Abstract
The tripartite motif (TRIM)–containing proteins are involved in many cellular functions such as cell signaling, apoptosis, cell differentiation, and immune modulation. TRIM5 proteins, including TRIM5α and TRIM-Cyp, are known to possess antiretroviral activity against many different retroviruses. Besides being retroviral restriction factors, TRIM5 proteins participate in other cellular functions that have recently emerged in the study of TRIM5α. In this review, we discuss properties of TRIM5α such as cytoplasmic body formation, protein turnover, and trafficking. Also, we discuss recent insights into innate immune modulation mediated by TRIM5α, highlighting the various functions TRIM5α has in cellular processes.
Keywords: rhTRIM5alpha, Cytoplasmic bodies, HIV-1, Restriction factor, Signaling 2
Introduction
TRIM5α is a cellular protein that binds to determinants present on a retroviral capsid soon after the entry of the viral core into the cytoplasm of a target cell. This restriction occurs in a species-specific manner, the best characterized example being the TRIM5α protein from rhesus macaques (rhTRIM5α), which potently inhibits HIV-1 infection [1]. It is becoming increasingly clear that TRIM5α does not simply bind to the retroviral capsid and mediate its effects autonomously without other associated cellular proteins. Rather, the proteins that associate with TRIM5α and the signaling pathways it activates are intimately tied to its ability to restrict retroviral infection. This review will focus on our understanding of how the cellular biology of TRIM5α governs its ability to act as an antiviral restriction factor.
TRIM Family of Proteins
The tripartite motif (TRIM) family of proteins is defined by three domains present in all TRIM family members: RING, BBox2, and coiled-coil (CC) [2]. These motifs can be functionally divided into two activities. Firstly, an E3 ubiquitin ligase activity mediated by the N-terminal RING domain. Secondly, self association activity collectively mediated by the BBox2 and CC domains, and at least in the case of TRIM5α, residues within the L2 region connecting the CC and C-terminal B30.2/PRYSPRY domain. This tendency to self-associate is a defining feature of TRIM family proteins [2], and likely a key aspect of the biology of these of proteins, as discussed in more detail below. The tendency to self-associate has also made this family of proteins quite difficult to study, as TRIM proteins are generally not very soluble in solution at concentrations needed for many standard biochemical and molecular techniques.
TRIM5α proteins contain an additional C-terminal B30.2/PRYSPRY domain that binds to capsid and mediates specific recognition and restriction of retroviruses by TRIM5α proteins from different species [1, 13]. For example, while human TRIM5α (huTRIM5α) does not restrict HIV-1, as does its counterpart from rhesus macaques (rhTRIM5α), a single amino-acid change (P332R) is sufficient to lead to HIV-1 restriction by human TRIM5α [14]. B30.2/PRYSPRY has been subjected to intense positive selection during primate evolution, underscoring its critical role in this process [15]. Space constraints prevent us from describing in detail the many noteworthy studies which describe how variations within this region define the spectrum of retroviral restriction of individual TRIM5α proteins by modulating the ability to bind retroviral capsid cores, but we refer interested readers to more extensive reviews of this topic [16–18]. Additionally, in some monkey species, the C-terminal SPRY domain has been functionally replaced by a cyclophilin A (CypA) domain [19–22]. These proteins, termed TRIM-Cyp proteins, retain the ability to bind retroviral capsids through the interaction between CypA and the CypA-binding loop of the viral capsid protein.
TRIM5 Multimerization
As TRIM5 self-association is mediated by numerous regions of the protein, self-association can functionally be reduced to low-order (dimerization) and higher-order multimerization. Dimerization is required for higher-order multimerization of TRIM5 proteins, as evidenced by the fact that mutations that perturb dimerization abrogate TRIM5 higher-order multimerization [7].
Dimerization
Defining the low-order multimerization state of TRIM5α has proven to be complex. TRIM5α was originally reported to be a trimer [10], although subsequent studies have determined that this finding was a result of an aberrant electrophoretic mobility of TRIM5α dimers, which are actually the low-order multimerization state of TRIM5 proteins [8, 9]. To circumvent this and other experimentally confounding aspects of TRIM5α, a recombinant TRIM5α variant was constructed, in which the RING domain of TRIM5α was replaced with the RING domain of TRIM21 (TRIM5-21R) [8, 9]. This protein still effectively restricts HIV-1 and has proven to be much more amenable to biochemical purification and subsequent analysis. The dimeric form of TRIM5-21R can directly bind restriction-sensitive viral capsid, while the monomeric form could not [9], demonstrating the functional requirement of this low-order dimerization in TRIM5α retroviral restriction.
