Abstract
What happens to a single, presynaptically quiescent synapse among a population of active synapses? In this issue of Neuron, Ehlers and colleagues show that, far from being eliminated, these inactive synapses are primed for potentiation and incorporation into a new neural circuit through an upregulation of NR2B-containing NMDA receptors.
Circuits in the developing brain become functional through orchestrated elimination of undesirable synapses and the selective strengthening of synapses that appropriately drive their postsynaptic partner. While this process requires rapid forms of synaptic plasticity, neither the emergence of a functional circuit nor its continued maintenance would be possible if the properties of synaptic plasticity were fixed. Theoretical studies suggest that there must also be a slower process, termed “metaplasticity,” that adjusts the ability to strengthen and weaken synapses based on the recent history of neural activity (Abraham, 2008). Consider, for example, an active synapse that consistently drives a neuron to fire action potentials. In the absence of a homeostatic mechanism such as synaptic scaling (Nelson and Turrigiano, 2008) or metaplasticity (Abraham, 2008), this type of synapse would potentiate (strengthen) to its maximum capacity through frequency- or timing-dependent plasticity mechanisms, rendering the synapse both disproportionately strong and unable to exhibit additional potentiation. Such bounded synapses create a severe limitation on the information storage (e.g., memory) capacity of neurons. Homeostatic mechanisms such as metaplasticity can overcome this limitation. By increasing the requirements for synaptic potentiation, metaplasticity can prevent runaway potentiation in strong, active synapses. Metaplastic processes can also make it easier for quiescent synapses to be strengthened by even small increases in synaptic activity, and hence encode new information carried by this activity. In addition to maintaining synapses within a dynamic range of functionality, metaplasticity is also thought to allow neural networks to store memories (Abraham, 2008).
The properties of metaplasticity have been poorly understood, yet this information is crucial for understanding the role(s) and spatial scale upon which metaplasticity operates. Much of the theoretical and experimental groundwork for metaplasticity has suggested that the properties of synaptic plasticity adjust in a cell-wide manner and that this can help tune neurons to respond to select features of the environment (Kirkwood et al., 1996). Metaplasticity can also be induced in an input-specific manner (Abraham, 2008). Because previous attempts to study input-specific metaplasticity have typically used extracellular or other strong stimulation protocols, it has been difficult to determine whether the induction of metaplasticity requires changes in the firing of postsynaptic action potentials and/or the coincident activation of a minimal number of synapses.
To gain the first insights into whether metaplasticity can occur at single synapse, Ehlers and colleagues took advantage of an approach that allowed them to presynaptically silence single synapses in a sea of otherwise normally active synapses (Lee et al., 2010). This was accomplished in cultured neurons by sparsely transfecting presynaptic cells with a construct that simultaneously marked presynaptic terminals (with synaptophysin-GFP) and suppressed neurotransmitter release (with tetanus toxin light chain). When a postsynaptic neuron was labeled with a red fluorophore (mCherry), the small number of presynaptically silenced synapses onto that neuron could be visually distinguished from active synapses (Figure 1). Using two-photon microscopy and glutamate uncaging to visualize and stimulate single synapses, the authors then probed postsynaptic glutamate receptor function in silenced synapses and their active neighbors (Figure 2). What the authors found was surprising—while the silenced synapses exhibited normal currents mediated by AMPA receptors, there was a large increase in the postsynaptic Ca2+ transients and the amount of charge carried by NMDA receptors. Because the bidirectional control of subtype-specific NMDA receptor functions powerfully regulates the properties of synaptic plasticity (Lau and Zukin, 2007), the authors examined whether changes in NMDA receptor function might be due to a change in the synaptic abundance of NMDA receptor subtypes. NMDA receptors expressed at excitatory synapses of the forebrain are tetramers consisting of NR1 and either NR2A or NR2B subunits. Whereas immature hippocampal neurons express primarily NR2B-containing NMDA receptors, mature neurons express primarily NR2A-containing NMDA receptors (Lau and Zukin, 2007). This developmental switch in NMDA receptor subunit composition is functionally important, as more immature NMDA receptor subtypes have longer decay time constants (Cull-Candy and Leszkiewicz, 2004) and are thus capable of integrating synaptic currents across broader time intervals. In addition to their longer currents, NR2B-containing NMDA receptors carry more Ca2+ current per unit charge (Sobczyk et al., 2005) and are preferentially tethered to the plasticity protein CaMKII (Barria and Malinow, 2005).
