Abstract
Glycogen synthase kinase-3β (GSK3β) may play an important role in the brain of patients with major depressive disorder (MDD); therefore, we investigated whether the GSK3β gene is involved in the etiology of MDD and whether it affects MDD endophenotypes. Three single-nucleotide polymorphisms (SNPs) (rs6438552, rs7633279, and rs334558) were genotyped in 559 MDD patients and 486 healthy controls. To explore quantitative traits of MDD, we analyzed the association of these SNPs with the factor scores of the 17-item Hamilton Depression Rating Scale (HAMD-17) and the Hamilton Anxiety Rating Scale (HAMA). We also determined the effects of these SNPs on the measurement of the P300 wave. Although no significant association between GSK3β SNPs and MDD was found, some genotypes and haplotypes were associated with anxiety symptoms in MDD. The three SNPs were associated with the HAMA total score and with the HAMD anxiety and somatization factor score (p<0.05). Three-locus haplotype analysis showed the C-T-G carriers to have a strong association with the HAMA total score (p=0.032). Moreover, the P300 latency and amplitude were also associated with GSK3β genotypes. The individuals with the T allele genotype, both in rs6438552 and rs7633279, have a longer P300 latency than those carrying the C/C (p=0.04) and A/A genotype (p=0.013). The individuals with the G/G genotype in rs334558 have a lower amplitude than those carrying the A allele genotype (p=0.007). Our findings show, for the first time, that GSK3β polymorphisms may play an important role in MDD endophenotypes, especially in anxiety symptoms.
Introduction
Major depressive disorder (MDD) is one of the most prevalent and costly neuropsychiatric diseases, with a lifetime prevalence of 12–17% (Wittchen et al., 1992). Although the etiology of MDD remains unclear, a genetic component is likely to contribute to its development, with heritability ranging from 40–50% (Bierut et al., 1999; Sullivan et al., 2000). Thus, identification of MDD susceptibility genes can contribute, not only to understanding the etiology of the disease but also to the development of individualized prevention and treatment strategies. With the development of hypotheses concerning mental illness and neural plasticity, much interest has recently been shown in glycogen synthase kinase-3β (GSK3β), a key regulator of neuronal function (Lachman et al., 2007; Meng et al., 2008; Tsai et al., 2008).
GSK3β was identified in the early 1980s as a key enzyme in the regulation of the glycogen synthesis. It is an ubiquitous cellular serine–threonine kinase that phosphorylates and inactivates glycogen synthase (Embi et al., 1980; Chin et al., 2005; Balaraman et al., 2006). In addition to glucose metabolism, GSK3β is also involved in the regulation of critical intracellular signaling pathways that affect multiple processes, including embryogenesis, gene expression, cell cycle, apoptosis, and cell survival (Grimes and Jope, 2001; Jope and Bijur, 2002; Muyllaert et al., 2008). GSK3β, an isoform of GSK3, is highly abundant in the brain and is highly expressed in neural tissue where its expression is regulated during development (Grimes and Jope, 2001). In addition to its function as a negative regulator of glycogen synthesis, GSK3β now has become recognized as an enzyme with broad influence. In neurons, GSK3β takes part in cytoskeletal organization and remodeling. It plays an important role in the BDNF pathway, and is thereby involved in mechanisms of synaptic plasticity, neurogenesis, and resilience to neuronal injury (Grimes and Jope, 2001; Gould and Manji, 2005; Muyllaert et al., 2008). GSK3β may be implicated in the pathogenesis of MDD, and there are many studies showing that inhibition of GSK3β activity may affect the therapeutic effects of antidepressants. Treatment with antidepressants was found to inhibit GSK3β activity in the mouse brain (Li et al., 2004; Roh et al., 2005). Lithium, a mood stabilizer that enhances the action of antidepressants, was found to inhibit GSK3β in vitro, in the mouse brain (Klein and Melton, 1996; Stambolic et al., 1996) and in human peripheral blood mononuclear cells (Li et al., 2007). Furthermore, Tsai et al. (2008) have reported an association between genetic variation in GSK3β and response to 4 weeks of selective serotonin reuptake inhibitor antidepressant therapy in Chinese individuals with MDD.
In a previous study, we had observed a weak potential association between a GSK3β-associated single-nucleotide polymorphism (SNP), rs6782799, and MDD (Yang et al., 2010; Zhang et al., 2010). For this reason, we have further investigated the association between the GSK3β and MDD in a Chinese population using an enlarged sample size and an increased number of SNPs. In addition, we analyzed the association between the GSK3β polymorphisms and endophenotypes in MDD patients.
