Abstract
The present study on long-term outcome of presymptomatic testing for Machado-Joseph disease (MJD) aimed to evaluate the psychological well-being and the familial satisfaction of subjects that 5 years prior received an unfavorable result in the predictive testing (PT). The study included 47 testees of Azorean origin (23 from the island of Flores and 24 from S. Miguel) that completed the fourth evaluation session of the MJD protocol, and undertook a neurological examination at the moment of participation in the study. Nearly 50% of testees were symptomatic at the time of the study. Psychological well-being of the 47 participants was evaluated using the Psychological General Well-Being Index (PGWB). The family satisfaction scale by adjectives was applied to obtain information on family dynamics. The average PGWB score of the total participants was of 73.3, a value indicative of psychological well-being. Nearly half of the testees presented scores indicating psychological well-being, whereas scores indicating moderate (28.9%) or severe (23.7%) stress were found in the remaining. The average score in the PGWB scale was lower in symptomatic than in asymptomatic subjects; moreover, the distinct distribution of the well-being categories seen in the two groups shows an impact of the appearance of first symptoms on the psychological state. Motives for undertaking the test, provided 5 years prior, failed to show an impact in well-being. The average score for familial satisfaction was of 134, a value compatible with high familial satisfaction, which represented the most frequent category (59.6%). Results demonstrate that well-being and family satisfaction need to be monitored in confirmed carriers of the MJD mutation. The inclusion of acceptance studies, after PT, as well as the development of acceptance training actions, should be of major importance to anticipate the possibility of psychological damage.
Introduction
Machado-joseph disease, also known as spinocerebellar ataxia type 3 (MJD/SCA3; MIM #109150), represents the most common form of spinocerebellar ataxia worldwide (Schöls et al., 2004; Paulson, 2007). MJD is an autosomal dominant neurodegenerative disorder of late onset (mean of 40.2 years), involving predominantly the cerebellar, pyramidal, extrapyramidal, motor neuron, and oculomotor systems (Coutinho, 1992). The marked phenotypic variability observed among MJD patients has justified their classification into three main clinical types, which are distinct with respect to the age at onset and neurological signs, implying variable degrees of incapacity (D'Abreu et al., 2010). Although certain MJD manifestations (such as ataxia or dystonia) can be alleviated with the use of specific drugs, presently there is no effective treatment to delay or to halt the progression of the disease. Recent advances in gene therapy, using interference RNA or antisense oligonucleotides in both cellular and animal models have been made [reviewed in Bettencourt and Lima (2011)]. In 1994, the identification of an expanded and unstable CAG repeat in the coding region of the ATXN3 gene, located at 14q32.1 (Takiyama et al., 1993; Kawaguchi et al., 1994), led to the observation that when this CAG tract was sized above a certain threshold (consensually more than 52 CAG units), it becomes pathological. Direct detection of the MJD mutation was then made feasible, and the molecular diagnosis for this disease was validated (Maciel et al., 1995). Predictive testing (PT) and prenatal diagnosis (Sequeiros et al., 1998), as well as preimplantation genetic diagnoses (Drüsedau et al., 2004) are currently possible for MJD.
