Abstract
The volume of tissue that can be engineered is limited by the extent to which vascularization can be stimulated within the scaffold. The ability of a scaffold to induce vascularization is highly dependent on its rate of degradation. We present a novel approach for engineering poly (ethylene glycol) diacrylate (PEGDA) hydrogels with controlled protease-mediated degradation independent of alterations in hydrogel mechanical and physical properties. Matrix metalloproteinase (MMP)-sensitive peptides containing one (SSite) or three (TriSite) proteolytic cleavage sites were engineered and conjugated to PEGDA macromers followed by photopolymerization to form PEGDA hydrogels with tethered cell adhesion ligands of YRGDS and with either single or multiple MMP-sensitive peptide domains between cross links. These hydrogels were investigated as provisional matrices for inducing neovascularization, while maintaining the structural integrity of the hydrogel network. We show that hydrogels made from SSite and TriSite peptide-containing PEGDA macromers polymerized under the same conditions do not result in alterations in hydrogel swelling, mesh size, or compressive modulus, but result in statistically different hydrogel degradation times with TriSite gels degrading in 1–3 h compared to 2–4 days in SSite gels. In both polymer types, increases in the PEGDA concentration result in decreases in hydrogel swelling and mesh size, and increases in the compressive modulus and degradation time. Furthermore, TriSite gels support vessel invasion over a 0.3–3.6 kPa range of compressive modulus, while SSite gels do not support invasion in hydrogels above compressive modulus values of 0.4 kPa. In vitro data demonstrate that TriSite gels result in enhanced vessel invasion areas by sevenfold and depth of invasion by twofold compared to SSite gels by 3 weeks. This approach allows for controlled, localized, and cell-mediated matrix remodeling and can be tailored to tissues that may require more rapid regeneration and neovascularization.
Introduction
The fundamental principle underlying the success of engineered scaffolds for the replacement and restoration of damaged and/or diseased tissues is proper vascularization to support tissue growth.1 The success of engineered tissues has been limited to thin or avascular tissues such as skin or cartilage.2–4 Engineering tissues of larger volume requires the formation of rapid and stable neovascularization (new blood vessel formation) for oxygen and nutrient transport, since cells and tissues located further than 200 hundred microns from the nearest capillaries undergo hypoxia and apoptosis.5 Therefore, the volume of tissue that can be engineered is limited by the extent to which blood vessels can be stimulated to form within the scaffold.
The successful design of biomaterial scaffolds is highly dependent on their ability to promote rapid and stable neovascularization before complete material degradation.6 Therefore, when engineering scaffolds for the replacement of tissue, the scaffold degradation rate should match the tissue regeneration rate.7 During the process of material degradation, the scaffold should maintain its structural integrity as well as provide chemical and mechanical cues to cells during the various stages of neovascularization and regeneration. Ideally, a scaffold should degrade in a manner allowing for cellular infiltration, lumen formation, and extracellular matrix (ECM) synthesis.8 For endothelial cells and supporting perivascular cells to form and stabilize vascular networks, they have to be able to adhere to, migrate within, and remodel their surrounding ECM. During this process, matrix metalloproteinases (MMPs) play a key role in mediating cell-induced proteolytic matrix degradation, remodeling, and controlled neovascularization.9,10
Synthetic polymeric hydrogels are attractive biomaterials for use in tissue engineering applications due to their inherent ease in tuning mechanical properties to match those of soft tissues. Among the classes of synthetic biomaterials, poly (ethylene glycol) diacrylate (PEGDA) hydrogels have been extensively investigated as scaffolds in tissue engineering due to their biocompatibility, hydrophilicity, resistance to nonspecific protein adsorption and cell adhesion, and ease of biochemical modification. To recapitulate the complexity of integrin-mediated cell adhesion and protease-mediated matrix remodeling during processes such as neovascularization, PEG hydrogels have been modified with immobilized cell adhesion peptides, growth factors, and MMP-sensitive peptides, which render the scaffolds susceptible to degradation and localized cell invasion by cell-secreted proteases. These hydrogels have been widely investigated for their ability to promote cell-mediated scaffold degradation and migration.11–15
Most studies focusing on designing systems with proteolytically-mediated matrix remodeling utilize MMP-sensitive peptide sequences found within the alpha chain of collagen type I (GPQG↓IWGQ).16,17 These sequences, however, do not degrade particularly fast, which may limit their ability to induce cellular infiltration and application for tissues that require faster remodeling. Recent studies have focused on enhancing the proteolytic degradation rate of PEG hydrogels by screening MMP-sensitive peptides with an increased catalytic activity; however, the effects of altered degradation rates on neovascularization within synthetic PEG hydrogels have yet to be investigated.18 Other studies have focused on the use of high molecular weight (MW)-containing PEG-peptide macromers containing multiple peptide repeats within the terminal acrylate groups to increase the concentration of proteolytically degradable cross-links within the hydrogel network upon polymerization.15 While this approach enhances hydrogel degradation rate, it may also lead to simultaneous variations in the mechanical properties of the hydrogel.
