developmental biology, we examined a variety of techniques for imaging and manipulation in a wide array of experiments. Embryos may freely rotate within their chorion at any stage of development, making it difficult for researchers to photograph them in a specific position or at a particular angle, or to microinject DNA, RNA, and chemicals in specific regions of the embryo. Consequently, we have developed an agarose bed system (Fig. 1) that allows us to easily maintain K. Marmoratus embryos within the camera frame, and rotate them for specific angles without removing the chorion. To achieve this, agarose is poured on glass capillaries of a specific diameter to mould grooves that hold the embryos in place without damaging them (Fig. 1A [v]).
Using this technique, we injected embryos at the two-cell stage of development to demonstrate that microinjection (e.g., DNA or RNA constructs) can be performed during early development with ease (Fig. 2A [i and ii]). Despite the relatively dark orange color of the blastomeres, injected material can be tracked without the use of dyes such as phenol red (Fig. 2A [ii]; black arrowhead). It is important to avoid injecting the embryos at an angle as the needle may bend and break due to the hard chorion. We also demonstrate microinjection of sytox green in the YSL, at the late-blastula stage (Fig. 2B [i and ii]). The YSL is located between the expanding blastoderm and the yolk (Mourabit et al. 2011), and embryos must be positioned at a specific angle to be injected correctly.
Although it is possible to enzymatically dechorionate embryos, this procedure can take several hours and injected or chemically treated embryos may be fragile and more susceptible to injury from the proteases used for dechorionation (pronase and hatching enzyme). Thus, we tested imaging methods that do not require removal of the chorion. Following injection, fluorescence of the yolk syncytial nuclei (YSN) was monitored using stereomicroscopy and compound microscopy. Using a stereomicroscope, embryos can easily be monitored using the agarose method described above (Fig. 2C), both for brightfield (Fig. 2C [i]) and fluorescence (Fig. 2C [iii]) imaging. When using an inverted microscope, embryos must be kept in place without the use of agarose as the samples need to be at the bottom of the slide or dish. Since the chorion is still present, and the embryo may move within it, one must be able to easily reach and rotate the embryo during microscopy. Thus, methyl cellulose was used for this purpose, as the viscosity keeps embryos in place and allows rotation of the sample, here focusing on a cluster of YSN (Fig. 2D). We demonstrate that despite the thick chorion, small structures such as YSN can be clearly observed with both differential interference contrast microscopy (DIC or Nomarski microscopy) (Fig. 2D [i]) and fluorescent microscopy (Fig. 2D [ii]) using a ×10 dry-objective lens. For imaging at higher magnification, however, it becomes necessary to remove the chorion for clear and focused micrographs (using a ×20 dry-objective lens) (Fig. 2E).
Some techniques, such as high-magnification and time-lapse imaging, require longer periods of detailed analyses during which embryos must be fixed in place without their chorion. Embedding in agarose is particularly useful for such procedures, and is described in the methods section and in Fig. 2B. We tested the efficiency of this embedding technique in K. Marmoratus by analyzing embryonic structures and cell behaviors using high magnification imaging with compound microscopes.
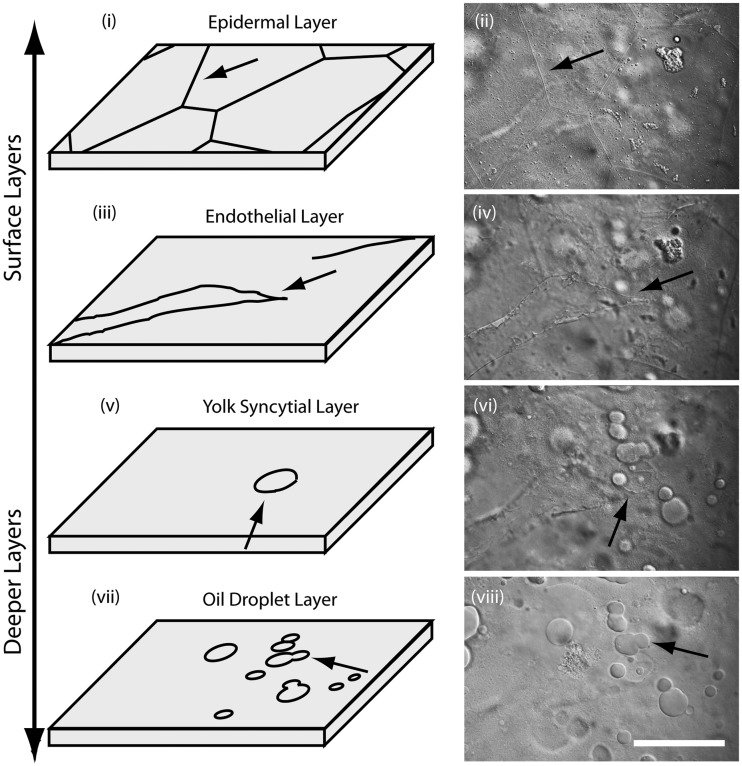
To assess detailed observations in embedded embryos, we examined embryonic structures on the different layers of the yolk during midsomitogenesis (∼ 3 days postfertilization) (Fig. 3). At the surface of the embryonic yolk, geometrical skin cells were developing to form the epidermal layer (Fig. 3 [i and ii]). Below this layer, filamentous endothelial cells were found forming vitelline vessels (Fig. 3 [iii and iv]). The next deeper layer was the YSL where relatively large YSN (∼20 µm) were observed (Fig. 3 [v and vi]). In the deepest layer, only oil droplets were seen (Fig. 3 [vii and viii]).