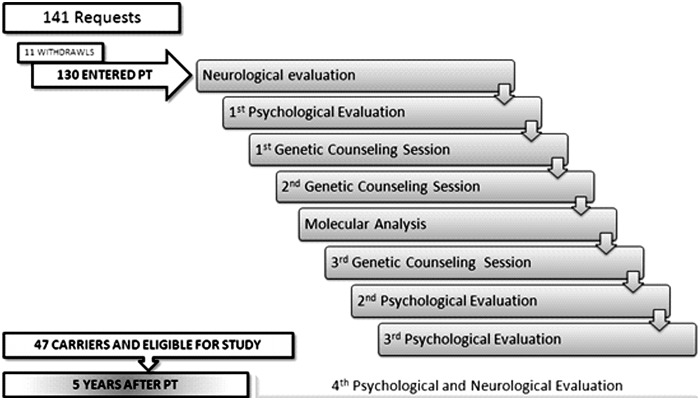
The geographic distribution of MJD is heterogeneous, and although it is classified as rare in the vast majority of countries, in a few populations, including the Azores archipelago (Portugal), the disease clusters. With a population of nearly 246,000 individuals (Instituto Nacional de Estatística, 2011), the Azores are formed by nine islands, which are divided into three geographical groups (eastern, central, and western). In the small western island of Flores, MJD reaches its highest prevalence, affecting 1 in 239 inhabitants (Bettencourt et al., 2008). A program for PT and genetic counseling (GC) for MJD that includes psychological evaluation and support, together with direct molecular testing, was initiated by the end of 1995 in Portugal (Sequeiros, 1996), based on the previously established Huntington's disease (HD) program (International HD Association [IHA] and the World Federation of Neurology [WFN] Research group on HD, 1994). The general protocol of this program aims to provide to adult at-risk individuals the access to the genetic information that can reduce the uncertainty about their genetic status, as well as to provide the necessary support to allow a healthy adaptation to the test results, so that psychological harm can be avoided (Sequeiros, 1996). The protocol (Fig. 1) includes four different psychological evaluations: initial evaluation (performed upon entering the program); second evaluation (undertaken ∼1 month after delivering the molecular test results); third evaluation (performed 1 year later); and fourth evaluation (undertaken ∼5 years after entering the PT program). A long-term follow-up is offered to all testees, as part of the test procedure. In the Azores, the PT and GC program has been conducted since 1996 by a local multidisciplinary team (Lima et al., 2001; Gonzalez et al., 2004). A component of this program has the main purpose of evaluating, in molecularly confirmed carriers of the MJD mutation, the psychological well-being, the individual and familial representations of the disease, as well as the family dynamics. In the first study concerning the impact of PT for MJD in the Azores, one of the main motives reported by testees for undertaking the test (Gonzalez et al., 2004) was the reduction of uncertainty (of being or not being a carrier). The analysis of the short-term impact (first-year follow-up) of the PT for MJD provided measures of depression and anxiety which were not clinically significant (Gonzalez et al., 2004). The aim of the present work was to assess, in subjects that ∼5 years prior received an unfavorable (carrier) result in the PT: (1) the psychological well-being; (2) familial satisfaction and occurrence of familial changes; and (3) the role played by a number of factors, such as presence of symptoms, in the general psychological well-being and familial satisfaction. Insights derived from this study should contribute to the improvement of the PT and GC program for MJD, and promote actions targeted to anticipate the possibility of psychological damage, therefore minimizing the negative impact of the PT in MJD families.
FIG. 1.
Program for predictive testing and genetic counseling for Machado-Joseph disease in the Azores Islands. The total number of requests, the number of subjects that undertook the program, as well as the number of participants in the present study are shown.
Methods
The data used in the present work were obtained at the fourth psychological evaluation session of the MJD protocol (Fig. 1), which took place ∼5 years after initiating the program. From the total of 130 subjects who entered the program in the Azores, during the period 1998–2001, 50 consultants (19 men and 31 women), who received a result of carrier in the direct molecular test accepted to participate in this study, after being contacted through the Department of Neurology of the Hospital of Ponta Delgada (São Miguel Island). Carriers that participated in the study represent nearly 80% of the testees that received a result of carrier. Twenty-four of the individuals were from S. Miguel, whereas 26 were from Flores Island. All subjects had been submitted to a neurological examination before entering the PT (Fig. 1), which confirmed them as being asymptomatic at that time. To provide an objective criterion concerning the presence of symptoms, a neurological exam was performed at the moment of participation in this study (Fig. 1). From the total 50 subjects who undertook the fourth psychological evaluation session, three were excluded from the present study, because they were unavailable for this neurological examination and, therefore, their status for the disease at the time of the study could not be confirmed. Participants' motives for being tested had been assessed during the pretest phase. During this stage, motives presented by at-risk subjects upon entering the PT program were categorized as: (1) plan for the future; (2) be sure; and (3) decide to have children.