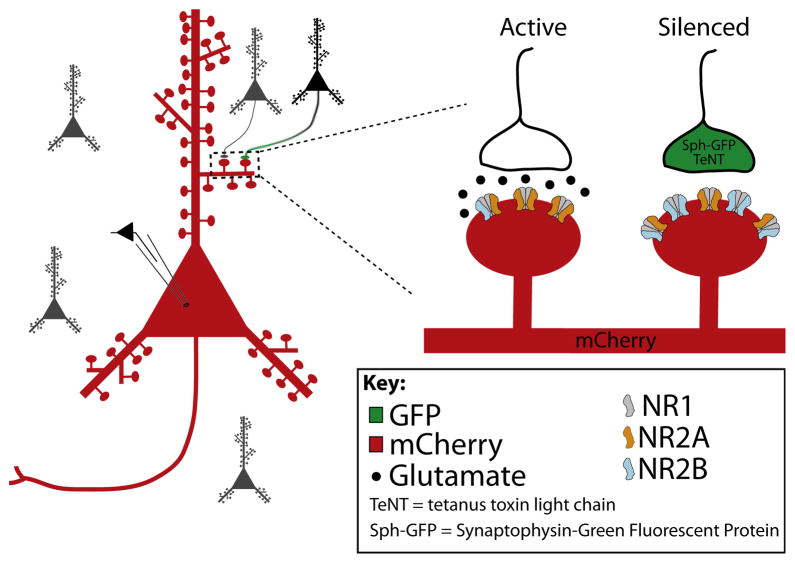
Figure 1. Visualization of Isolated, Silenced Synapse.
A small subset of cultured neurons were transfected with a viral construct containing synaptophysin-GFP (Sph-GFP), to visualize presynaptic terminals, and tetanus toxin light chain (TeNT), to greatly diminish neurotransmitter release. Individual postsynaptic neurons were transfected with a red fluorophore (mCherry), allowing the silenced synapses to be visually identified by overlap of red and green signals.
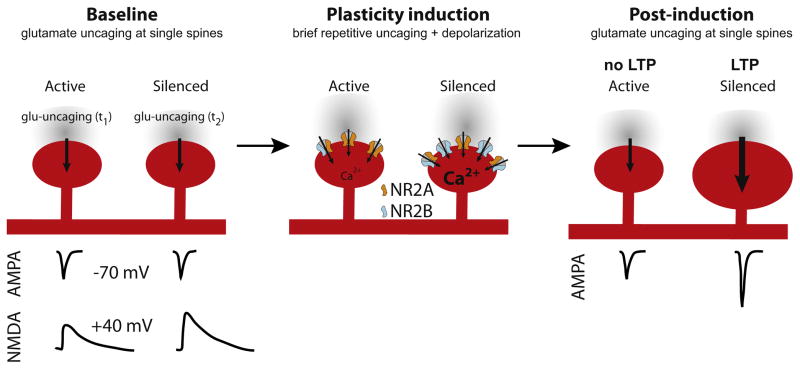
Figure 2. Silenced Synapses Are Primed for Potentiation.
Two-photon uncaging of glutamate at single synapses allowed synaptic properties and plasticity to be assessed. Glutamate uncaging at individual spines revealed similar AMPA-receptor-mediated synaptic currents at active and silenced synapses (left panel), but enhanced NMDA receptor synaptic currents at silenced synapses. Weak bursts of glutamate uncaging paired with postsynaptic depolarization (a “subthreshold” stimulus at active synapses, middle panel) produced long-term potentiation and spine enlargement at silenced, but not active, synapses (right panel), likely due to enhanced NMDA EPSCs and greater fractional NR2B at silenced synapses.