Higher-Order Oligomerization
TRIM5 proteins also exhibit higher-order oligomerization. Using a protein cross-linking reagent such as glutaraldehyde or EGS, TRIM5α is crosslinked into numerous high-molecular weight species [7, 10, 12, 23–26]. While the apparent molecular weight of these crosslinked species is often variable, at least one study has found that biochemical crosslinking yields a species with a molecular weight consistent with a hexameric form of TRIM5α [26]. The BBox2 domain appears to be a critical determinant in mediating the higher-order multimerization. A hydrophobic patch on the surface of the B-box 2 domain is necessary for the higher-order multimerization of TRIM5α as well as capsid binding and retroviral restriction [4, 23, 25, 27]. These observations suggest an important role for higher-order multimerization in the ability of TRIM5α to restrict retroviral infection. It is well accepted that the C-terminal B30.2/PRYSPRY domain (in the case of TRIM5α) or CypA domain (in the case of TRIM-Cyp proteins) are the regions of the protein that directly bind viral capsid determinants. Therefore, the finding that interfering with the ability of TRIM5α to form higher-order multimers, disrupts the ability to bind in vitro assembled capsid cores suggests that binding of TRIM5 to these complexes requires cooperativity between individual TRIM5 dimers. In such a model, the binding of the first TRIM5 protein to the viral capsid enhances the ability of subsequent TRIM5 proteins to be recruited to the complex and bind the capsid in a C-terminal domain–dependent fashion.
We have also recently identified residues within the Linker 2, which are necessary for the ability of TRIM5α to self-associate and restrict HIV-1 infection [18]. However, the higher-order multimerization mediated by the L2 region appears, in some ways, distinct from the higher-order multimerization mediated by the BBox2 region. There are two stretches of amino acid residues in L2 which, if altered, abrogate the ability of TRIM5α to restrict HIV-1 infection. TRIM5α variants harboring mutations in these regions also exhibit a diffuse localization in cells and fail to localize to cytoplasmic bodies. However, biochemical crosslinking does not reveal an apparent defect in the ability of these mutants to form higher-order multimers [18].
A recent study by Ganser-Pornillos et al. [28•] has provided valuable insight into the nature of the assemblies formed by TRIM5α. Using negative-stain EM and biochemical studies, these authors demonstrated that TRIM5-21R spontaneously forms two-dimensional hexagonal arrays. These TRIM5 arrays are complementary in symmetry and dimension to the retroviral capsid. The authors suggest that retroviral capsid binding and higher-order assemblies are coupled. Their model proposes that the B-Box2 domain mediates tripodial protein extensions observed in the lattice, while the L2 region mediates the self-association of opposing dimers needed to achieve hexagonal symmetry [28•].
TRIM5α Cytoplasmic Bodies: Are all Bodies Created Equal?
The paper which first characterized the TRIM family of proteins observed that most TRIM family members localized to discrete accumulations of proteins of diverse shapes and sizes in the nucleus and the cytoplasm [2]. TRIM5α localizes to structures termed cytoplasmic bodies [1]. The importance of these cytoplasmic bodies (CBs) to retroviral restriction has been controversial since the first discovery of TRIM5α’ s antiviral activity. Our studies have shown that TRIM5α cytoplasmic bodies are highly dynamic structures that rapidly exchange between cytoplasmic bodies and a diffuse cytoplasmic fraction [29], suggesting that these accumulations to which TRIM5α localizes are not simply dead end aggregates. We have also used live cell imaging to observe the de novo formation of rhTRIM5α cytoplasmic bodies around fluorescently labeled HIV-1 virions [30•]. However, two studies show that preexisting cytoplasmic bodies are not required for TRIM5α’ s antiretroviral activity. One study used geldanamycin, which is an Hsp90 inhibitor, prior to infection in order to reduce or eliminate TRIM5α cytoplasmic body formation. In this study geldanamycin treatment did not affect the ability of the TRIM5α proteins to restrict HIV-1 infection despite its ability to disrupt cytoplasmic bodies [31]. Another study utilized a cell line expressing epitope tagged TRIM-Cyp which does not form cytoplasmic bodies in the absence of restriction-sensitive virus and found that this cell line was able to efficiently restrict HIV-1 infection [32]. While these studies did not determine if CBs formed following encounter with restriction-sensitive virus, they collectively demonstrate that the preexisting pool of CBs is not required for retroviral restriction.
The disagreement regarding the role that CBs play in restriction is complicated both by the inability to assess the localization of endogenous TRIM5 proteins and also a lack of consensus regarding the definition of what constitutes a cytoplasmic body. Because reliable antibodies do not exist that can with certainty assess the localization of TRIM5α proteins in cells, most studies assessing its localization have relied on the expression of epitope-tagged forms of the protein. This raises the concern that the localization observed in epitope-tagged cell lines or following transient transfection may be an artifact of protein overexpression and may not resemble the localization of endogenous protein. The formation of these structures is certainly dependent on expression levels, as there are striking differences in the localization of epitope-tagged TRIM5α proteins in transiently transfected cells or cells expressing TRIM5α from a stably integrated retroviral vector (Fig. 1). Transient transfection yields very large and protein dense TRIM5α accumulations in cells, while a more diffuse localization with fewer, smaller cytoplasmic puncta are commonly observed in stably expressing cell lines. Notably, transiently transfected cells inhibit retroviral infection poorly if at all, while stably expressing cells, which express TRIM5α at much lower levels, inhibit retroviral infection much more potently (EMC, unpublished observations). This suggests that the localization of TRIM5α following transient transfection is aberrant in a way that disrupts the antiviral function of TRIM5α.