The psychological well-being of the testees was evaluated using the Psychological General Well-Being Index (PGWB) scale (Dupuy, 1984). PGWB is a scale composed of 22 items, which evaluates anxiety, depressed mood, positive well-being, self-control, general health, and vitality. In accordance to the score obtained, it is possible to define three different categories: severe stress (0–60), moderate stress (61–72), and psychological well-being (73–110). Open questionnaires were used with the purpose of allowing participants to describe the impact of the PT result, the meaning and implications of the disease and the way they deal with it in their lives. With these questionnaires, we aimed to analyze the answers of the individuals by using direct questions referring to the impact, confrontation, and representation of the disease. Semistructured interviews were also conducted; these aimed to evaluate several aspects related with the disease, namely, changes that could have occurred after the PT, as well as familial indicators (family reactions to the test result, family reorganization at the level of its structure, routines and dynamics, levels and types of communication as well as patterns of interaction). Answers obtained in the questionnaire were compared with those provided subsequently at the interview. To obtain information on the family dynamics of the participants, the “Family satisfaction scale by adjectives” (ESFA) (Mairal and López-Yarto, 2003) was used. ESFA establishes the levels of family satisfaction by the use of adjectives. Levels of satisfaction were classified, according to the scores obtained into very low (<100), low (101–117), average-low (118–125), average-high (129–133), high (134–138) and very high (>138).
Differences in age between MJD subjects with symptoms and asymptomatic carriers were tested using the Mann–Whitney U test. Differences in categorical variables were assessed using the Fisher's exact test. Statistical analysis was performed using the software SPSS, version 15.0 (StatSoft, Inc., 2006). A p-value bellow 0.05 was considered as statistically significant.
Results
Table 1 presents baseline sociodemographic data of participants in the study (N=47). The majority of subjects were women (61.7%; 29/47), whose over-representation in the group of testees had already been reported in our previous work (Gonzalez et al., 2004). The analysis of the age distribution of subjects (average of 39.0±13.4 [standard deviation, SD] years) indicates that the majority of the individuals belong to age groups 1 and 2 (20–30 years and 31–40 years of age, respectively). When asked if the subjects would perform the test, if again faced with that decision, 96.1% of the subjects (100% in Flores Island) answered affirmatively.
Table 1.
Sociodemographic Characteristics of the Participants in the Present Study (N=47)
| Gender | |
| Women | 29 (61.7%) |
| Men | 18 (38.3%) |
| Age group (years) | |
| 1 (20–30) | 15 (31.9%) |
| 2 (31–40) | 17 (36.2%) |
| 3 (41–50) | 6 (12.8%) |
| 4 (51–60) | 4 (8.5%) |
| 5 (61–72) | 5 (10.6%) |
| Island of birth | |
| Flores | 23 (48.9%) |
| São Miguel | 24 (51.1%) |
| Educational level | |
| Grammar School (1–4) | 13 (32.5%) |
| High School (5–9) | 18 (45%) |
| High School (10–12) | 8 (20%) |
| University | 1 (2.5%) |
| Not available | 9 |
Five years after performing the test, symptoms were already present in 48.9% (23/47) of the subjects included in the study. The average onset and the main neurological features displayed by the subjects with symptoms at the time of the study are shown in Table 2. Cerebellar signs and oculomotor alterations were the two most frequent findings, in accordance to the natural history of MJD (Coutinho, 1992). Differences in age composition between the symptomatic and the asymptomatic group were statistically significant (Mann–Whitney U, Z=−2.589; p=0.01) (Table 3).
Table 2.
Main Neurological Findings in Symptomatic Testees (N=23)
| Age at onset (years) | |
| Mean (±SD) | 35.2±10.6 |
| Range | 18–55 |
| Frequency of neurological signs (%) | |
| Cerebellar | 93.8% |
| Pyramidal | 70.6% |
| Extrapyramidal | 12.5% |
| Peripheral | 6.3% |
| Oculomotor alterations | 88.2% |
SD, standard deviation.
Table 3.