Fig. 3.
Imaging the surface layers of yolk in K. marmoratus embryos. During midsomitogenesis, geometrically shaped skin cells covered the surface of the yolk (i and ii; arrows). Under the epidermal layer, newly formed blood vessels were observed (iii and iv; arrows). The next deeper layer was the YSL where YSN were observed (v and vi; arrows). The deepest layer only contained oil droplets (vii and viii). Scale bar = 50 µm.
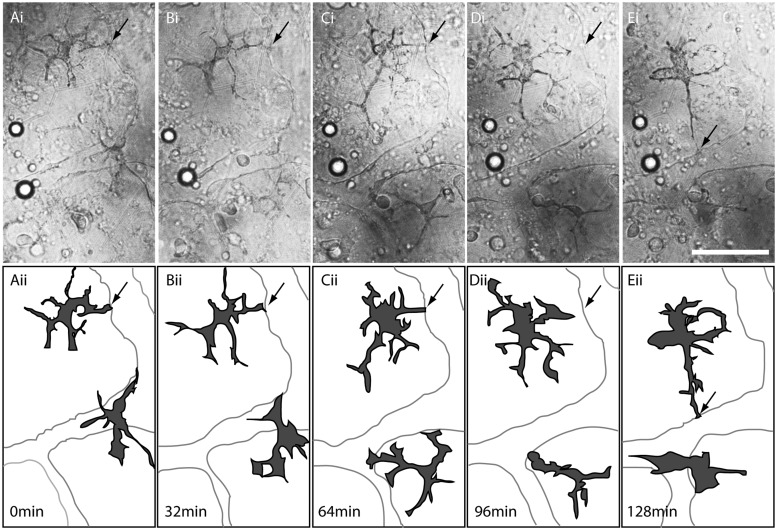
We further tested time-lapse imaging using embedded embryos, by monitoring morphological changes and migration of melanophores on the yolk surface in the endothelial layer. By late embryonic development, a network of vitelline vessels has formed over the yolk, and is covered by melanophores (Mourabit et al. 2011). Here, we demonstrate by time-lapse analyses that, interestingly, the melanophores dynamically change shape during their development (Fig. 4). Extending protrusions of the melanophores appear to touch the edge of vitelline vessels and then change their location to come in contact with another edge of the vitelline vessels (see progression from A to E in Fig. 4).
Fig. 4.
Time-lapse analysis of melanophores on the surface of the yolk in K. marmoratus embryos. (A [i])–(E [i]) DIC images of the endothelial layer on the yolk during the late embryonic development. (A [ii])–(E [ii]) Diagrams depicting positions and shapes of melanophores and vitelline vessels. A (i and ii) = 0 min; B (i and ii) = 32 min; C (i and ii) = 64 min; D (i and ii) = 96 min; and E (i and ii) = 128 min. Extending protrusions from the melanophores appear to come in contact with the edge of a vitelline vessel (A [i and ii]; B [i and ii]; C [i and ii]; arrows) and then detach (D [i and ii]; arrows) to touch another edge of the vitelline vessel (E [i and ii]; arrows). Scale bar = 50 µm.
Although the chorion in K. marmoratus was rougher and harder than in medaka and zebrafish, we demonstrated that it was still possible to microinject embryos with a fluorescent dye using glass microneedles. This injection method is quick and straightforward, and can be used for the injection of DNA, RNA, morpholinos, and other chemicals. We have also shown that embryos with intact chorions can be monitored by stereomicroscopy and compound microscopy for experiments in which dechorionation is not necessary or is less feasible. The data using dechorionated embryos indicate that high-resolution and time-lapse imaging can be achieved with K. marmoratus embryos.
We found that the surface of the yolk during somitogenesis provides an excellent platform for imaging analyses, as cells on the yolk appear to move on the same Z-plane, allowing tracing of a single cell over a period of time without changes in focus. In addition, cell density was not high, further contributing to the ease of tracing the positions and shapes of cells. We also observed that only a few kinds of cells were observed on the yolk during this period of development (e.g., endothelial cells, melanophores, erythrophores, and macrophages) and therefore the interactions between different types of cells were easily observable without labeling the cells. Finally, despite the large quantity of oil droplets present in K. Marmoratus yolk, we found that due to the large area of yolk, it was easy to find oil droplet-free areas to monitor cell behavior and morphology.