Fig. 1.
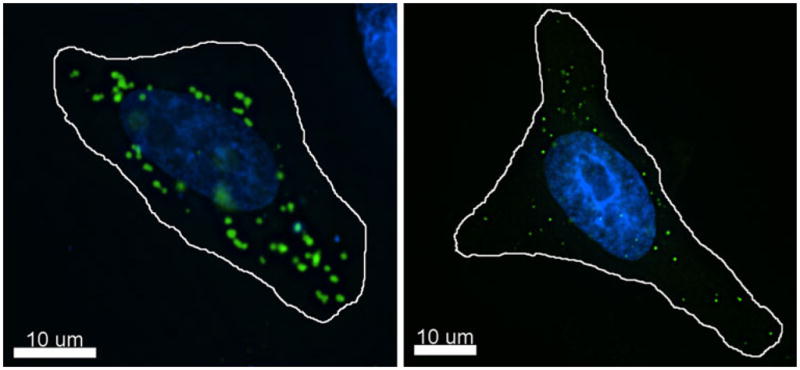
Subcellular localization of rhesus macaque TRIM5α. A, HeLa cells were seeded on glass coverslips and transiently transfected with YFP–rhTRIM5α using a PEI protocol. Z–stack images were collected and de-convolved using Deltavision deconvolution software. Individual channel images were superimposed to create the merged image. B, HeLa cells stably expressing YFP–rhTRIM5α from an integrated retroviral vector were seeded on coverslips. Z–stack images were collected and de-convolved using Deltavision deconvolution software. Individual channel images were superimposed to create the merged image
If the large aggregates formed following transient transfection are not biologically relevant structures, what about the smaller structures visible in stable cell lines? It is clear that the interaction between TRIM5α and viral capsids in the cytoplasm of target cells is relevant to TRIM5α restriction, so it seems very likely that the TRIM5α assemblies observed to form around HIV-1 capsids, visible by live and fixed cell imaging [30•], are relevant to the ability of TRIM5α to restrict retroviral infection. However, aside from the fact that they can be observed associating with viral capsids, these cytoplasmic concentrations of TRIM5α are indistinguishable from the puncta that exist in the absence of virus by fluorescent microscopy. It is possible that TRIM5α accumulations visible in the absence of virus represent protein assemblies that are fundamentally similar to those that contain a restricted retrovirus. The study of the hexameric lattices formed by TRIM5-21R support this notion [28•]. This study found that TRIM5-21R formed identical lattices in the presence or absence of restriction-sensitive virus, though the formation was enhanced by the addition of in vitro assembled viral capsid structures [28•]. It therefore seems possible that the puncta visible in cells in the absence of virus represent the in situ manifestation of this observation. Moving forward, it will be valuable to understand the differences and similarities between these microscopically similar cytoplasmic accumulations of TRIM5α, specifically with respect to the other proteins present in both structures. It may be that accumulations of protein which do not contain viral capsids represent small model systems that resemble the assemblies of TRIM5α that form around viral capsids. It may also be that these accumulations that exist in the absence of virus are more relevant to the capsid-independent signaling aspect of TRIM5α [33•, 34], described in more detail below. It is also not currently possible to exclude the possibility that these TRIM5α accumulations that exist in the absence of virus are indeed biologically irrelevant aggregates. Given the observations by Ganser-Pornillos et al. [28•], we favor the idea that these structures are similar in nature to the assemblies which form around restriction-sensitive virus.
When pondering the biological relevance of TRIM5α CBs, it may be relevant to consider the case of TRIM19, also known as promyelocytic leukemia (PML) protein. Unlike other TRIM family members, including TRIM5α, a number of antibodies exist which are commonly used to detect endogenously expressed PML by immunofluorescence [35–37]. These antibodies can be used to detect PML localizing to nuclear structures termed nuclear bodies (NBs), which are electrondense spheres of 0.1–1.0 μM in diameter [38]. Unlike the situation with TRIM5α, the relevance of these structures formed by PML is not a matter of debate. While the extensive literature on PML bodies is outside of the scope of this review, we refer the interested reader to numerous review articles describing the role of these bodies and these structures in cancer, antiviral defense, and their dynamic response to cellular stress and viral infection [38–40]. Notably, the study of PML NBs not only reveals many similarities between PML NBs and TRIM5α cytoplasmic bodies, they may also associate with each other in some cases, as discussed in more detail below.