Demographic Characteristics and Psychological Impact of Predictive Testing for Machado-Joseph Disease, According to the Presence of Symptoms in Testees
| Total (N=47) | Symptomatic (N=23) | Asymptomatic (N=24) | p-Valuea | |
|---|---|---|---|---|
| Age | ||||
| Median (range) | 36 (20–73) | 38 (22–73) | 32 (20–59) | 0.01 |
| Perform PT again, N (%) | ||||
| Yes | 45 (96%) | 22 (95.7%) | 23 (95.8%) | 1.0 |
| No | 2 (4%) | 1 (4.4%) | 1 (4.2%) | |
| Reasons to perform the PT (before PT), N (%) | ||||
| Plan for the future | 13 (28.9%) | 6 (26.1%) | 7 (31.8%) | 0.37 |
| Be sure | 21 (46.7%) | 13 (56.5%) | 8 (36.4%) | |
| Decide to have children | 11 (24.4%) | 4 (17.4%) | 7 (31.8%) | |
| Missing | 2 | 0 | 2 | |
| Well-being (PGWB), N (%) | ||||
| Severe stress | 9 (23.7%) | 8 (44.4%) | 1 (5.0%) | 0.02 |
| Moderate stress | 11 (28.9%) | 3 (16.7%) | 8 (40.0%) | |
| Psychological well-being | 18 (47.4%) | 7 (38.9%) | 11 (55.0%) | |
| Missing | 9 | 5 | 4 | |
| Familial changes (questionnaire), N (%) | ||||
| Detected | 8 (17.4%) | 5 (22.7%) | 3 (12.5%) | 0.45 |
| Not detected | 38 (82.6%) | 17 (77.3%) | 21 (87.5%) | |
| Missing | 1 | 1 | 0 | |
| Familial changes (interview), N (%) | ||||
| Detected | 19 (41.3%) | 14 (63.6%) | 5 (20.8%) | 0.006 |
| Not detected | 27 (58.7%) | 8 (36.4%) | 19 (79.2%) | |
| Missing | 1 | 1 | 0 | |
| Type of changes (interview), N (%)b | ||||
| Distancing | 8 (42.1%) | 7 (50.0%) | 1 (20.0%) | 0.34 |
| Approximation | 11(57.9%) | 7 (50.0%) | 4 (80.0%) | |
| Familial satisfaction (ESFA), N (%) | ||||
| Very low | 3 (6.4%) | 2 (8.7%) | 1 (4.2%) | 0.06 |
| Low | 2 (4.3%) | 2 (8.7%) | 0 (0%) | |
| Average–low | 4 (8.5%) | 4 (17.4%) | 0 (0%) | |
| Average–high | 6 (12.8%) | 1 (4.3%) | 5 (20.8%) | |
| High | 28 (59.6%) | 13 (56.5%) | 15 (62.5%) | |
| Very high | 4 (8.5%) | 1 (4.3%) | 3 (12.5%) | |
p-Value for the Mann–Whitney U test for age or the Fisher's exact test for categorical variables.
Relative to the subjects with familial changes detected through the interview.
PT, predictive testing; PGWB, Psychological General Well-Being Index; ESFA, family satisfaction scale by adjectives.
The average PGWB score of the total participants was of 73.3±15.5 [SD], a value indicative of psychological well-being. When considering well-being categories (Table 3), although 47.4% (18/38) of subjects presented scores indicating psychological well-being, scores not compatible with well-being were obtained for 52.6% of the participants, which displayed either moderate (28.9%; 11/38) or severe stress levels (23.7%; 9/38). The average score in the PGWB scale was lower in symptomatic (69.1±17.4 [SD]) than in asymptomatic subjects (76.9±12.87 [SD]). Furthermore, in the group with symptoms, there were relatively more individuals that displayed scores compatible with severe stress (8/18 in the symptomatic vs. 1/20 in the asymptomatic group). When organized by categories (Table 3), the scores of the PGWB scale showed statistically significant differences between these two groups (Fisher's exact test, p=0.02).
When grouping the participants by island of origin (S. Miguel vs. Flores), a higher mean score for the PGWB scale was observed in testees from Flores Island (78.3±13.9 [SD] for Flores; 70.0±15.8 [SD] for São Miguel). Differences observed, however, were not statistically significant. Motives for performing the PT, disclosed in the pretest phase, failed to show an impact in well-being.
For the total sample, the average score for familial satisfaction (ESFA scale) was of 134±18.3 [SD], a value in the lower extreme of high familial satisfaction. This last corresponded to the more represented category, with 59.6% (28/47) of the subjects (Table 3). The average ESFA score for symptomatic subjects was 131.0±19.5 [SD]; in the asymptomatic group, this value was of 136.8±16.9 [SD]. Globally, no statistically significant differences were detected between symptomatic and asymptomatic testees. When considering the island of origin of the subjects, it was noted that familial satisfaction was higher in Flores (average of 136.0±14.3 [SD]) than in S. Miguel (average of 132.0±21.6 [SD]), although this failed to produce significant differences.