In a recent study, we have investigated the effects of increased proteolytic cleavage site incorporation within PEGDA hydrogels entrapped with acidic soluble fibroblast growth factor (FGF-1) and immobilized RGD on fibroblast invasion and have shown that this approach leads to enhanced scaffold degradation and cellular invasion.19 In our previous work, a modification of a previously published multistep reaction protocol was used to generate (PEG-peptide)n macromers through the use of heterobifunctional acrylate succinimidyl valerate active esters (i.e., acrylate-PEG-SVA and SVA-PEG-SVA).20 This multistep conjugation process, however, results in the formation of multiple products that cannot be readily separated without extensive purification making it difficult to synthesize PEGDA macromers with a controllable number of cleavage sites, and thus effectively decouple hydrogel mechanical/physical properties from degradation.
Building upon this concept, we present a more robust and controllable method for obtaining hydrogels with multiple proteolytic cleavage sites between cross links that can be synthesized using a one-step conjugation process. Here, we have designed MMP-sensitive peptide sequences with multiple proteolytic cleavage sites that can be incorporated into the backbone of PEG and allow for enhanced scaffold degradation, while maintaining the mechanical (compressive modulus) and physical (swelling ratio and mesh size) properties of the hydrogel network. Using this approach, we are able to decouple alterations in matrix stiffness from scaffold degradation and independently investigate and quantify the effects of material properties on vascularization in vitro. Our data indicate that hydrogels modified with peptides containing multiple proteolytic cleavage sites between cross links result in significant increases in vessel invasion area and depth within the scaffolds over a wider range of mechanical properties as compared to hydrogels modified with peptides with a single proteolytic cleavage site between cross-links. This approach allows for controlled, localized, and cell-mediated matrix remodeling and neovascularization within PEG-based hydrogels and can be tailored to regenerate tissues that require more rapid degradation and neovascularization.
Materials and Methods
Peptide synthesis, design, and purification
The MMP-sensitive peptide sequences containing either one or three protease-sensitive cleavage site repeats, GGL↓GPAGGK (SSite, 712.8 g/mol) and GGL↓GPAGRGL↓GPAGDGL↓GPAGGK (TriSite, 1889.1 g/mol) (cleavage sites by cell-secreted collagenase enzymes within the peptide sequence are bolded and indicated between the leucine and glycine residues by ↓) were synthesized by solid-phase peptide synthesis using a Focus Xi model (AAPPtec, Louisville, KY) with standard FMOC chemistry. The aspartic acid and arginine amino acids were added to balance the overall hydrophilicity of the peptides. The resulting SSite and TriSite peptide sequences were designed to yield comparable properties with a net charge=1, isolelectric point=1, and average hydrophilicity=0.1. Amino acids were coupled with a N,N-diisopropylethylamine (DIEA) and O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate (HBTU) mixture. The FMOC group was deprotected with 20% piperidine in N,N-dimethylformamide (DMF). Peptides were cleaved from the resin and deprotected with trifluoroacetic acid (TFA)/triisopropylsilane/ddH2O (95:2.5:2.5). Peptides were precipitated in cold diethyl ether and desiccated overnight. The dried products were dissolved in ddH2O and lyophilized. Peptide MWs were confirmed by MALDI-TOF mass spectrometry and were purified by reverse-phase high-performance liquid chromatography to obtain purities of >95%. The final products were lyophilized and stored at −80°C. All amino acids, HBTU, DMF, and TFA were purchased from AAPPtec. DIEA, triisopropylsilane, and diethyl ether were purchased from Fisher Scientific (Hanover Park, IL). Piperidine was purchased from Sigma (St. Louis, MO).