Intracellular TRIM5α Trafficking
Studies of the intracellular mobility of TRIM5α proteins have revealed a dynamic protein which exhibits mobility at many levels. Fluorescence recovery after photobleaching (FRAP) and photo-activatable GFP (PA-GFP) studies have demonstrated that there is a rapid exchange of TRIM5α which occurs between proteins present in CBs and the diffuse cytoplasmic pool of TRIM5α [29]. Notably, the rate of TRIM5α exchange in these structures, is very comparable to the rate of exchange measured for PML NBs in similar studies [41, 42]. As is also the case with PML NBs [43, 44], TRIM5α CBs can be observed to exhibit dynamic fission and fusion behaviors, allowing the formation of multiple CBs from larger precursors or the coalescence of individual CBs into larger CBs [29]. However, the relevance of these dynamic behaviors is not well understood. We have observed that individual TRIM5α CBs can dynamically transfer TRIM5α onto HIV-1 virions during restriction [30•], and the relevance of this transfer must be considered in the context of the previously mentioned studies demonstrating that preexisting CBs are not required for retroviral restriction [31, 32]. It is also noteworthy that the movement of TRIM5α CBs is not random, possibly limiting it to regions where the incoming retroviral core is located.
TRIM5α CBs are transported along microtubules [29]. Live cell imaging experiments readily show TRIM5α CBs rapidly trafficking along curvilinear paths, and this trafficking is sensitive to the microtubule destabilizing agent nocodazole. The mechanism by which TRIM5α CBs engage the microtubule trafficking machinery is unclear. While other TRIM family proteins have been shown to associate with microtubules [45], the proposed mechanism of this interaction involves a C-terminal COS box domain not present in TRIM5α proteins [45]. Recent studies seem to suggest that the association between TRIM5α and microtubules may be relevant to restriction, as disruption of microtubules by nocodazole treatment reduces restriction of HIV-1 mediated by TRIM5α (Paulina Pawlica and Lionel Berthoux, personal communication). Incoming HIV-1 viral complexes have also been reported to be transported by the microtubule network during infection [46]. Association with microtubules may provide an opportunity for TRIM5α to effectively decrease the required area of retroviral surveillance, preferring to hunt for susceptible retroviral cores along the two-dimensional microtubule network rather than the vastly larger three-dimensional cytoplasm.
It was recently reported that some TRIM5α proteins traffic through the nucleus, even though their steady state localization is primarily cytoplasmic (Fig. 1). This study utilized leptomycin B (LMB), an inhibitor of CRM1-mediated nuclear export, to show that rhTRIM5α and huTRIM5α, but not owl monkey TRIM-Cyp and other primates, can accumulate in the nucleus when CRM1 nuclear export is inhibited [47]. Notably, nuclear accumulation of these proteins results in their localization to PML NBs. While the relevance of this nuclear trafficking is not well understood, it may be related to a recent study showing that SUMO interacting motifs (SIMs) present in TRIM5α are required for its ability to restrict HIV-1 infection [48]. SUMO-1 localization is predominantly nuclear (EMC, unpublished observations) and PML sumoylation is known to be important for the formation of PML NBs [39].
TRIM5 Turnover
While there appears to be a connection between TRIM5α turnover and its ability to restrict infection, this connection has proven to be less than straightforward. Pulsechase analysis and cycloheximide studies demonstrate the approximate half-life of TRIM5α to be slightly less than an hour [23, 49]. This rate of turnover is only slightly increased by the addition of proteasome inhibitors [23, 49], suggesting that a proteasome-independent pathway mediates the rapid turnover of TRIM5α in absence of restriction-sensitive virus. In two different studies, the ability of rhTRIM5α to restrict HIV-1 does not strictly correlate with the half-life of the protein in the absence of virus [18, 23].
Yet, it is known that inhibition of the proteasome by MG132 relieves the TRIM5α-mediated block of reverse transcription products while not affecting the ability to inhibit infection [49, 50]. Another study has demonstrated that, following addition of restriction-sensitive virus, TRIM5α is degraded in a proteasome-dependent fashion [51]. This data would suggest that the degradative fate of TRIM5α is altered in presence of virus. While it is unclear how this switch is mediated, we have recently reported that TRIM5α associates with the protein p62/sequestosome-1 [52], which is an adaptor protein that has been reported to facilitate both the proteasomal [53–55] and autophagic [56] degradation of cellular proteins. p62 somehow acts to stabilize TRIM5α expression, as p62 depletion by siRNA increases the rate of TRIM5α turnover in cells in the absence of virus [52].