Materials and Methods
Subjects
The patient group comprised 581 MDD patients (264 men and 317 women), with a mean age of 32.0±9.8 years, ranging from 18 to 65 years. Clinical diagnosis was made by at least two consultant psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for MD (American Psychiatric Association, 2000). All patients recruited for this study were also assessed with the Chinese Version of the Modified Structured Clinical Interview for DSM-IV TR Axis I Disorders Patient Edition (SCID-I/P, 11/2002 revision). We excluded potential participants who were pregnant or had significant medical conditions, unstable psychiatric features (e.g., suicidal), or a history of substance abuse or drug addiction within the previous 6 months, with the exception of nicotine dependence. Other Axis I comorbid disorders were not excluded.
The control group consisted of 486 healthy volunteers (217 men and 269 women), with a mean age of 32.8±8.6 years, ranging from 18 to 65 years. They were recruited from local communities or were people undergoing routine health check-ups. All control subjects were assessed using the SCID. Subjects with relevant physical diseases or a history of major psychiatric disorders or suicidal behavior were excluded, and those who had a first-degree relative with a history of severe mental disorder or suicidal behavior were also excluded.
The patients in our study were not receiving medical treatment currently. They were all on the first episode of disease. In addition, the 17-item Hamilton Depression Rating Scale (HAMD-17) and the Hamilton Anxiety Rating Scale (HAMA) were used to assess clinical characteristics. For exploratory purposes, we examined the factor scores of HAMD-17, including the anxiety/somatization factor (items 10, 11, 12, 13, 15, and 17), the dysgnosia factor (items 2, 3, and 9), the blocking factor (items1, 7, 8, and 14), and the sleep factor (items 4, 5, and 6). HAMA factor scores were also analyzed, including the somatic anxious factor (items 7, 8, 9, 10, 11, 12, and 13) and the mental anxious factor (items 1, 2, 3, 4, 5, 6, and 14). All subjects were of Han Chinese origin, came from the same geographical area in Northern China, and gave written informed consent. This study was approved by the Ethics Committee for Medicine of the First Hospital of Shanxi Medical University, China.
P300 wave event-related potential assessments
Of the subjects genotyped, 267 patients and 248 controls underwent P300 assessments. They sat alert with eyes closed while listening to stimuli presented biaurally through ear plugs. The P300 event-related potential component was assessed by a standard auditory odd-ball task. The stimuli were four hundred 80-dB tones with 20-ms duration; 20% were target stimuli (1500 Hz), and 80% were standard stimuli (1000 Hz). The participants were instructed to press a button in response to the target stimuli. Electroencephalogram (EEG) data were collected from the central electrode according to the 10/20 International System, referenced to the left ear. Eye movements were recorded from the outer canthus of each eye, above and below the left eye. Electron impedances were kept below 5Ω. For the recording of EEG activity, the analog/digital rate was 500 Hz, and the filter setting was 0.03 Hz (high pass) and 120 Hz (low pass). The EEG was segmented in epochs of 900-ms duration (−100 to 800 ms relative to stimulus onset). The P300 peaks were defined as the most positive point constituting a peak between 250 and 650 ms after stimulus by use of maximum-likelihood estimation. The measurement has been described previously (Xu et al., 2010).
SNP selection and genotyping
Three GSK3β SNPs were selected to test their disease association in the Chinese Han population, two of which are tag SNPs based on the information of the HapMap for the Han Chinese in a Beijing (CHB) population (www.hapmap.org). rs6438552 in intron 5 and rs7633279 in intron 8 have been reported as risk loci for MDD in other studies (Kwok et al., 2005). Further, we also investigated rs334558 located in promoter 5 for genetic analysis.
Leukocyte DNA was extracted using a standard phenol–chloroform method and quantified using a microspectrophotometer (NaNoDropND-1000; Thermo Scientific). Real-time quantitative polymerase chain reaction was performed using a TaqMan minor groove binder (MGB) probe and appropriate primers (Applied Biosystems). TaqMan MGB probes are oligonucleotides capable of binding to specific allelic loci. They have different fluorescent labels, denoted by VIC and FAM. On 384-well plates, the wet DNA method was used to manually add sample to the reaction plate. The reaction system is a 5-μL system, with each test well containing 2.5 μL of 2× TaqMan Master Mix (Applied Biosystems), 0.25 μL of 20× TaqMan genotyping assay mix (Applied Biosystems), 1.25 μL of ddH2O, and 1 μL of DNA at 50 ng/μL. A reference reaction was also run containing 1 μL of ddH2O instead of DNA. Reaction conditions were 95°C for 10 min, 95°C for 15 s, and 60°C for 60 s. Steps two and three were repeated 50 times. According to the PCR results, 5 to 10 additional assays were performed using different probes as necessary.
Statistical analysis
The Haploview program (version 4.1; Broad Institute of MIT and Harvard) (www.broad.mit.edu/mpg/haploview) was applied to test the genotypic distributions of SNPs for the Hardy–Weinberg equilibrium (HWE) and to estimate linkage disequilibrium (LD) between these three SNPs in which the LD strength was expressed by measurements D′ and r2. Allelic, genotypic, and haplotypic associations were analyzed using the UNPHASED program (version 3.1.5) (www.mrcbsu.cam.ac.uk/personal/frank/software/unphased). SPSS for Windows (version 15.0; SPSS) was used to perform data analyses. The effects of individual genotypes on P300 amplitude and P300 latency were also analyzed by Student's t-test (two-tailed). The significance level was set at a p-value of 0.05.
