Abstract
Expansion of autologous chondrocytes in vitro is used to generate adequate populations for cell-based therapies. However, standard (SD) culture methods cause loss of chondrocyte phenotype and dedifferentiation to fibroblast-like cells. Here, we use a novel surface expansion culture system in an effort to inhibit chondrocyte dedifferentiation. A highly elastic silicone rubber culture surface was continuously stretched over a 13-day period to 600% of its initial surface area. This maintained cells at a high density while limiting contact inhibition and reducing the need for passaging. Gene expression analysis, biochemical assays, and immunofluorescence microscopy of follow-on pellet cultures were used to characterize the results of continuous expansion (CE) culture versus SD cultures on rigid polystyrene. CE culture yielded cells with a more chondrocyte-like morphology and higher RNA-level expression of the chondrogenic markers collagen type II, aggrecan, and cartilage oligomeric matrix protein. Furthermore, the expression of collagen type I RNA and α-smooth muscle actin protein were significantly reduced, indicating suppression of fibroblastic features. Pellet cultures from CE chondrocytes contained more sulphated glycosaminoglycan and collagen type II than pellets from SD culture. Additional control cultures on static (unexpanded) silicone (SS culture) indicated that benefits of CE culture were partially due to features of the culture surface itself and partially due to the reduced passaging which that surface enabled through CE. Chondrocytes grown in CE culture may, therefore, be a superior source for cell-based therapies.
Introduction
Adult articular cartilage exhibits a poor regenerative capacity after injury, and this inability to regenerate is a major factor contributing to the development of joint degenerative disease after cartilage injuries.1–4 Cell-based therapies such as autologous chondrocyte implantation (ACI) are of interest to enhance the natural regenerative capacity, replace damaged cartilage, and inhibit progression of disease. ACI is a stepwise procedure that requires isolation of chondrocytes from a healthy tissue biopsy, population expansion in vitro, and implantation in a defect.5,6 Chondrocytes should be multiplied in culture to produce cell numbers that are sufficient for clinical application. For example, assuming roughly constant cell density and tissue thickness, if cells obtained from a cartilage biopsy of surface area 25 mm2 are used to repair a defect of surface area 10 cm2, an expansion factor of at least 40 is required. This estimate is roughly consistent with the suggestions of investigators involved in the development of ACI.6–8 Therefore, in vitro expansion of chondrocyte populations is a central feature of leading cell-based strategies for cartilage repair.
During population expansion, moderate chondrocyte densities should be maintained for efficient cell growth.9 Once chondrocytes become confluent, contact inhibition can lead to a loss of phenotype and reduced proliferation.9,10 This limited desirable range of cell densities means that in a standard (SD) culture, repeated passaging and reseeding of chondrocytes is required. Passaging of chondrocytes is associated with changes in the chondrocyte phenotype in as few as two passages,11,12 or “dedifferentiation” that is characterized by a loss of rounded morphology, decreased cartilage-specific gene expression, rapid proliferation, and increased fibrotic gene expression, that is, α-smooth muscle actin (α-SMA) and collagen 1. Ultimately, dedifferentiation reduces the efficiency of cell-based repair methods, because it decreases the capacity for implanted chondrocytes to regenerate functional cartilage tissue and necessitates additional protocols for redifferentiation toward the desired phenotype. Many factors promote chondrocyte dedifferentiation, including contact with a flat, rigid, two-dimensional culture surface,12,13 exposure to degradative enzymes during passaging,14,15 and abnormally rapid (for chondrocytes) proliferation.12,16 Improved understanding and control of these factors may, therefore, lead to significant simplifications and improvements in procedures required for cell-based cartilage repair.
To minimize and circumvent conditions promoting chondrocyte dedifferentiation during population expansion, we have developed a novel culture technique that facilitates more continuous growth of cells while limiting the effects of contact inhibition and reducing the necessity for passaging.17,18 In this new method, cells are grown on a continuously expanding, elastic dish that allows for an increase in the culture surface area as the cell population grows. Relatively high cell densities are maintained that promote efficient proliferation while confluence (and the need for passaging) is delayed until relatively high cell numbers are attained. This “continuous expansion” (CE) culture technique has been previously used to expand human mesenchymal stem cell (hMSC) populations more efficiently than by standard methods while maintaining a pluripotent stem cell phenotype and inhibiting an undesired fibrotic phenotype.17,18 We hypothesized that CE culture could also be beneficial for population expansion of primary chondrocytes, where maintenance of a chondrogenic phenotype and inhibition of dedifferentiation are desired.
Materials and Methods
Chondrocyte isolation
Knee joints from freshly slaughtered skeletally mature cows were obtained from a local slaughterhouse. Articular cartilage was cut from the femoropatellar groove with a scalpel, and chondrocytes were isolated according to established methods.19 Approximately 5 g of tissue was washed in sterile phosphate buffered saline (PBS) supplemented with antibiotics and cut into 2 mm pieces using a sterile scalpel. The tissue was transferred to a 50 mL conical tube containing 30 mL of chondrocyte growth medium (high-glucose Dulbecco's modified Eagle's medium (DMEM); 0.1 mM nonessential amino acids; 10 mM HEPES; 1 mM sodium pyruvate; 10% fetal bovine serum; and 1% penicillin-streptomycin-glycine solution) supplemented with 1.5 mg/mL collagenase type II (Invitrogen/Gibco) (sterile filtered). Samples were incubated overnight to allow complete digestion of extracellular matrix. The digested mixture was passed through a 100 μm filter (BD Biosciences) and centrifuged at 200 g for 5 min. The supernatant was removed, and pelleted chondrocytes were washed with sterile PBS and centrifuged again at 200 g for 5 min. The supernatant was removed, and cells were resuspended in 10 mL of chondrocyte growth medium. The cells were counted using a hemocytometer.