Innate Immune Signaling and E3 Ubiquitination Activity
Various TRIMs have been shown to play a part in host immunity and signaling pathways [57–64]. However until recently, it was not appreciated that TRIM5 proteins are capable of stimulating intracellular pathways. One study exploring this possibility observed that both human and rhesus TRIM5α are able to activate NF-κB–driven transcription in the absence of restriction-sensitive virus [34]. Another study from Pertel et al. [33•] similarly observed the ability of TRIM5 proteins to activate NK-κB and AP-1 transcription in a capsid-independent fashion. Both studies also observe that TRIM5 proteins can bind the signaling adapter proteins TAB2 and TAB3, though this binding was not strictly correlated with the ability to activate NK-κB, suggesting that the ability of TRIM5 proteins to activate this pathway may be multifaceted [33•, 34]. Notably, the study by Pertel and colleagues also observed that the basal signaling activity of TRIM5 proteins is stimulated by the addition of restriction-sensitive virus and that association with components of this signaling cascade, most notably TAK1, is required for efficient retroviral restriction [33•]. These authors found that TRIM5 synthesizes unanchored K63-linked ubiquitin chains which activate TAK1 autophosphorylation, further stimulating AP-1 and NF-κB signaling.
The observations that TRIM5 associates with TAK1 and synthesizes unanchored K63-linked ubiquitin raise a number of critical questions about TRIM5 proteins and restriction. It is known that TRIM5 recognizes incoming viral capsid within minutes of their entry into the cytoplasm. In the case of TRIM-Cyp, this recognition is irreversible with subsequent treatment of cells with cyclosporin A (CsA) [32]. On the surface, this seems incompatible with the idea that TRIM5 association with signaling molecules induces the de novo expression of a cofactor critical for restriction. Perhaps TRIM5 proteins sequester restriction-sensitive virions for some period while the products of the signaling cascades they activate are induced. If this sequestered complex was somehow resistant to CsA neutralization, this could explain the apparent discordance between these two studies. It is also possible that TAK1 has a more direct role in the poorly defined events that mediate restriction. For example, perhaps TAK1 phosphorylates a viral or cellular protein directly in a manner that is required for subsequent events in restriction, independent of its role in signal transduction. Further studies will clarify the interesting role TAK1 and related signaling proteins play in TRIM5-mediated restriction.
The study by Pertel et al. also demonstrates that the relationship between restriction and TRIM5 E3 ligase activity is more complex than previously appreciated. Examination of TRIM5 activity in vitro has demonstrated that TRIM5 can auto-ubiquitinate itself in the presence of the E2 enzyme UbcH5B [65]. A subsequent study demonstrated a similar capacity for TRIM5 auto-ubiquitination in the presence of UbcH5a [66]. However, the formation of unanchored K63-linked ubiquitin chains in the Pertel study required the E2 enzyme UBC13-UEV1A. Knockdown of UBC13 rescued HIV-1 and EIAV from TRIM5-mediated restriction. These results are not necessarily discordant, as both types of TRIM5 E3 ligase activity may be relevant to retroviral restriction, other aspects of the cell biology of TRIM5 proteins, or perhaps both. It seems feasible that TRIM5 ligase activity may be regulated in a spatiotemporal fashion. Perhaps encounter with a restriction-sensitive retrovirus facilitates the formation of an assembly that favors association with one particular E2 enzyme over another, thus altering the nature of TRIM5 E3 ligase activity exhibited by TRIM5 associated with a retroviral core as compared to the activity it exhibits when in the diffuse cytoplasmic pool of TRIM5 or in preexisting CBs that exist in the absence of viral capsid. Future studies will surely aim to test these possibilities, providing a more clear picture of how TRIM5 participation in signal transduction cascades and alterations in TRIM5 E3 ligase activity relate to the ability of TRIM5 proteins to restrict retroviral infection.
Conclusions
TRIM5 proteins are intrinsic restriction factors which bind to determinants present in retroviral capsids and prevent infection in a species-specific manner. However, it now seems clear that the antiviral activity of these proteins depends on dynamic interactions with additional cellular proteins. A better understanding of the cell biology of TRIM5alpha and related cellular factors will likely continue to provide critical insights into the restriction mechanism mediated by TRIM5 proteins.