Results
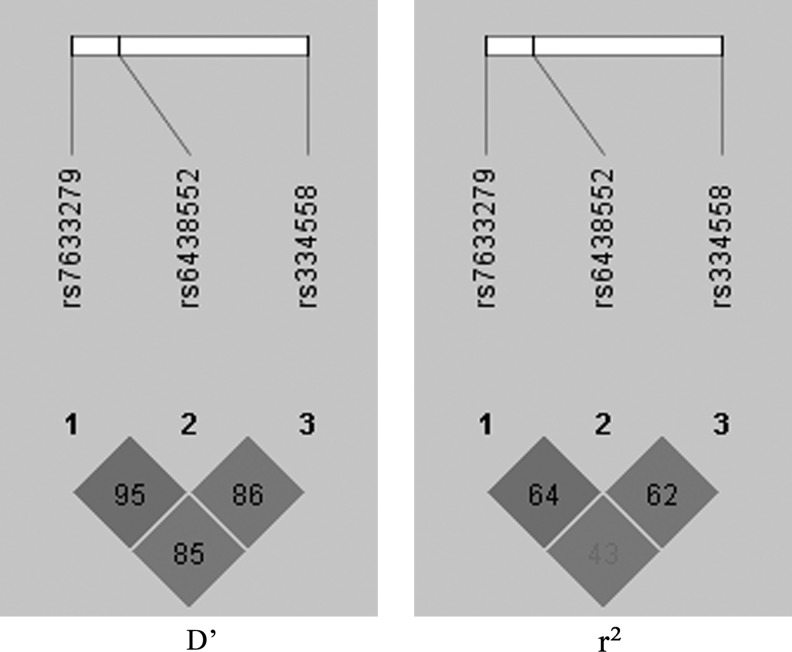
The genotypic distributions of the three GSK3β SNPs did not deviate from the HWE in the control group. The LD analysis was showed in Figure 1.
FIG. 1.
The linkage disequilibrium (LD) patterns of the three markers in the control group: D′ and r2.
Association with MDD risk
The mean age (t=−1.357, df=1062.8, p=0.175) and sex distributions (χ2=0.066, df=1, p=0.797) of the MDD patients and the healthy controls were similar. The genotype and allele distributions of the GSK3β SNPs (compared between the patient and control groups) are summarized in Table 1. As shown in Table 1, the analysis for single-locus effects showed no significant association between these SNPs and MDD (all p after permutation >0.05). The results of global case–control haplotypic analysis and comparisons of individual haplotypes between groups are presented in Table 2. However, there was no significant association between haplotypes and the risk of MDD (χ2=11.75, p=0.068, adjusted p=0.166).
Table 1.
Genotype Distributions and Allele Frequencies of Single-Nucleotide Polymorphisms in Major Depressive Disorder Patients and Healthy Controls
| SNP | Group | n | Genotype (%) | χ2 | p | Allele (%) | χ2 | p | OR (95% CI) | HWE-p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6438552 | C/C | C/T | T/T | C | T | ||||||||
| Patients | 496 | 155 (31.3) | 240 (48.4) | 101 (20.4) | 4.232 | 0.121 | 550 (55.4) | 442 (44.6) | 3.905 | 0.048a | 1.199 (1.001–1.436) | 0.646 | |
| Controls | 476 | 178 (37.4) | 214 (45.0) | 84 (17.6) | 570 (60.0) | 382 (40.1) | 0.160 | ||||||
| rs7633279 | T/T | T/A | A/A | T | A | ||||||||
| Patients | 526 | 138 (26.2) | 250 (47.5) | 138 (26.2) | 1.405 | 0.495 | 526 (50) | 526 (50) | 0.486 | 0.486 | 0.940 (0.789–1.119) | 0.257 | |
| Controls | 483 | 112 (23.1) | 244 (50.5) | 127 (26.3) | 468 (48.4) | 498 (51.6) | 0.803 | ||||||
| rs334558 | G/G | G/A | A/A | G | A | ||||||||
| Patients | 489 | 180 (36.8) | 222 (45.4) | 87 (17.8) | 2.678 | 0.262 | 582 (59.5) | 396 (40.5) | 2.805 | 0.094 | 1.173 (0.973–1.414) | 0.200 | |
| Controls | 444 | 185 (41.7) | 192 (43.2) | 67 (15.1) | 562 (63.3) | 326 (36.7) | 0.144 | ||||||
Adjusted p is 0.144 after 10000 permutation tests.
CI, confidence interval; HWE, Hardy–Weinberg equilibrium; OR, odds ratio; SNP, single-nucleotide polymorphism.
Table 2.