Culture surface materials and modifications
Chondrocytes were cultured on several different surfaces. These included (1) high-extension silicone rubber (HESR) dishes (Cytomec GmbH), which were continuously expanded (CE culture), (2) standard polystyrene culture dishes (SD culture), and (3) polystyrene culture dishes coated with approximately 1 mm of silicone rubber (SS culture–static silicone culture) (A-221-05 LSR, Factor II). All silicone rubber culture surfaces were chemically modified to promote cell adhesion as previously described.17,20–22 Briefly, the surface was rinsed with 30% sulphuric acid for 15 min and copiously washed with deionized water followed by silanization with 1% (3-aminopropyl) triethoxysilane (Sigma-Aldrich) for 2 h at 70°C. After another wash with water, the surface was functionalized with 6% (wt/wt) glutaraldehyde and then coated with 2 mL of monomeric rat tail collagen type I (50 μg/mL; Sigma-Aldrich). A partial characterization of modified silicone surfaces has been previously established.22 Standard polystyrene culture surfaces were left unmodified.
CE, SD and SS cultures
To initiate cultures, 10,000 chondrocytes per cm2 were seeded and subcultured in chondrocyte growth medium. CE cultures were performed on HESR dishes that were expanded from 12 to 76.8 cm2 over 10 days after a 3-day initial attachment period. Highly uniform surface expansion was performed using a motorized iris-like device (Cytomec GmbH). This 13-day period was defined as one generation, which corresponded to three conventional 1:2 passages in an SD culture on polystyrene (Fig. 1). The final surface area in CE culture equaled the total surface area of the third passage in SD culture (eight wells at 9.6 cm2 each). The difference in initial surface area (12 cm2 in CE culture vs. 9 cm2 in SD culture) was due to limitations of the HESR dishes; the final surface areas were kept equal in order to provide a comparison that could be more easily interpreted in the context of population expansions for cell-based therapies. Confluence at each passage in SD culture was ∼80%, and 0.25% Trypsin-ethylenediaminetetraacetic acid (EDTA) solution (Invitrogen/Gibco) was used. Unless otherwise noted, all tissue culture reagents were obtained from Invitrogen/Gibco.
FIG. 1.
Experimental design for CE culture. (A) An equal number of primary articular chondrocytes were seeded on a HESR dish for CE culture and one well of a six-well polystyrene plate for SD culture. After a 3-day attachment period, the CE culture was slowly but continuously expanded from 12 to 76.8 cm2 over 10.5 days. During this time, cells in the SD cultures were passaged at 1:2 every 3 days. The final surface area of the CE dish was equal to eight wells of the SD cultures. (B) Surface areas of CE culture HESR dish (solid line) and SD culture on polystyrene (dotted line) versus time. (C) Schematic drawings of the iris-like device on which HESR dishes were mounted, in the closed and open positions. CE, continuous expansion; HESR, high-extension silicone rubber; SD, standard.
To control for silicone surface chemistry in experiments, to reduce unnecessary passaging, and to reduce gross density differences (Fig. 2), polystyrene culture dishes (35, 55, and 100 mm) were coated with a silicone elastomer and functionalized with rat tail collagen type I (as above). These “static silicone” (SS) cultures were, therefore, performed on silicone rubber, but without any mechanical expansion. Overall, 10,000 cells per cm2 were seeded on a 35 mm dish (96,200 cells total). Initial control experiments (Fig. 2) were always passaged onto 35 mm throughout P5. In later studies, after 3 days of initial attachment and growth, the 35 mm dish was trypsinized and passaged (P1) to the 55 mm dish (coated and functionalized in the same way). After 5 days, chondrocytes were trypsinized again, passaged (P2) to the 100 mm dish, and cultured for another 5 days. This 13-day expansion protocol was considered one generation in SS culture.
FIG. 2.
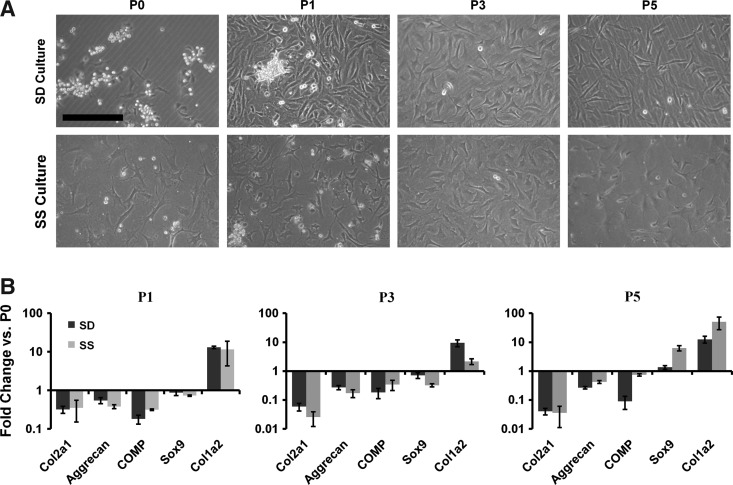
Phenotype comparison between articular chondrocytes cultured on polystyrene and a SS rubber surface. (A) Chondrocytes were cultured for five passages on SD and SS surfaces. Images were taken, and their gross morphology was compared. Scale bar: 250 μm. (B) qPCR revealed similar patterns of dedifferentiation of chondrocytes passaged on SD and SS surfaces. Dark bars represent polystyrene, and light bars represent silicone dishes. Mean±SEM (n=3). SS, static silicone; qPCR, quantitative polymerase chain reaction; SEM, standard error of the means.
At the end of each generation in CE, SD, or SS culture, cells were trypsinized (0.25% Trypsin-EDTA solution; Invitrogen), and counted. For a subsequent generation, 105 cells were then reseeded within each culture condition. 106 cells resulting from each generation were used for redifferentiation experiments, the remaining cells (1–2×106) were lysed in 1 mL of TRIzol reagent (Invitrogen), and RNA was isolated as described next.
Chondrocyte redifferentiation
At the end of each generation, ∼2×106 cells were centrifuged at 500 g for 10 min in 1.5 mL microfuge tubes to generate a pellet as previously described.22 Chondrocyte growth media were removed and replaced with 500 μL of osteo/chondrogenic differentiation medium (DMEM, 10% fetal bovine serum, 1.25 mM glutamine, 10 nM dexamethasone, 50 μM/mL ascorbic acid, 1 μM β-glycerophosphate, 5 μg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine, and 1% penicillin/streptomycin solution). This culture procedure and medium was modified from previous studies to avoid rapid cell death.17,23,24 Transforming growth factor beta (TGF-β) was intentionally excluded from this differentiation medium in order to emphasize the effects of CE versus SD culture without the concern of overwhelming growth factor stimulation. Pellets were incubated in centrifuge tubes for 6 days until they became firm, were then transferred to a six-well plate (which helped maintain viability), and incubated for an additional 6 days to observe chondrocyte outgrowth. The medium was changed every 2 days. Pellets were then fixed with 4% paraformaldehyde and prepared for cryosectioning.