Synthesis of cross linkable PEGDA-peptide macromers
PEGDA hydrogels were rendered degradable by the covalent incorporation of engineered peptides containing a single MMP-sensitive cleavage site repeat (SSite gels) or three MMP-sensitive cleavage site repeats (TriSite gels) as described above. Peptides were conjugated to acrylate-PEG-SVA (MW 8000 Da; Laysan Bio, Arab, AL) in 50 mM NaHCO3 (pH 8.0) in a 2:1 PEG:peptide molar ratio. Peptides were dissolved in 10 mL of 50 mM NaHCO3 buffer. Acrylate-PEG-SVA was dissolved in 14 mL of 50 mM NaHCO3 buffer, added drop-wise to the peptides, and allowed to stir for 4 h protected from light. The final products were dialyzed (10,000 Da MW cut-off) in 2 L ddH2O for 24 h (with water changes after 6 and 12 h). The resulting macromers, acrylate-PEG-peptide-PEG-acrylate (peptide=SSite or TriSite) were of similar MW (SSite=16,712.8 Da, TriSite=17,889.1 Da), but with different susceptibility to degradation due to the difference in the number of cleavage repeats within the terminal acrylate groups. Degradable PEGDA macromers were lyophilized and stored frozen at −20°C until use. Similarly, the cell adhesion ligand YRGDS was conjugated to PEG by reacting YRGDS with acrylate-PEG-SVA (MW 3400 Da) in a 1:1 PEG:peptide molar ratio in 50 mM NaHCO3 (pH 8.0). The solution was allowed to react for 4 h protected from light. The final product acrylate-PEG-YRGDS was dialyzed (2000 MW cut-off), lyophilized, and stored frozen at −80°C until use. MW determination of PEGDA-peptide macromers was confirmed with gel permeation chromatography performed by PolyAnalytik, Inc. (London, ON).
Hydrogel formation
Hydrogel precursor solutions were prepared in 1× phosphate-buffered saline (PBS) (pH 7.4) with 37 mM N-vinylpyrrolidone, 225 mM triethanolamine, and 0.05 mM of the initiator, eosin Y with final PEGDA concentrations of 3, 4, and 5% (weight/volume [w/vol]) and 15 mg/mL acrylate-PEG-YRGDS. Precursor solutions (100 μL/hydrogel) were photopolymerized by exposure to visible light (λ=514 nm) for 0.25 min using an Argon Ion Laser (Coherent, Inc., Santa Clara, CA) at a laser flux of 100 mW/cm2. All chemicals were purchased from Sigma.