When the authors examined the composition of NMDA receptors at silenced synapses, using both anatomical and electrophysiological measures, they found that there was a significant increase in the “immature,” NR2B-containing form of NMDA receptors. This change in NMDA receptor subunit composition had a profound impact on the expression of synaptic plasticity in silenced synapses. When silenced synapses were repeatedly activated by uncaging glutamate (to simulate new presynaptic activity), they were more easily strengthened than neighboring active synapses. That is, weak bursts of glutamate uncaging that failed to alter responses and synapse morphology in active synapses were capable of inducing long-term potentiation and enlarging dendritic spines in their silenced neighbors (Figure 2). These findings indicate that silenced synapses are primed to undergo both electrophysiological strengthening and anatomical growth to new synaptic activity.
The findings are significant because they demonstrate, for the first time, that metaplasticity can be spatially delimited at the level of single synapses. Moreover, the data provide compelling evidence that NR2B-containing NMDA receptors favor the induction of long-term potentiation, not long-term depression, an idea that has received considerable attention and been hotly debated (Morishita et al., 2006). While this paper was the first to show metaplasticity at the level of single synapses through modifications in NMDA receptor composition, previous studies have shown that experience-dependent modifications in NMDA receptor subunit composition can adjust the plasticity threshold in sensory neocortex (Philpot et al., 2001) and that changes in NMDA receptor phenotype can occur in an input-specific manner (Bellone and Nicoll, 2007). Considering that synapses typically have only a handful of NMDA receptors per synapse, these studies collectively indicate that synaptic activity tightly regulates NMDA receptor number and composition on a synapse-by-synapse basis and that these properties in turn regulate the plasticity capacity of individual synapses.
What is the purpose of endowing quiescent synapses with an explosive potential for rapid strengthening? While difficult to prove, it is tempting to speculate that these synapses are poised to be rapidly integrated into new neural circuits by activity-driven changes in synaptic activity. This concept provides new insight into how memories might be made—changes in synaptic activity may selectively strengthen extant but silenced synapses to bring them back “online” and render them capable of conveying information to the postsynaptic neuron. Aside from this speculation, it is clear that the individual synapses may themselves hold information, as an easily potentiated synapse is likely to have had a recent history of inactivity.
Like most provocative findings, the observations by Ehlers and colleagues (Lee et al., 2010) raise as many questions as they answer. We consider three of these here. First, how does silencing cause synapses to acquire more NMDA receptors and undergo a switch in phenotype from primarily NR2A- to primarily NR2B-containing? An increase in NR2B-containing receptors could occur by synaptic incorporation of preexisting NR2B-containing NMDA receptors. Alternatively, activity blockade could drive local translation at individual synapses. Local protein synthesis endows a neuron with the ability to spatially restrict protein expression within individual dendrites, dendritic branches, or each of thousands of synapses made by the neuron, thereby vastly increasing the computational capacity of the brain (Wang et al., 2010). Stimulus-induced changes in receptor expression can alter and refine circuit connectivity in a persistent manner. In this way, experience modifies our memories, behaviors, feelings, and thoughts such that nature and nurture combine to determine who we are as individuals. A previous study involving Aplysia sensory-motor neurons has shown that activity-dependent local translation can occur in a stimulus- and synapse-specific manner (Wang et al., 2009). Thus, it is conceivable that local translation underlies the synapse-specific expression of experience-driven metaplasticity. Although intriguing, the mechanism by which synapses decode specific signals and activity patterns is as yet unclear (for review, see Wang et al., 2010).