Reverse transcription and quantitative real-time polymerase chain reaction
RNA was extracted from chondrocytes using TRIzol Reagent (Invitrogen). After RNA extraction, 500 ng of total RNA was subject to cDNA synthesis using the qScript cDNA synthesis kit (Quanta Biosciences). Consequently, 1 μL of each cDNA sample was loaded per reaction (in duplicate) using PerfeCTa SYBR Green FastMix (Quanta Biosciences). Standard recommended polymerase chain reaction (PCR) protocols were performed (50°C for 2 min, 94°C for 10 min, 95°C for 30 s, and 60°C for 1 min, with steps 3 and 4 repeated for 40 cycles) using the ABI 7900 HT Fast Real-Time PCR System (Applied Biosystems). The average cycle count for each target gene was normalized to GAPDH to give the average delta count (ΔCt) using RQ SDS manager software (Applied Biosystems). Then, for each target gene, the average ΔCt reading from each experimental cDNA was subtracted from the average ΔCt from the comparative GAPDH endogenous control (ΔΔCt). The average fold change in the gene expression of experimental samples compared with controls was calculated by the 2−ΔΔCt method.25 Statistical significance in the fold changes in gene expression was determined using the Student's t-test (p<0.05). PCR primers for collagen type II, aggrecan, cartilage oligomeric matrix protein (COMP), Sox9, collagen type I, and GAPDH were generated exactly as described elsewhere.26
Western blotting
Chondrocytes were lysed in lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1 mM β-glycerophosphate, supplemented with complete EDTA-free protease inhibitor cocktail). Twenty micrograms of total protein were run on a 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to nitrocellulose membranes. Membranes were blocked in 5% bovine serum albumin (BSA) for 30 min and probed with antibodies against α-SMA (1:500; Abcam), and α-tubulin (Abcam; 1:2000), followed by incubation with anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5000; Cell Signalling). Membranes were then washed thrice in tris buffered saline-tween for 10 min. Western blots were developed using Super Signal West Pico Substrate (Thermo-Fisher) and Kodak BioMax MR film (Perkin Elmer).
Immunofluorescence and histological analysis
For histological analysis, pellet cultures were fixed in 4% paraformaldehyde and embedded in a tissue freezing medium (Triangle Biomedical Sciences). Frozen sections of 10 μm thickness were cut using a Leica CM3050 S cryomicrotome. Sectioned samples were stained with Alcian Blue for proteoglycan and counterstained with Nuclear Fast Red (Sigma). For immunofluorescence, samples were blocked in permeabilization buffer for 30 min (PBS, 0.1% Triton X-100 and 1% BSA). The permeabilized samples were then incubated with antibodies against phospho-histone H3 (1:250; Sigma), cleaved caspase 3 (1:250; Sigma), and collagen type II (1:100; Abcam) overnight at 4°C. The samples were washed thrice in PBS and then incubated with either Alexa Fluor 488 Goat anti-Mouse IgG (1:250; Invitrogen) or rhodamine-conjugated Goat anti-Rabbit IgG (1:250; Sigma) for 1 h at room temperature. The samples were washed and mounted with Fluoroshield with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) and visualized on an Olympus IX81 inverted fluorescence microscope. Morphological images were captured using a Zeiss Axiovert 40C microscope equipped with a Canon Powershot A640 digital camera attached to a Zeiss MC80DX 1.0×tube adapter.
Quantitation of cell proliferation and apoptosis
Isolated chondrocytes were seeded (10,000 cells/cm2) on sterilized 22 mm square uncoated coverslips (glass) or coverslips coated with 200 μL of silicone rubber functionalized with collagen I as just described (SS culture). Cells were fixed with 4% paraformaldehyde solution, blocked for 30 min in permeabilization buffer, and probed with antibodies against phospho-histone H3, and cleaved caspase 3 and immunofluorescence was performed as just described. All images were captured using a 10×objective with MAG Biosystems Software 7.5 (Photometrics). Three random positions per slide were captured from three independent experiments. Positively stained nuclei were counted and plotted as a percent of total nuclei.
Results
Chondrocyte growth on SS surfaces
To test for effects of modified silicone culture surfaces on cell phenotype in the absence of expansion, chondrocytes were cultured over five passages on modified silicone rubber in SS culture and compared with SD culture on polystyrene. Chondrocyte attachment in both SS and SD cultures typically occurred by 3 days after seeding, at which point experiments began (day 0). Cell morphology appeared similar for the two culture conditions during passaging (Fig. 2A). RNA-level expression of the cartilage-specific genes collagen type II, aggrecan, and COMP declined with each passage number for both culture conditions, while collagen type I expression increased (Fig. 2B). Expression of the master regulator of chondrogenesis Sox9 showed no consistent changes during passaging for either culture condition.
To examine the influences on proliferation and apoptosis, chondrocytes cultured on SS-coated coverslips or uncoated glass coverslips were subjected to immunohistochemistry for phospho-histone H3 and cleaved caspase-3 (Fig. 3). A significant reduction in phospho-histone H3 (proliferating cells) was observed in SS compared with the control (Fig. 3A). However, there was no apparent difference in caspase-3 (actively apoptotic cells) between the two culture conditions (Fig. 3B). When cultured on SS, chondrocyte phenotype and dedifferentiation patterns were, therefore, similar to SD culture.
FIG. 3.
Quantification of chondrocyte proliferation and apoptosis on SS culture surfaces. Primary articular chondrocytes were cultured on glass or silicone-coated coverslips for 3 and 6 days. Fixed cells were probed with antibodies against (A) phospho-histone H3 (proliferation marker) or (B) active caspase-3 (apoptosis marker), and immunofluorescence microscopy was performed. Positively stained cells were counted and compared with total cells (4′,6-diamidino-2-phenylindole [DAPI] stained). (A) The percentage of proliferating chondrocytes was significantly less on SS surfaces for both 3- and 6-day cultures (p<0.05; Student's t-test). (B) No significant differences were detected in chondrocyte apoptosis in SS cultures. Dark bars represent chondrocytes grown on glass coverslips, and light bars represent SS coverslips. Mean±SEM (n=3).