Evaluation of hydrogel swelling and mesh size
Hydrogel swelling was calculated by measuring the ratio of the mass of the swollen gel (Ms) 24 h postphotopolymerization to the mass of the dried gel (MD). The mass of the swollen hydrogel was measured by removing excess fluid from the hydrogel surface. The mass of the dried hydrogel was measured after drying the hydrogels in a vacuum oven for 3 days at 50°C. The hydrogel swelling ratio (Q) was calculated as:
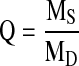 |
The mesh size (ξ) of the swollen hydrogel network was calculated based on the rubber elasticity theory as previously published21:
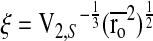 |
where V2,S is the polymer volume fraction of the hydrogel in the swollen state, which is also equal to the reciprocal of the swelling ratio:
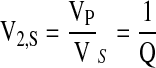 |
In the above equation, VP and VS represent the hydrogel volume in the dried and swollen state, respectively. The root mean square unperturbed end to end distance of the polymer chain between cross links 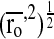 is given by:
is given by:
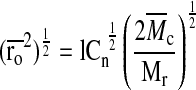 |
where l is the average bond length between the C–C and C–O bonds in the PEG repeat unit (l=1.46 Å), Cn is the characteristic ratio of PEG (Cn=4) and Mr is the MW of the PEG repeat unit (Mr=44 g/mol). The average MW between cross links, 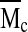 , in the hydrogel network is calculated according to22:
, in the hydrogel network is calculated according to22:
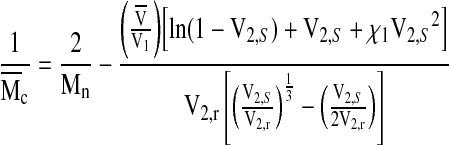 |
where 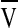 is the specific volume of the polymer,
is the specific volume of the polymer, 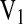 is the molar volume of the solvent, and
is the molar volume of the solvent, and 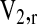 is the polymer volume fraction in the relaxed state immediately after polymerization before equilibrium swelling.
is the polymer volume fraction in the relaxed state immediately after polymerization before equilibrium swelling.
Evaluation of hydrogel mechanical properties
Compression experiments were conducted using a TA RSA3 mechanical tester (TA Instruments, New Castle, DE) controlled by TA Orchestrator software. PEGDA hydrogels were allowed to reach equilibrium swelling for 24 h before mechanical testing. Hydrogels were compressed at a constant strain rate of 0.5 mm/min23 using a 10 N load cell. The compressive modulus was calculated from the slope of the linear region of the stress versus strain curve at less than 10% strain (r2>98%), as previously reported.24,25
Quantification of hydrogel degradation kinetics
The degradation profiles of MMP-sensitive PEGDA hydrogels were obtained by incubation with collagenase enzyme solution from Clostridium hystolyticum (Sigma). Each hydrogel after equilibrium swelling was incubated at 37°C with 1 mL of 10 μg/mL collagenase in PBS with 1 mM CaCl2 with enzyme changes every 24 h. The change in wet weight of the hydrogels was measured over time.
Cell maintenance
Human umbilical vein endothelial cells (HUVECs) were maintained in endothelial basal media supplemented with 2% fetal bovine serum, bovine brain extract, epidermal growth factor (EGF), hydrocortisone, ascorbic acid, and gentamicin. Human umbilical artery smooth muscle cells (HUASMCs) were maintained in smooth muscle basal media supplemented with 5% fetal bovine serum, insulin, FGF, EGF, and gentamicin. Cells were cultured in an incubator at 37°C and 5% CO2. Both HUVECs and HUASMCs used in the experiments were between passages 5 and 10. Both cell lines and cell culture reagents were purchased from Lonza (Walkersville, MD).
Neovascularization model in vitro
A previously published in vitro neovascularization assay consisting of a co-culture of HUVECs and HUASMCs was used to assess neovascularization within PEGDA hydrogels.26–29 Co-culture cell spheroids were prepared with 2500 HUVECs and 2500 HUASMCs in endothelial basal media supplemented with 2% fetal bovine serum, bovine brain extract, EGF, hydrocortisone, ascorbic acid, and gentamicin containing 0.24% (w/v) carboxymethylcellulose (Sigma). Spheroids were seeded into a nonadherent 96-well round bottom plate at 37°C and 5% CO2 for 24 h. The resulting spheroids were encapsulated in the hydrogels by placing the spheroid in the prepolymer solution and polymerizing into the hydrogel network. Spheroids were imaged at week 0 (24 h postencapsulation), 1, 2, and 3 with an Axiovert 200 inverted microscope using a 5× objective (1.255 μm/pixel) (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Vessel invasion into the surrounding hydrogels was monitored by tracing the projected area of the spheroid using AxioVision 4.5 Image Analysis software. At weeks 0, 1, 2, and 3, hydrogel samples were fixed with 4% paraformaldehyde, stained for F-actin with Alexa Fluor 546-phalloidin (25 U/mL) (Invitrogen, Eugene, OR), and analyzed with confocal microscopy. Depth of invasion into hydrogels after 3 weeks was quantified from Phalloidin- stained spheroids that were imaged using a PASCAL laser scanning microscope system from Carl Zeiss MicroImaging, Inc. with a 5× objective (3.571 μm/pixel). Images were taken as a series of z-sections at 10 μm intervals. Serial images were imported into Axiovision 4.5 (Carl Zeiss) for 3D volume rendering.