Estimated Haplotype Frequencies and Association Significance
| |
|
|
|
Frequency (%) |
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|
| Haplotype | rs6438552 C/T | rs7633279 T/A | rs334558 G/A | Patients n=581 | Controls n=486 | χ2 | p | OR (95% CI) | Global p (χ2) |
| 1 | C | T | G | 49.79 | 77.01 | 5.342 | 0.021 | 1 (1–1) | 0.068 (11.75) |
| 2 | C | T | A | 8.579 | 5.484 | 0.549 | 0.459 | 2.419 (0.686–8.539) | |
| 3 | C | A | G | 387.5 | 414.4 | 0.643 | 0.423 | 1.446 (0.978–2.137) | |
| 4 | C | A | A | 35.18 | 20.11 | 4.267 | 0.039 | 2.706 (1.376–5.32) | |
| 5 | T | T | G | 70.38 | 57.1 | 1.368 | 0.242 | 1.906 (1.139–3.19) | |
| 6 | T | T | A | 295.2 | 292.4 | 0.365 | 0.546 | 1.562 (1.049–2.324) | |
| 7 | T | A | G | 5.373 | 5.49 | 0.005 | 0.945 | 1.513 (0.419–5.467) | |
Association with MDD endophenotypes
To explore endophenotypes of MDD, we analyzed SNP association with subfactors of HAMD-17 and HAMA in MDD patients (Table 3). As shown in Table 3, GSK3β genotypes were associated with the HAMA total score and two of the factor scores (all p<0.05). The T/T genotype in rs6438552 and the A/A genotype in both rs7633279 and rs334558 all associate with a higher anxiety score than other genotypes. Similarly, we also found an association between the anxiety/somatization factor score in HAMD and the genotypes of rs6438552: p=0.002, rs7633279: p=0.027, but not of rs334558 (p>0.05). The three-locus haplotype analysis showed the C-T-G carriers to have a strong association with the HAMA total score (p=0.002, adjusted p=0.032) (Table 4). In addition, we also found a strong association between the rs334558 genotype and the sleeping factor of HAMD (p=0.047, adjusted p=0.009) (Table 3), and with the haplotype (global p<0.001, adjusted p=0.008) (Table 4).
Table 3.
GSK3β Genotype Associations with Factor Scores in HAMA and HAMD-17
| |
rs6438552 |
rs7633279 |
rs334558 |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Mean score |
|
|
Mean score |
|
|
Mean score |
|
|
|
||||||
| Factors | CC (154) | CT (238) | TT (99) | χ2 | p | TT (136) | TA (247) | AA (138) | χ2 | p | GG (179) | GA (220) | AA (85) | χ2 | p | Adjusted p |
| HAMA-T | 17.96 | 16.98 | 19.35 | 11.13 | 0.004 | 18.46 | 16.86 | 18.99 | 12.75 | 0.002 | 17.78 | 17.66 | 19.65 | 7.083 | 0.029 | 0.010 |
| HAMA-1 | 6.20 | 5.58 | 6.95 | 10.25 | 0.006 | 6.34 | 5.66 | 6.66 | 7.017 | 0.030 | 6.15 | 5.92 | 7.11 | 6.449 | 0.040 | 0.018 |
| HAMA-2 | 11.76 | 11.40 | 12.40 | 7.121 | 0.028 | 12.13 | 11.20 | 12.34 | 14.12 | 0.001 | 11.63 | 11.75 | 12.54 | 5.13 | 0.077 | 0.003 |
| HAMD-T | 22.61 | 22.02 | 22.79 | 3.436 | 0.179 | 22.45 | 21.93 | 23.02 | 7.017 | 0.058 | 22.74 | 22.45 | 23.11 | 0.804 | 0.669 | 0.138 |
| HAMD-1 | 7.06 | 6.42 | 7.39 | 12.82 | 0.002 | 6.85 | 6.53 | 7.28 | 7.203 | 0.027 | 7.13 | 6.81 | 7.18 | 2.286 | 0.319 | 0.007 |
| HAMD-2 | 4.01 | 4.09 | 3.74 | 3.436 | 0.179 | 3.79 | 4.16 | 3.86 | 4.13 | 0.127 | 4.17 | 4.01 | 3.71 | 7.239 | 0.027 | 0.060 |
| HAMD-3 | 7.39 | 7.58 | 7.75 | 2.038 | 0.361 | 7.71 | 7.51 | 7.42 | 1.013 | 0.603 | 7.45 | 7.64 | 7.80 | 0.834 | 0.659 | 0.615 |
| HAMD-4 | 3.46 | 3.32 | 3.49 | 1.03 | 0.598 | 3.53 | 3.20 | 3.71 | 5.838 | 0.054 | 3.31 | 3.46 | 3.84 | 9.323 | 0.047 | 0.009 |
GSK3β, glycogen synthase kinase-3β; HAMD, Hamilton depression rating scale; HAMA, Hamilton anxiety rating scale; HAMA-T, HAMA total; HAMA-1, HAMA somatic anxious factor; HAMA-2, HAMA mental anxious factor; HAMD-T, HAMD Total; HAMD-1, HAMD-17 anxiety and somatization factor; HAMD-2, HAMD-17 dysgnosia factor; HAMD-3, HAMD-17 blocking factor; HAMD-4, HAMD-17 sleep factor.