Although less likely, it is also feasible that a synapse to nuclear signal could regulate NR2B gene transcription. Transcription would be followed by translation, assembly and targeting of newly synthesized NR1/NR2B receptors to previously silenced, “tagged” synapses. NR2B transcription is activated by cyclic AMP response element binding protein (CREB) and repressed by restrictive element 1 gene silencing factor (REST), both of which act by epigenetic mechanisms. Given the differences in the properties and signaling through NR2A and NR2B subtypes (Sobczyk et al., 2005), the switch in NMDA receptor phenotype could alter synaptic signaling to ERK-MAPK signaling, which impacts on many downstream targets including CREB. This, in turn, would promote a positive feedback loop whereby synaptic silencing would increase NR2B-containing receptors, which would activate CREB, which in turn would promote expression of new NR2B subunits. In the study by Lee et al., synaptic silencing occurs over many days, providing ample time for alterations in gene transcription to occur. Such a mechanism would invoke epigenetic remodeling of NMDA receptor number and subunit composition at single synapses in response to highly localized and spatially restricted external cues.
Second, are silenced synapses primed solely as a consequence of alterations in NMDA receptor strength, or does activity blockade independently alter the abundance and/or localization of other postsynaptic proteins and the intracellular signaling cascades downstream of NMDA receptors? Previous work by Ehlers showed that global activity blockade induces long-lasting changes in the molecular composition of the postsynaptic density through the ubiquitin-proteasome system and that these changes are bidirectional and reversible (Ehlers, 2003). In addition, activity blockade regulates alternative mRNA splicing, favoring the appearance of the C2′ variant of the NR1 subunit, which accelerates NMDA receptor forward trafficking at the endoplasmic reticulum (ER export) by virtue of a motif within the C2′ splice cassette, which recognizes and binds COPII (Mu et al., 2003). Thus, there are many conceivable mechanisms, perhaps acting in concert, for regulating synapse-specific and activity-driven changes in the properties of synaptic plasticity.
Third, is the change in NMDA receptor number and composition causally related to the lowered threshold for LTP (priming) induced in response to synaptic silencing? A direct test of causality could be achieved by applying subsaturating concentrations of an NMDA receptor antagonist (e.g., AP5) to silenced synapses to normalize NMDA EPSCs to a similar magnitude observed in the active synapses and then measuring the ability of a weak stimulus to elicit LTP in silenced synapses. A positive finding that this treatment prevented LTP in silenced synapses would suggest that a change in NMDA receptor current amplitude was causally related to the priming of silenced synapses. Given that NR2B-containing NMDA receptors are preferentially tethered to CaMKII (Barria and Malinow, 2005), it may be possible that, even with partial blockade of NMDA receptor current, an increase in CaMKII activity could drive phosphorylation and synaptic incorporation of the AMPA receptor subunit GluR1 (i.e., LTP).
In summary, Ehlers and colleagues (Lee et al., 2010) use infection of a tetanus toxin light chain to silence isolated synapses and uncaging of glutamate at single spines to reveal priming of individual synapses for LTP. The authors show that the underlying molecular mechanism involves an increase in NMDA receptor number and a switch in NMDA receptor phenotype from NR2A-containing to NR2B-containing receptors. Alterations in postsynaptic NMDA receptors are associated with an increase in post-synaptic Ca2+ and increase in the NMDA component of the EPSC. These findings are significant in that they show for the first time that metaplasticity, once thought to be a process involving a global change in the biophysical properties of entire neural networks, can occur within a single synapse. Understanding how changes in NMDA receptor number and subtype alter the threshold for potentiation is likely to cast light on the molecular mechanisms involved in plasticity and priming in general and increase our understanding of how neural networks participate in higher cognitive function, including learning and memory, and how their dysregulation causes neuropsychiatric disorders, including autism.
References
- Abraham WC. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MG, Bear MF. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lee M-C, Yasuda R, Ehlers MD. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Neuropharmacology. 2006;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DO, Kim SM, Zhao Y, Hwang H, Miura SK, Sossin WS, Martin KC. Science. 2009;324:1536–1540. doi: 10.1126/science.1173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DO, Martin KC, Zukin RS. Trends Neurosci. 2010;33:173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]