Footnotes
Disclosure: No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–53. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 2.Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, et al. The tripartite motif family identifies cell compartments. Embo J. 2001;20:2140–51. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-Griffero F, Qin XR, Hayashi F, Kigawa T, Finzi A, Sarnak Z, et al. A B-box 2 surface patch important for TRIM5alpha self-association, capsid binding avidity, and retrovirus restriction. J Virol. 2009;83:10737–51. doi: 10.1128/JVI.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Sodroski J. The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol. 2008;82:11495–502. doi: 10.1128/JVI.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Song B, Xiang SH, Sodroski J. Functional interplay between the B-box 2 and the B30.2(SPRY) domains of TRIM5alpha. Virology. 2007;366:234–44. doi: 10.1016/j.virol.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J Am Chem Soc. 2002;124:6063–76. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 7.Javanbakht H, Yuan W, Yeung DF, Song B, Diaz-Griffero F, Li Y, et al. Characterization of TRIM5alpha trimerization and its contribution to human immunodeficiency virus capsid binding. Virology. 2006;353:234–46. doi: 10.1016/j.virol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Kar AK, Diaz-Griffero F, Li Y, Li X, Sodroski J. Biochemical and biophysical characterization of a chimeric TRIM21-TRIM5alpha protein. J Virol. 2008;82:11669–81. doi: 10.1128/JVI.01559-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langelier CR, Sandrin V, Eckert DM, Christensen DE, Chandrasekaran V, Alam SL, et al. Biochemical characterization of a recombinant TRIM5alpha protein that restricts human immunodeficiency virus type 1 replication. J Virol. 2008;82:11682–94. doi: 10.1128/JVI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mische CC, Javanbakht H, Song B, Diaz-Griffero F, Stremlau M, Strack B, et al. Retroviral restriction factor TRIM5alpha is a trimer. J Virol. 2005;79:14446–50. doi: 10.1128/JVI.79.22.14446-14450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Yeung DF, Fiegen AM, Sodroski J. Determinants of the Higher Order Association of the Restriction Factor TRIM5{alpha} and Other Tripartite Motif (TRIM) Proteins. J Biol Chem. 2011;286:27959–70. doi: 10.1074/jbc.M111.260406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sastri J, O’Connor C, Danielson CM, McRaven M, Perez P, Diaz-Griffero F, et al. Identification of residues within the L2 region of rhesus TRIM5alpha that are required for retroviral restriction and cytoplasmic body localization. Virology. 2010;405:259–66. doi: 10.1016/j.virol.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stremlau M, Perron M, Welikala S, Sodroski J. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J Virol. 2005;79:3139–45. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol. 2005;15:73–8. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 15.Sawyer SL, Wu LI, Emerman M, Malik HS. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc Natl Acad Sci U S A. 2005;102:2832–7. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson WE, Sawyer SL. Molecular evolution of the antiretroviral TRIM5 gene. Immunogenetics. 2009;61:163–76. doi: 10.1007/s00251-009-0358-y. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama EE, Shioda T. Anti-retroviral activity of TRIM5 alpha. Rev Med Virol. 2010;20:77–92. doi: 10.1002/rmv.637. [DOI] [PubMed] [Google Scholar]
- 18.Sastri J, Campbell EM. Recent insights into the mechanism and consequences of TRIM5alpha retroviral restriction. AIDS Res Hum Retroviruses. 2011;27:231–8. doi: 10.1089/aid.2010.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman RM, Hall L, Kirmaier A, Pozzi LA, Pery E, Farzan M, et al. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog. 2008;4:e1000003. doi: 10.1371/journal.ppat.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–73. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- 21.Virgen CA, Kratovac Z, Bieniasz PD, Hatziioannou T. Independent genesis of chimeric TRIM5-cyclophilin proteins in two primate species. Proc Natl Acad Sci U S A. 2008;105:3563–8. doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson SJ, Webb BL, Ylinen LM, Verschoor E, Heeney JL, Towers GJ. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci U S A. 2008;105:3557–62. doi: 10.1073/pnas.0709003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Griffero F, Kar A, Perron M, Xiang SH, Javanbakht H, Li X, Sodroski J. Modulation of Retroviral Restriction and Proteasome Inhibitor-resistant Turnover by Changes in the TRIM5{alpha} B-box 2 Domain. J Virol. 2007 doi: 10.1128/JVI.00703-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz-Griffero F, Li X, Javanbakht H, Song B, Welikala S, Stremlau M, et al. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology. 2006;349:300–15. doi: 10.1016/j.virol.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Griffero F, Vandegraaff N, Li Y, McGee-Estrada K, Stremlau M, Welikala S, et al. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology. 