Table 4.
GSK3β Haplotype Associations with Factor Scores in HAMA and HAMD-17
| |
p (χ2) |
|
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | CTG | CTA | CAG | CAA | TTG | TTA | TAG | χ2 | Global p | Adjusted p |
| HAMA-T | 0.002 (9.149) | 0.386 (0.751) | 0.460 (0.547) | 0.053 (3.757) | 0.283 (1.155) | 0.027 (4.891) | 0.881 (0.022) | 17.15 | 0.009 | 0.032 |
| HAMA-1 | 0.004 (8.201) | 0.76 (0.093) | 0.402 (0.704) | 0.032 (4.575) | 0.648 (0.209) | 0.141 (2.168) | 0.398 (0.713) | 14.89 | 0.021 | 0.052 |
| HAMA-2 | 0.014 (6.027) | 0.040 (4.217) | 0.658 (0.195) | 0.225 (1.472) | 0.130 (2.289) | 0.012 (6.27) | 0.499 (0.458) | 17.65 | 0.007 | 0.133 |
| HAMD-T | 0.120 (2.412) | 0.671 (0.181) | 0.804 (0.061) | 0.826 (0.048) | 0.467 (0.530) | 0.277 (1.182) | 0.252 (1.31) | 5.211 | 0.517 | 0.601 |
| HAMD-1 | 0.043 (4.087) | 0.478 (0.503) | 0.959 (0.003) | 0.720 (0.128) | 0.319 (0.994) | 0.628 (0.236) | 0.140 (2.181) | 7.324 | 0.292 | 0.295 |
| HAMD-2 | 0.058 (3.593) | 0.069 (3.303) | 0.027 (4.887) | 0.020 (5.413) | 0.909 (0.013) | 0.067 (3.351) | 0.292 (1.111) | 18.2 | 0.006 | 0.170 |
| HAMD-3 | 0.731 (0.119) | 0.134 (2.247) | 0.072 (3.245) | 0.327 (0.962) | 0.260 (1.27) | 0.026 (4.986) | 0.116 (2.473) | 12.72 | 0.048 | 0.193 |
| HAMD-4 | 0.009 (6.807) | 0.475 (0.510) | 0.368 (0.810) | 0.001 (10.98) | 0.144 (2.136) | 0.044 (4.043) | 0.008 (6.948) | 29.96 | 0.000 | 0.008 |
Association with the P300 wave event-related potential
Compared with control subjects, patients with MDD displayed significantly longer P300 latency (controls=299.0±32.6 ms, patients=307.1±41.9 ms, p<0.001) and lower P300 amplitude (controls=8.4±4.1 μV, patients=7.9±3.8 μV, p<0.001).
We also estimated genotype association with P300 latency and amplitude (Table 5). When subjects were grouped according to GSK3β genotypes, the P300 latency and amplitude were also associated with genotypes of the three SNPs. The individuals with the T genotype in rs6438552 (C/T+T/T genotype) and rs7633279 (A/T+T/T genotype) have a longer P300 latency than those carrying the C/C (p=0.040) and A/A genotypes (p=0.013). The individuals with the G/G genotype in rs334558 showed a lower amplitude than those carrying the A genotype (p=0.007).
Table 5.