Phenotypic analysis of CE culture
Chondrocytes in CE culture had a more rounded and a less spindle-like morphology than in SD culture, indicating a reduction in the dedifferentiation into fibroblast-like cells (Fig. 4A). At the end of generation G1, that is, completion of the first 13 day expansion protocol, real-time quantitative PCR revealed a significantly higher expression of the cartilage-specific genes collagen type II, aggrecan, and COMP in CE versus SD culture (Fig. 4C). These trends remained consistent through generations G2 and G3 (Fig. 4C), and were statistically significant for aggrecan at the end of generation G2 and for collagen type II and COMP at the end of generation G3. In contrast, the fibrotic marker collagen type I was significantly down-regulated in CE versus SD culture at the end of all three generations (Fig. 4C). Cell lysates from CE and SD cultures were subjected to SDS-PAGE and Western blot probing for the fibrotic marker α-SMA. SD culture lysates revealed a steady induction of α-SMA through the three generations, which was inhibited in the CE culture lysates for generations G1 and G2 (Fig. 5). At the end of each generation, cell counting revealed fewer total chondrocytes in CE culture compared with SD culture (Fig. 4B).
FIG. 4.
Primary articular chondrocytes were grown for 39 days in SD or CE culture. (A) At the end of each generation, chondrocyte morphology was compared in SD and CE cultures. Scale bar: 500 μm. (B) Cells were counted at the end of each generation, and doublings were calculated. (C) qPCR was performed by comparing gene expression in CE culture with SD culture. Error bars represent SEM. Statistical significance was determined by the Student's t-test within each generation, where (*) indicates p<0.05, and (#) indicates p<0.06. Twelve, six, and five independent experiments were performed for generations G1, G2, and G3, respectively.
FIG. 5.
Chondrocytes were collected and lysed at the end of each generation of SD and CE culture, and 20 μg total protein from each sample was subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis and Western blot analysis. Samples were probed for the fibrotic maker alpha smooth muscle actin (α-SMA). α-tubulin was used a loading control.
Chondrocyte redifferentiation after CE culture
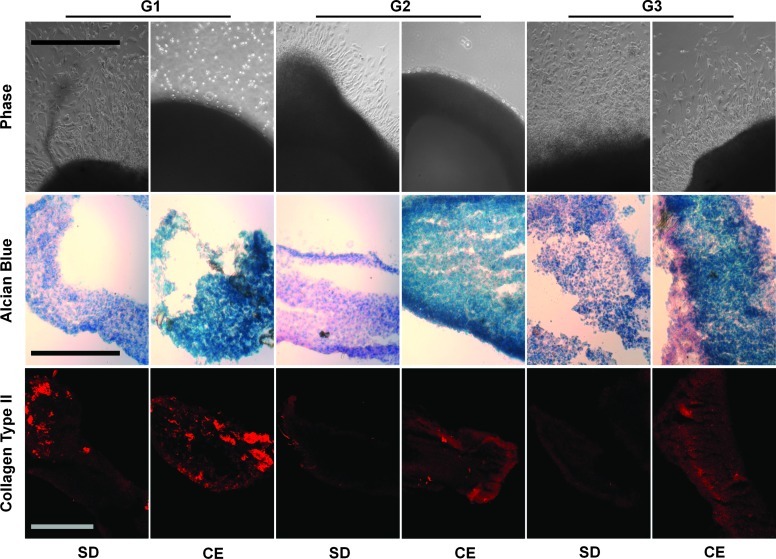
To assess the potential of cultured chondrocytes to redifferentiate into cartilage-like tissue, we subjected cells from all three generations of CE and SD cultures to three-dimension (3D) pellet cultures in an osteo-chondrogenic medium. After an initial 6 days, the pellets were moved to a six-well dish in order to assess chondrocyte outgrowth before histological analysis. The pellets derived from SD cultures strongly adhered to the culture dish during the final 6 days of the 12-day pellet culture. In contrast, the pellets derived from CE cultures only weakly adhered. Moreover, chondrocyte migration from adhered pellets was clearly evident for all three generations of SD culture but only for generation G3 of CE culture (Fig. 6). The CE pellets contained dramatically more sulphated glycosaminoglycans for all three generations compared with the SD pellets, as evident by Alcian blue staining. After generation of G1, both CE and SD pellets stained strongly for collagen type II immunofluorescence. However, after generations G2 and G3, collagen type II immunofluorescence was only detectable in CE pellet cultures. CE culture chondrocytes, therefore, exhibited greater potential for redifferentiation into cartilage-like tissue.
FIG. 6.