When answering to the questionnaire, 17.4% of testees (8/46) reported the occurrence of familial changes after the PT, whereas the remaining 82.6% reported the lack of familial changes (“nothing has changed since I have known that I am a carrier of this disease”). It was observed, nevertheless, that at the interview, 41.3% (19/46) of the subjects reported familial changes, when asked “Have changes occurred in your family life, since you know the result of PT?” or “Describe what has changed in your life or in the life of your relatives.” Therefore, discrepancies were observed, with the frequency of modifications in family dynamics being clearly higher at the interviews (Table 3). In the questionnaire, symptomatic subjects more frequently reported the occurrence of changes, when compared to asymptomatic; however, this difference was not statistically significant. Noteworthy, the dissimilarity in the report of familial changes between the two groups reached a statistical significance at the interview, with symptomatic testees more frequently reporting changes. (Fisher's exact test; p=0.006). Subjects from S. Miguel and Flores showed no differences on what concerns familial changes, either at the interview or in the questionnaire.
When considering the testees who reported the occurrence of changes, 42.1% (8/19) described their familial changes as “distancing,” whereas the remaining reported “approximation.” Changes denote, on one hand, the existence of family approximation, characterized by a higher support and collaboration, with the family becoming the major support; also, situations in which the spouses assume so intensively their role of caregivers that they anticipate the needs of the carriers/patients, and even merge their lives with the carriers/patients, becoming a “merged caregiver” (Rolland, 1994): “we have become closer…I wake up thinking what he may need for the day, and even before he says it I have already got it.” On the other hand, situations of deterioration of the family and conjugal relations were also observed, leading, in extreme cases, to situations of domestic violence “from that moment on we lived badly, a lot of discussions and fighting, violence….” The distancing between members of the couple and the lack of communication “I don't speak with my family about MJD, it's like a taboo” is another of the modifications observed. Furthermore, the isolation of the individuals is yet another aspect of the modifications detected in the interviews “I live alone; I isolate myself from my family.”
Discussion
Levels of uptake of the PT for MJD in the Azores Islands, reported by Gonzalez et al. (2004), were in the order of 21%. This value is in accordance with the one referred in the literature for HD which, in Europe, is less than 20% (Gargiulo et al. 2009). The fact that almost all the participants answered, 5 years after receiving an unfavorable test result, that they would again perform the PT seems to indicate that the test is, overall, perceived as a gain.
Five years after undertaking the PT, the average PGWB score of the total participants was of 73.2, a value indicative of psychological well-being and similar to the one ascertained in a sample of healthy individuals from the general Portuguese population (Leite et al., 2002). In the symptomatic group, however, the average score in the PGWB scale was indicative of moderate stress. The distribution of the well-being categories was distinct between testees with and without symptoms: in the group with symptoms, there were relatively more individuals with moderate and severe stress. We can therefore confirm that the presence of symptoms is a major determinant of the general well-being of carriers of the MJD mutation. This finding is in accordance with results reported by Timman et al. (2004) for HD, in a study that analyzed the 7–10-year psychological effects of PT for HD; based on several indicators, Timman et al. conclude that carriers became more pessimistic when approaching the age at onset, and that an accurate assessment of the impact of the predictive test requires a prolonged follow-up.
Contrarily to similar studies in HD, in which a strong predictive value with respect to long-term distress is given to the motive for testing, in the present study, motives presented by testees at the entrance in the PT failed to show an impact on levels of well-being.
A higher mean for the PGWB scale was observed in testees from Flores Island. The fact that all individuals reassessed in Flores assume they would again undertake the test, if faced with that decision, reinforces what was already postulated by our group (Gonzalez et al., 2004): in Flores, at-risk individuals undertake the PT as a way of dealing with the social stigma of the disease, seeking proof of their status as noncarriers as a condition of affirmation to the community and a way to ensure social integration. The risk, however, lies in the possibility that in face of a positive outcome, community suspicions are confirmed and carriers enter the area of the stigma previously established. Nevertheless, our interpretation is that the certainty of the condition of testees from Flores Island allows them to develop strategies to cope or avoid the stigma. In S. Miguel Island, the social stigma of the disease is not so clear, which leads us to think that there are different family communication models in these two islands. We postulate that this occurs due to cultural factors inherent to each of the islands; in S. Miguel, the stigma is confined to the family circle itself, and the fear of speaking about MJD within the family results in the “Myth of Silence” phenomenon.