GSK3β Genotype Associations with P300 Latency and Amplitude
| Measures | Genotype | n |
Mean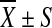
|
95% CI | F | p | Genotype | n |
Mean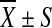
|
t | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6438552 | |||||||||||
| LAT | CC | 65 | 296.00±37.28 | 286.76–305.24 | 3.558 | 0.030 | CC | 65 | 296.00±37.28 | −2.067 | 0.040 |
| CT | 106 | 304.17±41.71 | 296.14–312.20 | CT+TT | 157 | 307.72±38.90 | |||||
| TT | 51 | 315.10±31.38 | 306.27–323.92 | ||||||||
| Total | 222 | 304.29±38.72 | 299.17–309.41 | ||||||||
| AMP | CC | 65 | 8.00±3.61 | 7.10–8.89 | 1.527 | 0.220 | CC | 65 | 8.00±3.61 | −1.662 | 0.098 |
| CT | 106 | 9.19±4.74 | 8.27–10.10 | CT+TT | 157 | 8.97±4.68 | |||||
| TT | 51 | 8.51±4.54 | 7.23–9.79 | ||||||||
| Total | 222 | 8.68±4.40 | 8.10–9.26 | ||||||||
| rs7633279 | |||||||||||
| LAT | TT | 65 | 308.31±32.24 | 300.32–316.30 | 3.196 | 0.043 | AA | 56 | 292.36±40.62 | 2.516 | 0.013 |
| TA | 113 | 306.57±40.81 | 298.96–314.17 | TT+TA | 178 | 307.20±37.82 | |||||
| AA | 56 | 292.36±40.62 | 281.48–303.24 | ||||||||
| Total | 234 | 303.65±38.94 | 298.63–308.67 | ||||||||
| AMP | TT | 65 | 9.03±4.07 | 8.03–1.04 | 1.617 | 0.201 | AA | 56 | 292.36±40.62 | 1.798 | 0.074 |
| TA | 106 | 9.12±4.88 | 8.21–10.03 | TT+TA | 178 | 307.20±37.82 | |||||
| AA | 51 | 7.87±3.88 | 6.83–8.91 | ||||||||
| Total | 222 | 8.80±4.45 | 8.229.37 | ||||||||
| rs334558 | |||||||||||
| LAT | TT | 65 | 308.31±32.24 | 300.32–316.30 | 3.196 | 0.043 | GG | 77 | 298.34±39.76 | −1.523 | 0.129 |
| TA | 113 | 306.57±40.81 | 298.96–314.17 | GA+AA | 151 | 306.82±39.78 | |||||
| AA | 56 | 292.36±40.62 | 281.48–303.24 | ||||||||
| Total | 234 | 303.65±38.94 | 298.63–308.67 | ||||||||
| AMP | TT | 65 | 9.03±4.07 | 8.03–1.04 | 1.617 | 0.201 | GG | 77 | 7.82±3.78 | −2.731 | 0.007 |
| TA | 106 | 9.12±4.88 | 8.21–10.03 | GA+AA | 151 | 9.40±4.80 | |||||
| AA | 51 | 7.87±3.88 | 6.83–8.91 | ||||||||
| Total | 222 | 8.80±4.45 | 8.229.37 | ||||||||
LAT, latency; AMP, amplitude.
Discussion
Several lines of evidence indicate that GSK3β is a good candidate for MDD susceptibility. In this study, we investigated whether common SNPs in the GSK3β gene were associated with MDD and its endophenotypes. Significant association with MDD was not shown for alleles and genotypes of a single locus. Analysis of three-locus GSK3β haplotypes was subsequently performed, but still no meaningful association was found. We therefore investigated whether the GSK3β genotypes and haplotypes were associated with endophenotypes of MDD. Our findings showed that three GSK3β SNPs are strongly associated with anxiety symptoms and with the P300 measures, both in genotypes and haplotypes. In particular, the T allele of rs6438552 may be a risk allele, because it was associated not only with the anxiety symptoms of MDD but also with the delayed P300 latency. Moreover, there was a risk trend in the case–control study of rs6438552, although it was not statistically significant (p=0.048, adjusted p=0.144).
In a recent study, Tsai et al. (2008) recruited 230 Chinese MDD patients and also found no significant association of MDD with the alleles and genotypes of single locus or four-locus haplotypes (rs334558, rs13321783, rs2319398, and rs6808874). However, three of the four polymorphisms investigated were significantly associated with an antidepressant therapeutic effect (p=0.002–0.011). Compared with the study of Tsai et al., we used a relatively large sample size and had more power to detect associations. The present findings may be more reliable, although they should still be interpreted with caution. As mentioned above, in a previous study, we observed a weak potential association between a GSK3β-associated SNP and MDD, while the combined effects of the BDNF and GSK3β genes were associated with MDD (Zhang et al., 2010). Furthermore, an interaction between BDNF and GSK3β may modify the relationship between negative life events and MDD in Chinese individuals (Yang et al., 2010). This was concordant with our present findings, because no strong association between a GSK3β-associated SNP and MDD was found. Moreover, we could infer that the GSK3β gene may not participate in the etiology of MDD on its own, but that the gene–gene interactions of GSK3β and other genes may play an important role.
The clinical symptoms of MDD vary greatly among individuals, so a single genetic association may not fully explain its complexity. Therefore, in future studies, it will be necessary to pay close attention to the endophenotypes of MDD. To our knowledge, this is the first report of evidence that the GSK3β gene is associated with the risk of anxiety symptoms in MDD in a Chinese population sample. However, in recent years, many studies have shown that a brain-derived neurotrophic factor (BDNF) gene polymorphism (rs6265) is associated with the anxiety symptoms of MDD (Jiang et al., 2005; Lang et al., 2005; Chen et al., 2006; Hunnerkopf et al., 2007; Enoch et al., 2008). BDNF regulates neuronal survival in the central nervous system via the phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B (PKB) pathway, and activated PKB regulates a number of cell survival-related proteins, such as GSK3β (Huang and Reichardt, 2001). As a component in the final steps of the BDNF pathway, GSK3β may be involved in the same pathophysiological mechanism of MDD as BDNF. This association is consistent with previous findings concerning BDNF and suggests that the whole BDNF system may be associated with the anxiety symptoms in MDD.