In summary, we demonstrate that K. marmoratus is an excellent model for embryological research using imaging and micromanipulation techniques. In combination with its self-fertilizing ability, this fish has the potential to become a significant model animal for a variety of fields within developmental biology, allowing us to study embryology from its basic principles to the mechanisms of genetic diseases and environmental impacts.
Funding
S.M. is funded by a PhD studentship provided by the Natural Environment Research Council in the United Kingdom. This work was supported by an NIH Conference (Grant R13HD070622) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development; SICB through the DCE, DCPB, DAB and the C. Ladd Prosser Fund; and the College of Agriculture and Natural Resources, University of Maryland.
References
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MFW, Fisher EMC. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Grizzle JM, Thiyagarajah A. Skin histology of Rivulus ocellatus marmoratus: apparent adaptation for aerial respiration. Copeia. 1987;1987:237–40. [Google Scholar]
- Harrington R. Oviparous hermaphroditic fish with internal self-fertilization. Science. 1961;134:1749–50. doi: 10.1126/science.134.3492.1749. [DOI] [PubMed] [Google Scholar]
- Harrington R. Environmentally controlled induction of primary male gonochorists from eggs of self-fertilizing hermaphroditic fish Rivulus marmoratus Poey. Biol Bull. 1967;132:174–99. doi: 10.2307/1539887. [DOI] [PubMed] [Google Scholar]
- Harrington R. Delimitation of thermolabile phenocritical period of sex determination and differentiation in ontogeny of normally hermaphroditic fish Rivulus marmoratus Poey. Physiol Zool. 1968;41:447–60. [Google Scholar]
- Harrington R. How ecological and genetic factors interact to determine when self-fertilizing hermaphrodites of Rivulus marmoratus change into functional secondary males, with a reappraisal of modes of intersexuality among fishes. Copeia. 1971;1971:389–432. [Google Scholar]
- Jarne P, Auld JR. Animals mix it up too: the distribution of self-fertilization among hermaphroditic animals. Evolution. 2006;60:1816–24. doi: 10.1554/06-246.1. [DOI] [PubMed] [Google Scholar]
- Kallman KD, Harrington RW. Evidence for the existence of homozygous clones in the self-fertilizing hermaphroditic teleost Rivulus marmoratus (Poey) Biol Bull. 1964;126:101–14. [Google Scholar]
- Kanamori A, Yarnamura A, Koshiba S, Lee JS, Orlando EF, Hori H. Methyltestosterone efficiently induces male development in the self-fertilizing hermaphrodite fish, Kryptolebias marmoratus. Genesis. 2006;44:495–503. doi: 10.1002/dvg.20240. [DOI] [PubMed] [Google Scholar]
- King JAC, Abel DC, Dibona DR. Effects of salinity on chloride cells in the euryhaline cyprinodontid fish Rivulus marmoratus. Cell Tissue Res. 1989;257:367–77. [Google Scholar]
- Mackiewicz M, Tatarenkov A, Taylor DS, Turner BJ, Avise JC. Extensive outcrossing and androdioecy in a vertebrate species that otherwise reproduces as a self-fertilizing hermaphrodite. Proc Natl Acad Sci USA. 2006;103:9924–28. doi: 10.1073/pnas.0603847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourabit S, Edenbrow M, Croft DP, Kudoh T. Embryonic development of the self-fertilizing mangrove killifish Kryptolebias marmoratus. Dev Dyn. 2011;240:1694–1704. doi: 10.1002/dvdy.22668. [DOI] [PubMed] [Google Scholar]
- Sakakura Y, Soyano K, Noakes DLG, Hagiwara A. Gonadal morphology in the self-fertilizing mangrove killiish, Kryptolebias marmoratus. Ichthyol Res. 2006;53:427–30. [Google Scholar]
- Tatarenkov A, Gao H, Mackiewicz M, Taylor DS, Turner BJ, Avise JC. Strong population structure despite evidence of recent migration in a selfing hermaphroditic vertebrate, the mangrove killifish (Kryptolebias marmoratus) Mol Ecol. 2007;16:2701–11. doi: 10.1111/j.1365-294X.2007.03349.x. [DOI] [PubMed] [Google Scholar]
- Tatarenkov A, Ring BC, Elder JF, Bechler DL, Avise JC. Genetic composition of laboratory stocks of the self-fertilizing fish Kryptolebias marmoratus: a valuable resource for experimental research. PLos One. 2010;5:e12863. doi: 10.1371/journal.pone.0012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DS, Davis WP, Turner BJ. Rivulus marmoratus: ecology of distributional patterns in Florida and the central Indian-River Lagoon. Bull Mar Sci. 1995;57:202–207. [Google Scholar]
- Turner BJ, Fisher MT, Taylor DS, Davis WP, Jarrett BL. Evolution of ‘maleness’ and outcrossing in a population of the self-fertilizing killifish, Kryptolebias marmoratus. Evol Ecol Res. 2006;8:1475–86. [Google Scholar]