The experience with HD has shown that the analysis of the impact of the PT requires a contextual approach in which the family dynamics can be taken into account (Decruyenaere et al., 2003). For the total sample, the average score for familial satisfaction (ESFA scale) was of 134 (±18.3), a value in the lower extreme of the high familial satisfaction category. When considering the island of origin of the subjects, it was noted that familial satisfaction was higher in Flores than in S. Miguel.
Although perceiving the PT as a gain, a high frequency of the participants presents moderate to severe levels of stress. The values obtained in this study for the fraction of individuals who are stressed or severely stressed indicate a need to implement psychotherapeutic actions. Thus, to promote a higher level of well-being, psychological support should be provided in a consistent form, with the aim of coworking with the PT participants in the acceptance of their carrier and subsequently symptomatic conditions as well as coping strategies. Furthermore, particular attention should be given to the role of the family, friends, as well as social support networks. Through the interviews conducted in this study, different family dynamics modifications, reflecting distinct organizational forms, were described. On one side, the existence of a family approximation, with more family support, collaboration, and cohesion, implying, sometimes, situations of merged caregivers. On the other hand, family distancing, lack of communication and support, leading sometimes to violence and separation/divorce situations. Seclusion situations were, furthermore, identified. Comparison of the information provided in the questionnaires with the one disclosed at the interviews revealed some incongruity, showing that these events are often minimized or denied by the individuals, which lead us to think of a possible social desirability of the testees, with respect to an idealized form of how he/she would like his family to be perceived.
Evidences provided by this study highlights the need to proceed with the well-being and family satisfaction monitoring in subjects confirmed as carriers of the MJD mutation; furthermore, the inclusion of acceptance studies, to be conducted after delivering the PT result should be considered, and will be of crucial importance upon appearance of first symptoms. In fact, recent studies on physiological well-being have highlighted the relevant role of acceptance as a decisive mediator factor in the management of negative emotions when the individuals and families confront themselves with difficult experiences or deal with adversities (Didonna, 2011). Therefore, the development of acceptance training actions in carries of the MJD mutation should be of major importance aiming to anticipate the possibility of psychological damage, and the minimization of the negative impact of the PT result.
Acknowledgments
This work was supported by “PRI-DMJ” (funded by Regional Government of the Azores). C.B. (SFRH/BPD/63121/2009) is a recipient of the Post-doctoral grant from Fundação para a Ciência e a Tecnologia. N.K. (M3.1.3/F/004/2009) is recipient of a Post-doctoral grant from Secretaria Regional da Ciência, Tecnologia e Equipamentos.
Author Disclosure Statement
No competing financial interest exists.