The current data and previously published reports support the assertion that GSK3β is involved in the development of MDD in a Han Chinese population, but that it is not directly involved in the pathomechanism. This is likely to reflect the complexity of psychiatric disorders, which are thought to be caused by multiple genetic factors, each providing a small effect. Thus, rs6438552 in GSK3β may be directly involved in one of the MDD endophenotypes, the anxiety symptom.
To clarify the role of GSK3β in disease components or endophenotypes and to understand the function of GSK3β in MDD, we further examined the association between GSK3β and the P300 waveform of the MDD endophenotype. Generally, quantitative trait association studies (based on phenotypic or endophenotypic subgroups) offer several advantages over case–control studies, one of which is a 4-fold to 8-fold increase in statistical power, thus greatly decreasing the required sample size to achieve sufficient statistical power (Wang et al., 2005; Potkin et al., 2009). Our results of longer P300 latency in MDD patients compared to healthy controls confirm those of previous reports (Bruder et al., 1991; Himani et al., 1999; Karaaslan et al., 2003). Moreover, individuals with the T allele genotype, both in rs6438552 (C/T+T/T) and rs7633279 (A/T+T/T), have a longer P300 latency than those with the C/C or A/A genotype. The latency of P300 has been considered a measure of attentional resource allocation (Coull, 1998), and its prolongation has been discussed as an index of neurodegenerative processes (O'Donnell et al., 1995) affecting the callosal size and the efficiency of interhemispheric transmission (Johnson, 1993). On the other hand, the decrease in the P300 amplitude may also represent a neurocognitive vulnerability marker for the development of depression (Urretavizcaya et al., 2003; Zhang et al., 2007). In our study, the individuals with the G/G genotype in rs334558, which is associated with a decreased P300 amplitude, may have more difficulty in neurocognition.
Our results suggest the possibility that MDD patients carrying the T allele genotype in rs6438552 might have more difficulties with the speed of perception and cognitive processing of auditory stimuli, possibly due to a neurodegenerative process leading to impaired interhemispheric transmission. This is in accordance with the presumed function of GSK3β in neural conduction. Overall, these findings suggest that the GSK3β SNP, rs6438552, may convey risk for MDD by disrupting neural connectivity, possibly white-matter integrity, leading to slower cognitive processing. This is speculative at present and needs to be confirmed by further neurobiological experiments.
In summary, GSK3β may be a susceptibility factor for MDD, and a significant association locus may be rs6438552; it was associated with the anxiety symptoms of MDD, and its genotypes were related to P300 latency. Further study of this association is needed to replicate this finding in ethnically different samples and in larger sample sizes.
Acknowledgments
We sincerely thank the patients, their families, and the healthy volunteers for their participation, and all the medical staff involved in specimen collecting.
This project was supported by the grants from the National Natural Science Foundation of China (No. 30770770, 30971054, and 81171290); the Science Foundation for Youths of Shanxi (No. 2011021036-1); the Science Foundation for Youths of Shanxi Medical University (No. 02201017), the National 973 Program (No.2010CB529603), the Beijing Natural Science Foundation (No.7102109), and the Fok Ying Tong Education Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington: 2000. Text Revision. [Google Scholar]
- Balaraman Y. Limaye AR. Levey AI. Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer's disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63:1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ. Heath AC. Bucholz KK, et al. Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Arch Gen Psychiatry. 1999;56:557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Bruder GE. Towey JP. Stewart JW, et al. Event-related potentials in depression: influence of task, stimulus hemifield and clinical features on P3 latency. Biol Psychiatry. 1991;30:233–246. doi: 10.1016/0006-3223(91)90108-x. [DOI] [PubMed] [Google Scholar]
- Chen ZY. Jing D. Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin PC. Majdzadeh N. D'Mello SR. Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res Mol Brain Res. 2005;137:193–201. doi: 10.1016/j.molbrainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, fMnctional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Embi N. Rylatt DB. Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Enoch MA. White KV. Waheed J. Goldman D. Neurophysiological and genetic distinctions between pure and comorbid anxiety disorders. Depress Anxiety. 2008;25:383–392. doi: 10.1002/da.20378. [DOI] [PubMed] [Google Scholar]
- Gould TD. Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- Grimes CA. Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Himani A. Tandon OP. Bhatia MS. A study of P300-event related evoked potential in the patients of major depression. Indian J Physiol Pharmacol. 1999;43:367–372. [PubMed] [Google Scholar]