Chondrocytes collected at the end of each generation were subjected to redifferentiation in pellet cultures containing an osteo/chondrogenic differentiation medium. Firm pellets were transferred to six-well plates for an outgrowth assay. Upper row: Chondrocyte outgrowth from pellets. Middle row: Alcian blue histological staining on frozen sections from CE pellet cultures compared with SD pellets. Lower row: Immunofluorescence microscopy was performed on SD and CE pellet cultures while probing for collagen type II content. Scale bar: 250 μm. Color images available online at www.liebertpub.com/tea
Reduced passaging enhances chondrocyte phenotype in CE cultures
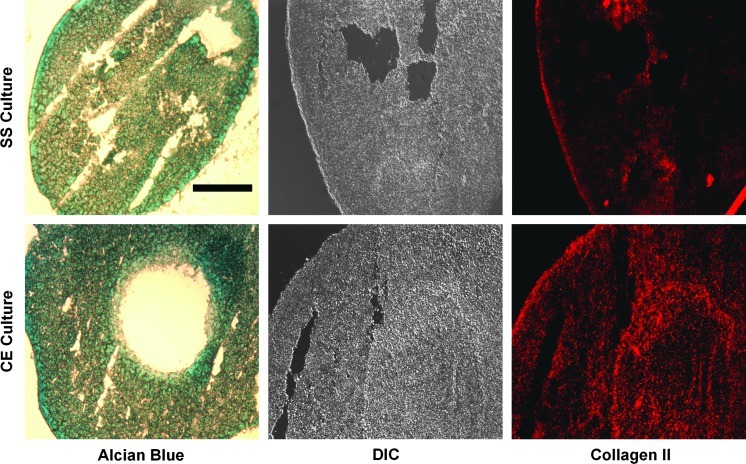
To isolate experimental variables that could influence differences between SD and CE culture, such as unnecessary passaging, cell densities, and culture surface stiffnesses, we designed an intermediate control (Fig. 7A). In this experiment, culture surfaces consisted of silicone-coated polystyrene functionalized with collagen type I on which chondrocytes were passaged to consecutively larger dishes. This SS condition allowed for a growth environment which was similar to that of CE cultured chondrocytes with regard to surface chemistry and mechanical stiffness, but was not mechanically extended. After two generations of growth, no differences in chondrocyte morphology or population doublings were observed (Fig. 7B, C). Real-time quantitative PCR revealed a significantly higher expression of collagen type II and aggrecan mRNA in CE versus SS cultures, and there was a trend for reduced collagen type I expression (Fig. 7D). It is noteworthy that in one experiment, collagen type I was below detection limits for the SS culture. There were no differences observed for COMP or Sox9 expression. CE and SS culture chondrocytes from G2 were redifferentiated in pellet cultures as just described. CE pellets appeared to contain slightly more sulphated glycosaminoglycan (GAG) than SS pellets, as evidenced by Alcian stain (Fig. 8, left panels). Collagen type II protein content was greatly enhanced in CE pellets versus SS pellets (Fig. 8, right panels).
FIG. 7.
Primary articular chondrocytes were grown for two generations in functionalized SS or CE culture. (A) Controls consist of two passages on consecutively larger functionalized silicone-coated dishes, for each generation. (B) Morphology of chondrocytes after two generations of SS or CE culture. Scale bar: 250 μm. (C) Cells were counted at the end of each generation, and population doublings were determined. (D) RNA was extracted after two generations and was converted to cDNA. qPCR was performed by comparing gene expression in CE culture with SS culture. Error bars represent SEM. Statistical significance was determined by the Student's t-test, where (*) indicates p<0.05, n=3.
FIG. 8.
Redifferentiation of SS and CE culture chondrocytes. Left panels show Alcian blue histological staining on frozen sections from CE pellet cultures compared with SS pellets. Immunofluorescence microscopy was performed on SS and CE pellet cultures probing for collagen type II content (right panels; differential interference contrast [DIC] images in middle). Scale bar: 250 μm. Color images available online at www.liebertpub.com/tea
Discussion
CE culture of primary bovine chondrocytes inhibited the dedifferentiation typically observed with SD culture techniques.11,12 Analyses of cell morphology, cartilage-specific gene expression, fibrotic gene expression, and cell numbers strongly indicated that CE culture preserves the chondrocyte phenotype during population expansion. SD culture chondrocytes exhibited a spindle-like morphology that was characteristic of a more fibroblast-like phenotype27–29 compared with the more rounded chondrocyte-like appearance evident in CE culture. Consistent with these observations, RNA-level expression of collagen type II, aggrecan, and COMP were consistently up-regulated in CE versus SD culture, while RNA-level expression of collagen type I and protein-level expression of α-SMA were down-regulated, indicative of less dedifferentiation to a fibroblast-like phenotype in CE culture. Consistent with improved preservation of the chondrocyte phenotype in CE versus SD culture, significantly fewer chondrocytes were obtained from CE culture at the end of each generation. Considering that chondrocytes do not readily proliferate within adult cartilage under normal physiological conditions while dedifferentiated chondrocytes are fibroblast-like and can rapidly proliferate,12,16 this result further supports the conclusion that CE culture helps maintain the chondrocyte phenotype. Furthermore, since three generations of CE culture achieved an overall expansion of the cell population by approximately 26,250-fold (25-fold, 35-fold, and 30-fold in generations G1, G2, and G3, respectively), it is clear that ample numbers of chondrocytes can, nevertheless, be generated in CE culture for clinical application in cell-based therapies.
As evidenced by reduced pellet adhesion to culture dishes, cell outgrowth from pellets, and expression of cartilage extracellular matrix proteins, chondrocytes from CE culture were superior to those from SD culture with regard to their ability to redifferentiate toward a mature chondrocyte phenotype. Pellets from SD culture readily attached to culture surfaces and exhibited dramatic outgrowth of chondrocytes, indicating they had transitioned to a more fibroblastic cell type.30–32 In contrast, pellets from CE culture were better able to generate neotissue with more nonadhesive, cartilage-like characteristics. Both SD and CE pellets were initially able to produce GAG and collagen type II; however, only CE pellets maintained this production throughout three generations. Taken together, these results indicate that CE culture produces cells that are markedly superior to those obtained from SD culture, with regard to their efficiency at redifferentiating into functional chondrocytes and generating de novo cartilage-like tissue.
The aim of the comparison between CE and SD culture was to contrast the CE culture technique against the current “gold standard” for cell culture: polystyrene dishes. However, the substantial technical differences between these two methods introduced several variables that could have influenced the resulting differences in the quality of expanded chondrocyte populations. Therefore, SS cultures passaged on consecutively larger dishes were employed as an intermediate control. This limited unnecessary passaging also provided a similar culture surface in terms of chemistry and mechanical stiffness. The only differences between CE and SS cultures were, therefore, the application of mechanical surface expansion in CE culture, compensated by more frequent enzymatic passaging in SS culture. In two generations, SS cultures were passaged five times compared with once in CE cultures, and CE cultures exhibited a significantly superior retention of chondrogenic phenotype versus SS cultures, and also a superior redifferentiation capacity for the production of cartilage-like neotissue. In light of data showing that passaging of chondrocytes plays a significant role in dedifferentiation,11,12,15,33 the present results indicate that the mechanical expansion aspect of CE culture and its reduction of the need for passaging specifically contribute to enhancement of the phenotype of chondrocytes cultured for cell-based therapies.