2006;351:404–19. doi: 10.1016/j.virol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Nepveu-Traversy ME, Berube J, Berthoux L. TRIM5alpha and TRIMCyp form apparent hexamers and their multimeric state is not affected by exposure to restriction-sensitive viruses or by treatment with pharmacological inhibitors. Retrovirology. 2009;6:100. doi: 10.1186/1742-4690-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Griffero F, Kar A, Lee M, Stremlau M, Poeschla E, Sodroski J. Comparative requirements for the restriction of retrovirus infection by TRIM5alpha and TRIMCyp. Virology. 2007;369:400–10. doi: 10.1016/j.virol.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Ganser-Pornillos BK, Chandrasekaran V, Pornillos O, Sodroski JG, Sundquist WI, Yeager M. Hexagonal assembly of a restricting TRIM5alpha protein. Proc Natl Acad Sci U S A. 2011;108:534–9. doi: 10.1073/pnas.1013426108. This paper visualized the TRIM5α lattice, mediated by the ability of TRIM5α to self-associate, which likely forms around restriction-sensitive viral capsids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell EM, Dodding MP, Yap MW, Wu X, Gallois-Montbrun S, Malim MH, et al. TRIM5 alpha cytoplasmic bodies are highly dynamic structures. Mol Biol Cell. 2007;18:2102–11. doi: 10.1091/mbc.E06-12-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Campbell EM, Perez O, Anderson JL, Hope TJ. Visualization of a proteasome-independent intermediate during restriction of HIV-1 by rhesus TRIM5alpha. J Cell Biol. 2008;180:549–61. doi: 10.1083/jcb.200706154. This paper visualized the association between TRIM5α cytoplasmic bodies during restriction, demonstrating that the ability of TRIM5α to self associate around viral complexes, manifested at the formation of cytoplasmic bodies, plays an important role in retroviral restriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song B, Diaz-Griffero F, Park DH, Rogers T, Stremlau M, Sodroski J. TRIM5alpha association with cytoplasmic bodies is not required for antiretroviral activity. Virology. 2005;343:201–11. doi: 10.1016/j.virol.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Caballero D, Hatziioannou T, Zhang F, Cowan S, Bieniasz PD. Restriction of human immunodeficiency virus type 1 by TRIM-CypA occurs with rapid kinetics and independently of cytoplasmic bodies, ubiquitin, and proteasome activity. J Virol. 2005;79:15567–72. doi: 10.1128/JVI.79.24.15567-15572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grutter MG, Luban J. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472:361–5. doi: 10.1038/nature09976. This study identified the connection between the ability of TRIM5 proteins to restrict retroviral infection and its ability to associate with proteins involved in signal transduction, most notably TAK1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tareen SU, Emerman M. Human Trim5alpha has additional activities that are uncoupled from retroviral capsid recognition. Virology. 2011;409:113–20. doi: 10.1016/j.virol.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyck JA, Maul GG, Miller WH, Jr, Chen JD, Kakizuka A, Evans RM. A novel macromolecular structure is a target of the promyelocyteretinoic acid receptor oncoprotein. Cell. 1994;76:333–43. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 36.Puvion-Dutilleul F, Chelbi-Alix MK, Koken M, Quignon F, Puvion E, de The H. Adenovirus infection induces rearrangements in the intranuclear distribution of the nuclear body-associated PML protein. Exp Cell Res. 1995;218:9–16. doi: 10.1006/excr.1995.1125. [DOI] [PubMed] [Google Scholar]
- 37.Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, et al. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994;76:345–56. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 38.Lallemand-Breitenbach V, de The H. PML nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000661. doi: 10.1101/cshperspect.a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everett RD, Chelbi-Alix MK. PML and PML nuclear bodies: implications in antiviral defence. Biochimie. 2007;89:819–30. doi: 10.1016/j.biochi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Geoffroy MC, Chelbi-Alix MK. Role of promyelocytic leukemia protein in host antiviral defense. J Interferon Cytokine Res. 2011;31:145–58. doi: 10.1089/jir.2010.0111. [DOI] [PubMed] [Google Scholar]
- 41.Dong S, Stenoien DL, Qiu J, Mancini MA, Tweardy DJ. Reduced intranuclear mobility of APL fusion proteins accompanies their mislocalization and results in sequestration and decreased mobility of retinoid X receptor alpha. Mol Cell Biol. 2004;24:4465–75. doi: 10.1128/MCB.24.10.4465-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera OJ, Song CS, Centonze VE, Lechleiter JD, Chatterjee B, Roy AK. Role of the promyelocytic leukemia body in the dynamic interaction between the androgen receptor and steroid receptor coactivator-1 in living cells. Mol Endocrinol. 2003;17:128–40. doi: 10.1210/me.2002-0165. [DOI] [PubMed] [Google Scholar]