- Huang EJ. Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnerkopf R. Strobel A. Gutknecht L, et al. Interaction between BDNF Val66Met and dopamine transporter gene variation influences anxiety-related traits. Neuropsychopharmacology. 2007;32:2552–2560. doi: 10.1038/sj.npp.1301383. [DOI] [PubMed] [Google Scholar]
- Jiang X. Xu K. Hoberman J, et al. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr. On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30:90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Jope RS. Bijur GN. Mood stabilizers, glycogen synthase kinase-3beta and cell survival. Mol Psychiatry. 2002;7(Suppl 1):S35–S45. doi: 10.1038/sj.mp.4001017. [DOI] [PubMed] [Google Scholar]
- Karaaslan F. Gonul AS. Oguz A, et al. P300 changes in major depressive disorders with and without psychotic features. J Affect Disord. 2003;73:283–287. doi: 10.1016/s0165-0327(01)00477-3. [DOI] [PubMed] [Google Scholar]
- Klein PS. Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JB. Hallupp M. Loy CT, et al. GSK3B polymorphisms alter transcription and splicing in Parkinson's disease. Ann Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- Lachman HM. Pedrosa E. Petruolo OA, et al. Increase in GSK3beta gene copy number variation in bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:259–265. doi: 10.1002/ajmg.b.30498. [DOI] [PubMed] [Google Scholar]
- Lang UE. Hellweg R. Kalus P, et al. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- Li X. Friedman AB. Zhu W, et al. Lithium regulates glycogen synthase kinase-3beta in human peripheral blood mononuclear cells: implication in the treatment of bipolar disorder. Biol Psychiatry. 2007;61:216–222. doi: 10.1016/j.biopsych.2006.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Zhu W. Roh MS, et al. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J. Shi Y. Zhao X, et al. No significant association between the genetic polymorphisms in the GSK-3 beta gene and schizophrenia in the Chinese population. J Psychiatr Res. 2008;42:365–370. doi: 10.1016/j.jpsychires.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Muyllaert D. Kremer A. Jaworski T, et al. Glycogen synthase kinase-3beta, or a link between amyloid and tau pathology? Genes Brain Behav. 2008;7(Suppl 1):57–66. doi: 10.1111/j.1601-183X.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF. Faux SF. McCarley RW, et al. Increased rate of P300 latency prolongation with age in schizophrenia. Electrophysiological evidence for a neurodegenerative process. Arch Gen Psychiatry. 1995;52:544–549. doi: 10.1001/archpsyc.1995.03950190026004. [DOI] [PubMed] [Google Scholar]
- Potkin SG. Turner JA. Guffanti G, et al. Genome-wide strategies for discovering genetic influences on cognition and cognitive disorders: methodological considerations. Cogn Neuropsychiatry. 2009;14:391–418. doi: 10.1080/13546800903059829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh MS. Eom TY. Zmijewska AA, et al. Hypoxia activates glycogen synthase kinase-3 in mouse brain in vivo: protection by mood stabilizers and imipramine. Biol Psychiatry. 2005;57:278–286. doi: 10.1016/j.biopsych.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Stambolic V. Ruel L. Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. Neale MC. Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tsai SJ. Liou YJ. Hong CJ, et al. Glycogen synthase kinase-3beta gene is associated with antidepressant treatment response in Chinese major depressive disorder. Pharmacogenomics J. 2008;8:384–390. doi: 10.1038/sj.tpj.6500486. [DOI] [PubMed] [Google Scholar]
- Urretavizcaya M. Moreno I. Benlloch L, et al. Auditory event-related potentials in 50 melancholic patients: increased N100, N200 and P300 latencies and diminished P300 amplitude. J Affect Disord. 2003;74:293–297. doi: 10.1016/s0165-0327(02)00016-2. [DOI] [PubMed] [Google Scholar]
- Wang WY. Barratt BJ. Clayton DG. Todd JA. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet. 2005;6:109–118. doi: 10.1038/nrg1522. [DOI] [PubMed] [Google Scholar]
- Wittchen HU. Essau CA. von Zerssen D, et al. Lifetime and six-month prevalence of mental disorders in the Munich Follow-Up Study. Eur Arch Psychiatry Clin Neurosci. 1992;241:247–258. doi: 10.1007/BF02190261. [DOI] [PubMed] [Google Scholar]
- Xu Y. Liu H. Li F, et al. A polymorphism in the microRNA-30e precursor associated with major depressive disorder risk and P300 waveform. J Affect Disord. 2010;127:332–336. doi: 10.1016/j.jad.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Yang C. Xu Y. Sun N, et al. The combined effects of the BDNF and GSK3B genes modulate the relationship between negative life events and major depressive disorder. Brain Res. 2010;1355:1–6. doi: 10.1016/j.brainres.2010.07.079. [DOI] [PubMed] [Google Scholar]
- Zhang K. Yang C. Xu Y, et al. Genetic association of the interaction between the BDNF and GSK3B genes and major depressive disorder in a Chinese population. J Neural Transm. 2010;117:393–401. doi: 10.1007/s00702-009-0360-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Hauser U. Conty C, et al. Familial risk for depression and p3b component as a possible neurocognitive vulnerability marker. Neuropsychobiology. 2007;55:14–20. doi: 10.1159/000103571. [DOI] [PubMed] [Google Scholar]