A factor by which CE culture inhibits dedifferentiation, therefore, appears to be associated with reduced passaging and limited exposure to degradative enzymes. Considering that passaging involves repeated nonspecific degradation of cell-surface proteins and receptors and the frequent need for wholesale re-establishment of cell-surface attachments, this is perhaps not surprising. The present findings, therefore, suggest that less disruptive methods for the detachment of chondrocytes from a culture surface and from each other may contribute to enhanced phenotype retention during growth, such as the case for endothelial cells for cornea tissue engineering.34 Minimization of the need for passaging also has important practical advantages for cell-based therapies. In addition to inhibition of dedifferentiation, CE methods are more automatized, thereby reducing the need for human intervention and handling of cells. These features likely decrease the risk of error and bacterial contamination over long-term cultures.
Mechanical factors contributing to chondrocyte phenotype preservation in CE culture may also include mechanical signaling. Mechanotransduction through integrin receptors and stretch-activated ion channels is involved in the maintenance of chondrogenic phenotypes,35–37 and this signaling may play a role in the pro-chondrogenic effects of CE culture. Many reports suggest that dynamic strain or compression can have positive effects on the phenotype of cultured chondrocytes,38,39 particularly regarding proteoglycan synthesis.40,41 It is important to note that these previous studies typically apply dynamic mechanical stimuli at amplitudes and frequencies which are quite different from those involved in CE culture. In contrast to situations involving oscillatory dynamic strain,38–41 CE culture chondrocytes experience very slow but very high amplitude (over 600%) stretch applied steadily and monotonically over the course of several days. Therefore, mechanotransduction stimuli in CE culture are characterized by a longer time scale than those of cell proliferation and remodeling of cell-substrate attachments. Therefore, it is possible that the nature and relative importance of mechanotransduction are different in CE culture as compared with typical applications of the oscillatory dynamic strain. Further studies conducted on the mechanotransduction pathways active during the slow, continuous strain may, therefore, yield important mechanistic insights into how CE culture preserves the chondrogenic phenotype compared with conventional passaging. In addition, opportunities remain for the optimization of CE culture by the superimposed dynamic stimulation of cells during growth.
Due to the ease with which its biochemical surface properties and geometry can be manipulated, silicone rubber is a widely used biomaterial in tissue engineering applications, including soft tissue-like compliant surfaces, expansion of stem cells, nanofilms for scaffolds, and growth of chondrocytes.17,42–47 It has been shown that the elastic properties of silicone elastomers can have important influences on cell differentiation.48,49 However, in the absence of CE, chondrocytes grown on silicone rubber dedifferentiated similarly to SD culture; the only differential effect of silicone rubber was an initial reduction in proliferation, which is consistent with previous studies.50–52 Previous studies have also indicated an initial reduction in MSC proliferation on HESR,17 which was subsequently overcome in prolonged CE culture. These findings, therefore, indicate that culture on a silicone surface does not by itself significantly alter a chondrocyte phenotype compared with the effects of SD culture. Future work may explore alternate coatings of the silicone rubber surface that may further support the preservation of a chondrocyte phenotype and the suppression of fibrotic gene expression in CE culture.
The cell signaling mechanisms involved in more advanced chondrocyte dedifferentiation downstream of increased enzyme-mediated passaging (in SD and SS cultures vs. CE cultures) remain unclear, but several candidate mechanisms have been identified. Interleukin-1 (IL-1) production increases with chondrocyte passaging,11 and IL-1 signaling is directly involved in chondrocyte dedifferentiation.53 Other studies have indicated that passaged and dedifferentiated chondrocytes display degradation of the receptor of hyaluronic acid, CD44.54 Disruption of CD44 signaling in chondrocytes results in receptor cleavage, decreased expression of chondrogenic genes, and decreased production of sulphated-GAG,55 all phenomena that are associated with dedifferentiation. IL-1 activity and CD44 expression may, therefore, be of interest for future investigations, to characterize the mechanisms by which CE culture improves the maintenance of cartilage phenotype over SD culture methods.
To enhance the redifferentiation of chondrocytes in 3D pellet cultures, the growth factor TGF-β is typically added to the chondrogenic medium.17,23,24 To gain a clear indication of the redifferentiation potential of CE culture chondrocytes, we purposely avoided the addition of TGF-β so as not to overwhelm the cells with cytokine stimulation. Without TGF-β, CE chondrocytes clearly displayed an advantage over SD chondrocytes during pellet culture, indicating a potentially cost-effective means of chondrogenic maintenance. In addition, the elimination of the need for TGF-β may also have important clinical implications that are associated with reducing the possibility of undesired growth factor delivery to patients.
The proposed culture methods (CE culture) represent a significant departure from the SD methods used for chondrocyte expansion. The implementation of these methods, therefore, requires the introduction of new techniques and technologies at the heart of a laboratory that is dedicated to chondrocyte expansion. Given the economic and therapeutic importance of the final product, these fundamental changes to decades-old methods of cell culture may be, nevertheless, warranted. The beneficial effects in terms of significant improvement of the chondrocyte phenotype indicate that disruptive technologies of this nature may provide the improvements that are necessary for routine clinical implementation of cell-based therapies for cartilage repair.
Acknowledgments
This work was supported by the Collaborative Health Research Programme (CIHR/NSERC) grant #1004005 to T.M.Q. and B.H., and Natural Sciences and Engineering Research Council (NSERC) Discovery Grant #342320-07 to T.M.Q., a Canada Research Chair in Soft Tissue Biophysics to T.M.Q., and the Canadian Institutes of Health Research grant #210820 to B.H. S.C. is a fellow of the CIHR cell signals training program and holds a Harron's fellowship. D.H.R. performed the research; D.H.R. and T.M.Q. designed the research study; D.H.R., G.K. and S.C. analyzed data; D.H.R. and T.M.Q. wrote the article; and M.M. and B.H. extensively reviewed and revised the article.
Disclosure Statement
One of the authors (T.M.Q.) is a shareholder in the company Cytomec GmbH, which makes the iris-like devices for cell culture on high extension surfaces used in this study. There are no further conflicts of interest.