- 43.Chen YC, Kappel C, Beaudouin J, Eils R, Spector DL. Live cell dynamics of promyelocytic leukemia nuclear bodies upon entry into and exit from mitosis. Mol Biol Cell. 2008;19:3147–62. doi: 10.1091/mbc.E08-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eskiw CH, Dellaire G, Mymryk JS, Bazett-Jones DP. Size, position and dynamic behavior of PML nuclear bodies following cell stress as a paradigm for supramolecular trafficking and assembly. J Cell Sci. 2003;116:4455–66. doi: 10.1242/jcs.00758. [DOI] [PubMed] [Google Scholar]
- 45.Short KM, Cox TC. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem. 2006;281:8970–80. doi: 10.1074/jbc.M512755200. [DOI] [PubMed] [Google Scholar]
- 46.McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, et al. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159:441–52. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Griffero F, Gallo DE, Hope TJ, Sodroski J. Trafficking of some old world primate TRIM5alpha proteins through the nucleus. Retrovirology. 2011;8:38. doi: 10.1186/1742-4690-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arriagada G, Muntean LN, Goff SP. SUMO-interacting motifs of human TRIM5alpha are important for antiviral activity. PLoS Pathog. 2011;7:e1002019. doi: 10.1371/journal.ppat.1002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc Natl Acad Sci U S A. 2006;103:7465–70. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson JL, Campbell EM, Wu X, Vandegraaff N, Engelman A, Hope TJ. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J Virol. 2006;80:9754–60. doi: 10.1128/JVI.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rold CJ, Aiken C. Proteasomal degradation of TRIM5alpha during retrovirus restriction. PLoS Pathog. 2008;4:e1000074. doi: 10.1371/journal.ppat.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Connor C, Pertel T, Gray S, Robia SL, Bakowska JC, Luban J, et al. p62/sequestosome-1 associates with and sustains the expression of retroviral restriction factor TRIM5alpha. J Virol. 2010;84:5997–6006. doi: 10.1128/JVI.02412-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christian F, Anthony DF, Vadrevu S, Riddell T, Day JP, McLeod R, et al. p62 (SQSTM1) and cyclic AMP phosphodiesterase-4A4 (PDE4A4) locate to a novel, reversible protein aggregate with links to autophagy and proteasome degradation pathways. Cell Signal. 2010;22:1576–96. doi: 10.1016/j.cellsig.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Geetha T, Seibenhener ML, Chen L, Madura K, Wooten MW. p62 serves as a shuttling factor for TrkA interaction with the proteasome. Biochem Biophys Res Commun. 2008;374:33–7. doi: 10.1016/j.bbrc.2008.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol. 2004;24:8055–68. doi: 10.1128/MCB.24.18.8055-8068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–96. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Bougrini J, Dianoux L, Chelbi-Alix MK. PML positively regulates interferon gamma signaling. Biochimie. 2011;93:389–98. doi: 10.1016/j.biochi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–20. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 59.Ishii T, Ohnuma K, Murakami A, Takasawa N, Yamochi T, Iwata S, et al. SS-A/Ro52, an autoantigen involved in CD28-mediated IL-2 production. J Immunol. 2003;170:3653–61. doi: 10.4049/jimmunol.170.7.3653. [DOI] [PubMed] [Google Scholar]
- 60.Kim JY, Ozato K. The sequestosome 1/p62 attenuates cytokine gene expression in activated macrophages by inhibiting IFN regulatory factor 8 and TNF receptor-associated factor 6/NF-kappaB activity. J Immunol. 2009;182:2131–40. doi: 10.4049/jimmunol.0802755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong HJ, Anderson DE, Lee CH, Jang MK, Tamura T, Tailor P, et al. Cutting edge: autoantigen Ro52 is an interferon inducible E3 ligase that ubiquitinates IRF-8 and enhances cytokine expression in macrophages. J Immunol. 2007;179:26–30. doi: 10.4049/jimmunol.179.1.26. [DOI] [PubMed] [Google Scholar]
- 62.Ryu YS, Lee Y, Lee KW, Hwang CY, Maeng JS, Kim JH, et al. TRIM32 protein sensitizes cells to tumor necrosis factor (TNFalpha)-induced apoptosis via its RING domain-dependent E3 ligase activity against X-linked inhibitor of apoptosis (XIAP) J Biol Chem. 2011;286:25729–38. doi: 10.1074/jbc.M111.241893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi M, Deng W, Bi E, Mao K, Ji Y, Lin G, et al. TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat Immunol. 2008;9:369–77. doi: 10.1038/ni1577. [DOI] [PubMed] [Google Scholar]
- 64.Yu S, Gao B, Duan Z, Xu W, Xiong S. Identification of tripartite motif-containing 22 (TRIM22) as a novel NF-kappaB activator. Biochem Biophys Res Commun. 2011;410:247–51. doi: 10.1016/j.bbrc.2011.05.124. [DOI] [PubMed] [Google Scholar]
- 65.Yamauchi K, Wada K, Tanji K, Tanaka M, Kamitani T. Ubiquitination of E3 ubiquitin ligase TRIM5 alpha and its potential role. FEBS J. 2008;275:1540–55. doi: 10.1111/j.1742-4658.2008.06313.x. [DOI] [PubMed] [Google Scholar]
- 66.Lienlaf M, Hayashi F, Di Nunzio F, Tochio N, Kigawa T, Yokoyama S, et al. Contribution of E3-Ubiquitin Ligase Activity to HIV-1 Restriction by TRIM5{alpha}rh: Structure of the RING Domain of TRIM5{alpha} J Virol. 2011;85:8725–37. doi: 10.1128/JVI.00497-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