References
- Bettencourt C. Lima M. Machado-Joseph Disease: from first descriptions to new perspectives. Orphanet J Rare Dis. 2011;6:35. doi: 10.1186/1750-1172-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt C. Santos C. Kay T, et al. Analysis of segregation patterns in Machado-Joseph disease pedigrees. J Hum Genet. 2008;53:920–923. doi: 10.1007/s10038-008-0330-y. [DOI] [PubMed] [Google Scholar]
- Coutinho P. Attempt of definition. PhD dissertation. University of Porto; Porto, Portugal: 1992. Machado-Joseph disease; p. 247. [Google Scholar]
- D'Abreu A. França MC. Paulson HL, et al. Caring for Machado-Joseph disease: current understanding and how to help patients. Parkinsonism Relat Disord. 2010;16:2–7. doi: 10.1016/j.parkreldis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decruyenaere M. Evers-Kiebooms G. Cloostermans T, et al. Psychological distress in the 5-year period after predictive testing for Huntington's disease. Eur J Hum Genet. 2003;11:30–38. doi: 10.1038/sj.ejhg.5200913. [DOI] [PubMed] [Google Scholar]
- Didonna F, editor. Clinical Manual of Mindfulness. Editorial Desclée De Brouwer; Bilbao, Spain: 2011. [Google Scholar]
- Drüsedau M. Dreesen JCFM. De Die-Smulders C, et al. Preimplantation genetic diagnosis of spinocerebellar ataxia 3 by (CAG)(n) repeat detection. Mol Hum Reprod. 2004;10:71–75. doi: 10.1093/molehr/gah008. [DOI] [PubMed] [Google Scholar]
- Dupuy HJ. The psychological general well-being (PGWB) index. In: Wenger NK, editor; Mattson ME, editor; Furberg CD, et al., editors. Assessment of Quality of Life in clinical trials of cardiovascular therapies. Le Jacq Publishing; New York: 1984. pp. 170–183. [DOI] [PubMed] [Google Scholar]
- Gargiulo M. Lejeune S. Tanguy ML, et al. Long-term outcome of presymptomatic testing in Huntington disease. Eur J Hum Genet. 2009;17:165–171. doi: 10.1038/ejhg.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. Lima M. Kay T, et al. Short-term psychological impact of predictive testing for Machado-Joseph disease: depression and anxiety levels in individuals at risk from the Azores (Portugal) Community Genet. 2004;7:196–201. doi: 10.1159/000082262. [DOI] [PubMed] [Google Scholar]
- Instituto Nacional de Estatística (INE) Censos. 2011. http://ine.pt/scripts/flex_v10/Main.html. [Sep;2011 ]. http://ine.pt/scripts/flex_v10/Main.html
- International HD Association (IHA) and the World Federation of Neurology (WFN) Research group on HD. Guidelines for the molecular genetics of the PT in HD. Neurology. 1994;44:1533–1536. [PubMed] [Google Scholar]
- Kawaguchi Y. Okamoto T. Taniwaki M, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Leite A. Paúl C. Sequeiros J. The pshychological well-being in individuals at risk for hereditary neurological disorders of late onset and controls. Psicologia, Saúde Doenças. 2002;3:113–118. [Google Scholar]
- Lima M. Kay T. Vasconcelos J, et al. Disease knowledge and attitudes toward predictive testing and prenatal diagnosis in families with Machado-Joseph disease from the Azores Islands (Portugal) Community Genet. 2001;4:36–42. doi: 10.1159/000051154. [DOI] [PubMed] [Google Scholar]
- Maciel P. Gaspar C. DeStefano AL, et al. Correlation between CAG repeat length and clinical features in Machado-Joseph disease. Am J Hum Genet. 1995;57:54–61. [PMC free article] [PubMed] [Google Scholar]
- Mairal JB. López-Yarto L. ESFA—Scale of Familial Satisfaction by Objectives. Tea Ediciones; Madrid, Spain: 2003. [Google Scholar]
- Paulson HL. Dominantly inherited ataxias: lessons learned from Machado-Joseph disease/spinocerebellar ataxia type 3. Semin Neurol. 2007;27:133–142. doi: 10.1055/s-2007-971172. [DOI] [PubMed] [Google Scholar]
- Rolland JS. Basic Books; New York: 1994. Families, Illness and Disability: An Integrative Treatment Model. [Google Scholar]
- Schöls L. Bauer P. Schmidt T, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- Sequeiros J. The Predictive Test for Machado-Joseph Disease. UnIGENe; Porto, Portugal: 1996. Classical and molecular genetics of Machado-Joseph disease; pp. 33–48. [Google Scholar]
- Sequeiros J. Maciel P. Taborda F, et al. Prenatal diagnosis of Machado-Joseph disease by direct mutation analysis. Prenat Diagn. 1998;18:611–617. [PubMed] [Google Scholar]
- StatSoft. Inc., S. SPSS for Windows: Release 15.0. SPSS Inc.; Chicago: 2006. [Google Scholar]
- Takiyama Y. Nishizawa M. Tanaka H, et al. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993;4:300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Timman R. Roos R. Maat-Kievit A, et al. Adverse effects of predictive testing for Huntington disease underestimated: longterm effects 7–10 years after the test. Health Psychology. 2004;23:189–197. doi: 10.1037/0278-6133.23.2.189. [DOI] [PubMed] [Google Scholar]