References
- 1.Natoli R.M. Athanasiou K.A. Traumatic loading of articular cartilage: mechanical and biological responses and post-injury treatment. Biorheology. 2009;46:451. doi: 10.3233/BIR-2009-0554. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D.D. Chubinskaya S. Guilak F. Martin J.A. Oegema T.R. Olson S.A., et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Driscoll S.W. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795. [PubMed] [Google Scholar]
- 4.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Batty L. Dance S. Bajaj S. Cole B.J. Autologous chondrocyte implantation: an overview of technique and outcomes. ANZ J Surg. 2011;81:18. doi: 10.1111/j.1445-2197.2010.05495.x. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 7.Gillogly S.D. Myers T.H. Reinold M.M. Treatment of full-thickness chondral defects in the knee with autologous chondrocyte implantation. J Orthop Sports Phys Ther. 2006;36:751. doi: 10.2519/jospt.2006.2409. [DOI] [PubMed] [Google Scholar]
- 8.Vijayan S. Bentley G. Combined autologous chondrocyte implantation (ACI) with supra-condylar femoral varus osteotomy, following lateral growth-plate damage in an adolescent knee: 8-year follow-up. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:5. doi: 10.1186/1758-2555-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolettas E. Muir H.I. Barrett J.C. Hardingham T.E. Chondrocyte phenotype and cell survival are regulated by culture conditions and by specific cytokines through the expression of Sox-9 transcription factor. Rheumatology. 2001;40:1146. doi: 10.1093/rheumatology/40.10.1146. [DOI] [PubMed] [Google Scholar]
- 10.Yu H. Grynpas M. Kandel R.A. Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials. 1997;18:1425. doi: 10.1016/s0142-9612(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z. Fitzgerald J.B. Xu J. Willers C. Wood D. Grodzinsky A.J., et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res. 2008;26:1230. doi: 10.1002/jor.20523. [DOI] [PubMed] [Google Scholar]
- 12.Darling E.M. Athanasiou K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Archer C.W. McDowell J. Bayliss M.T. Stephens M.D. Bentley G. Phenotypic modulation in sub-populations of human articular chondrocytes in vitro. J Cell Sci. 1990;97(Pt 2):361. doi: 10.1242/jcs.97.2.361. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre V. Peeters-Joris C. Vaes G. Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim Biophys Acta. 1990;1051:266. doi: 10.1016/0167-4889(90)90132-w. [DOI] [PubMed] [Google Scholar]
- 15.Homicz M.R. Schumacher B.L. Sah R.L. Watson D. Effects of serial expansion of septal chondrocytes on tissue-engineered neocartilage composition. Otolaryngol Head Neck Surg. 2002;127:398. doi: 10.1067/mhn.2002.129730. [DOI] [PubMed] [Google Scholar]
- 16.Giovannini S. Diaz-Romero J. Aigner T. Mainil-Varlet P. Nesic D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J Cell Physiol. 2010;222:411. doi: 10.1002/jcp.21965. [DOI] [PubMed] [Google Scholar]
- 17.Majd H. Wipff P.J. Buscemi L. Bueno M. Vonwil D. Quinn T.M., et al. A novel method of dynamic culture surface expansion improves mesenchymal stem cell proliferation and phenotype. Stem Cells. 2009;27:200. doi: 10.1634/stemcells.2008-0674. [DOI] [PubMed] [Google Scholar]
- 18.Majd H. Quinn T.M. Wipff P.J. Hinz B. Dynamic expansion culture for mesenchymal stem cells. Methods Mol Biol. 2011;698:175. doi: 10.1007/978-1-60761-999-4_14. [DOI] [PubMed] [Google Scholar]
- 19.Barbero A. Martin I. Human articular chondrocytes culture. Methods Mol Med. 2007;140:237. doi: 10.1007/978-1-59745-443-8_13. [DOI] [PubMed] [Google Scholar]
- 20.Wipff P.J. Majd H. Acharya C. Buscemi L. Meister J.J. Hinz B. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials. 2009;30:1781. doi: 10.1016/j.biomaterials.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Khayat G. Rosenzweig D.H. Quinn T.M. Low frequency mechanical stimulation inhibits adipogenic differentiation of C3H10T1/2 mesenchymal stem cells. Differentiation. 2012;83:179. doi: 10.1016/j.diff.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig D.H. Solar-Cafaggi S. Quinn T.M. Functionalization of dynamic culture surfaces with a cartilage extracellular matrix extract enhances chondrocyte phenotype against dedifferentiation. Acta Biomater. 2012 doi: 10.1016/j.actbio.2012.05.032. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Bernstein P. Sticht C. Jacobi A. Liebers C. Manthey S. Stiehler M. Expression pattern differences between osteoarthritic chondrocytes and mesenchymal stem cells during chondrogenic differentiation. Osteoarthritis Cartilage. 2010;18:1596. doi: 10.1016/j.joca.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z. McCaffery J.M. Spencer R.G. Francomano C.A. Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J Anat. 2004;205:229. doi: 10.1111/j.0021-8782.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Bosnakovski D. Mizuno M. Kim G. Takagi S. Okumura M. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 27.Brodkin K.R. Garcia A.J. Levenston M.E. Chondrocyte phenotypes on different extracellular matrix monolayers. Biomaterials. 2004;25:5929. doi: 10.1016/j.biomaterials.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 28.Guidry C. Isolation and characterization of porcine Muller cells. Myofibroblastic dedifferentiation in culture. Invest Ophthalmol Vis Sci. 1996;37:740. [PubMed] [Google Scholar]
- 29.Rocker D. Hesse F. Bader A. Wagner R. Intracellular nucleotide pools and ratios as tools for monitoring dedifferentiation of primary porcine hepatocytes in culture. Cytotechnology. 2006;51:119. doi: 10.1007/s10616-006-9019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J.T. Lee E.H. Chung K.H. Kang I.C. Lee D.H. Joo C.K. Transdifferentiation of cultured bovine lens epithelial cells into myofibroblast-like cells by serum modulation. Yonsei Med J. 2004;45:380. doi: 10.3349/ymj.2004.45.3.380. [DOI] [PubMed] [Google Scholar]
- 31.Qiu W. Murray M.M. Shortkroff S. Lee C.R. Martin S.D. Spector M. Outgrowth of chondrocytes from human articular cartilage explants and expression of alpha-smooth muscle actin. Wound Repair Regen. 2000;8:383. doi: 10.1111/j.1524-475x.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 32.Singh M. Sharma A.K. Outgrowth of fibroblast cells from goat skin explants in three different culture media and the establishment of cell lines. In Vitro Cell Dev Biol Anim. 2011;47:83. doi: 10.1007/s11626-010-9373-4. [DOI] [PubMed] [Google Scholar]
- 33.Darling E.M. Athanasiou K.A. Retaining zonal chondrocyte phenotype by means of novel growth environments. Tissue Eng. 2005;11:395. doi: 10.1089/ten.2005.11.395. [DOI] [PubMed] [Google Scholar]
- 34.Nitschke M. Gramm S. Gotze T. Valtink M. Drichel J. Voit B., et al. Thermo-responsive poly(NiPAAm-co-DEGMA) substrates for gentle harvest of human corneal endothelial cell sheets. J Biomed Mater Res A. 2007;80:1003. doi: 10.1002/jbm.a.31098. [DOI] [PubMed] [Google Scholar]
- 35.Millward-Sadler S.J. Wright M.O. Davies L.W. Nuki G. Salter D.M. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000;43:2091. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 36.Lee H.S. Millward-Sadler S.J. Wright M.O. Nuki G. Salter D.M. Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J Bone Miner Res. 2000;15:1501. doi: 10.1359/jbmr.2000.15.8.1501. [DOI] [PubMed] [Google Scholar]
- 37.Perkins G.L. Derfoul A. Ast A. Hall D.J. An inhibitor of the stretch-activated cation receptor exerts a potent effect on chondrocyte phenotype. Differentiation. 2005;73:199. doi: 10.1111/j.1432-0436.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- 38.Wong M. Siegrist M. Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 39.Holmvall K. Camper L. Johansson S. Kimura J.H. Lundgren-Akerlund E. Chondrocyte and chondrosarcoma cell integrins with affinity for collagen type II and their response to mechanical stress. Exp Cell Res. 1995;221:496. doi: 10.1006/excr.1995.1401. [DOI] [PubMed] [Google Scholar]
- 40.Chai D.H. Arner E.C. Griggs D.W. Grodzinsky A.J. Alphav and beta1 integrins regulate dynamic compression-induced proteoglycan synthesis in 3D gel culture by distinct complementary pathways. Osteoarthritis Cartilage. 2010;18:249. doi: 10.1016/j.joca.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villanueva I. Weigel C.A. Bryant S.J. Cell-matrix interactions and dynamic mechanical loading influence chondrocyte gene expression and bioactivity in PEG-RGD hydrogels. Acta Biomater. 2009;5:2832. doi: 10.1016/j.actbio.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castella L.F. Buscemi L. Godbout C. Meister J.J. Hinz B. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J Cell Sci. 2010;123:1751. doi: 10.1242/jcs.066795. [DOI] [PubMed] [Google Scholar]
- 43.Mhanna R.F. Voros J. Zenobi-Wong M. Layer-by-layer films made from extracellular matrix macromolecules on silicone substrates. Biomacromolecules. 2011;12:609. doi: 10.1021/bm1012772. [DOI] [PubMed] [Google Scholar]
- 44.Wu M.H. Wang H.Y. Liu H.L. Wang S.S. Liu Y.T. Chen Y.M., et al. Development of high-throughput perfusion-based microbioreactor platform capable of providing tunable dynamic tensile loading to cells and its application for the study of bovine articular chondrocytes. Biomed Microdevices. 2011;13:789. doi: 10.1007/s10544-011-9549-z. [DOI] [PubMed] [Google Scholar]
- 45.Wu M.H. Kuo C.Y. Application of high throughput perfusion micro 3-D cell culture platform for the precise study of cellular responses to extracellular conditions—effect of serum concentrations on the physiology of articular chondrocytes. Biomed Microdevices. 2011;13:131. doi: 10.1007/s10544-010-9478-2. [DOI] [PubMed] [Google Scholar]
- 46.Belanger M.C. Marois Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: a review. J Biomed Mater Res. 2001;58:467. doi: 10.1002/jbm.1043. [DOI] [PubMed] [Google Scholar]
- 47.Ni M. Tong W.H. Choudhury D. Rahim N.A. Iliescu C. Yu H. Cell culture on MEMS platforms: a review. Int J Mol Sci. 2009;10:5411. doi: 10.3390/ijms10125411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tenney R.M. Discher D.E. Stem cells, microenvironment mechanics, and growth factor activation. Curr Opin Cell Biol. 2009;21:630. doi: 10.1016/j.ceb.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Sank A. Chalabian-Baliozian J. Ertl D. Sherman R. Nimni M. Tuan T.L. Cellular responses to silicone and polyurethane prosthetic surfaces. J Surg Res. 1993;54:12. doi: 10.1006/jsre.1993.1003. [DOI] [PubMed] [Google Scholar]
- 51.Prasad B.R. Brook M.A. Smith T. Zhao S. Chen Y. Sheardown H., et al. Controlling cellular activity by manipulating silicone surface roughness. Colloids Surf B Biointerfaces. 2010;78:237. doi: 10.1016/j.colsurfb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Lynam E.C. Xie Y. Loli B. Dargaville T.R. Leavesley D.I. George G.A., et al. The effect of amphiphilic siloxane oligomers on fibroblast and keratinocyte proliferation and apoptosis. J Biomed Mater Res A. 2010;95:620. doi: 10.1002/jbm.a.32844. [DOI] [PubMed] [Google Scholar]
- 53.Hong E.H. Yun H.S. Kim J. Um H.D. Lee K.H. Kang C.M., et al. Nicotinamide phosphoribosyltransferase is essential for IL-1{beta}-mediated dedifferentiation of articular chondrocytes via SIRT1 and ERK complex signaling. J Biol Chem. 2011;286:28619. doi: 10.1074/jbc.M111.219832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chow G. Nietfeld J.J. Knudson C.B. Knudson W. Antisense inhibition of chondrocyte CD44 expression leading to cartilage chondrolysis. Arthritis Rheum. 1998;41:1411. doi: 10.1002/1529-0131(199808)41:8<1411::AID-ART10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi N. Knudson C.B. Thankamony S. Ariyoshi W. Mellor L. Im H.J., et al. Induction of CD44 cleavage in articular chondrocytes. Arthritis Rheum. 2010;62:1338. doi: 10.1002/art.27410. [DOI] [PMC free article] [PubMed] [Google Scholar]