Statistical analysis
All data are presented as mean±standard deviation. To determine statistical significance between groups, analysis of variance was performed followed by the Tukey's HSD test for multiple comparisons (SigmaStat 3.5). In all cases, probability (p) values<0.05 were considered statistically significant.
Results
Physical and mechanical characterization of MMP-sensitive PEGDA hydrogels
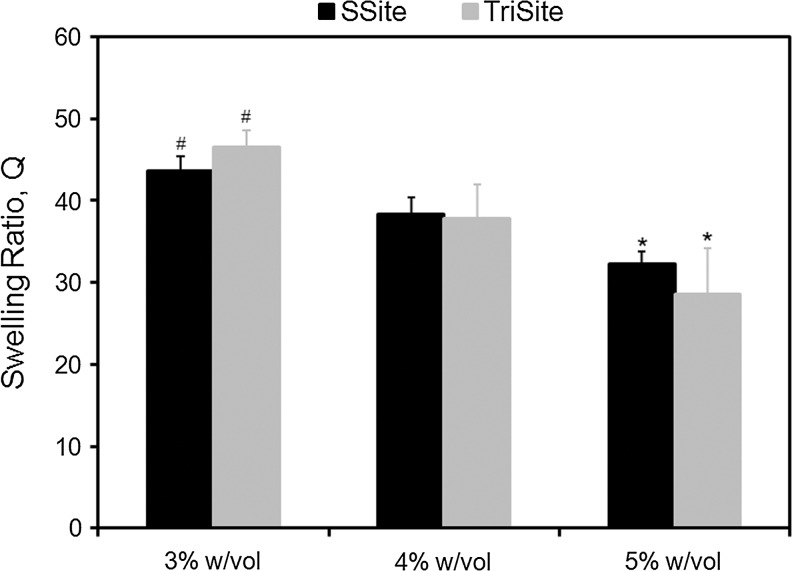
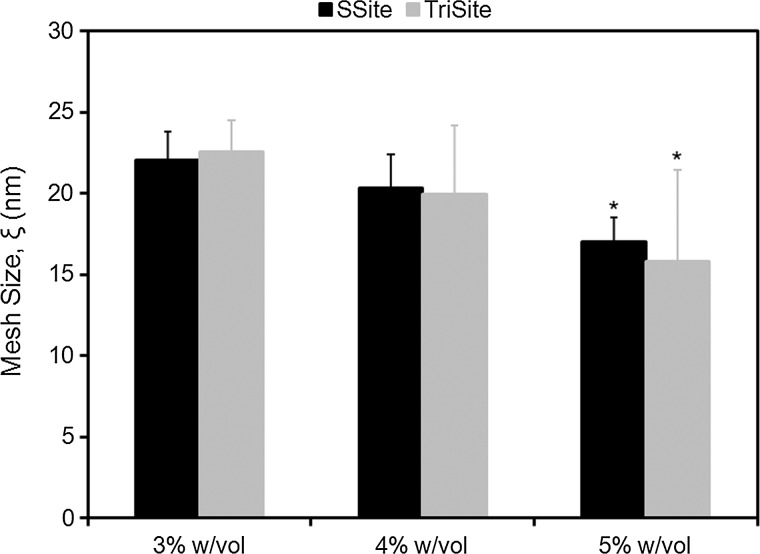
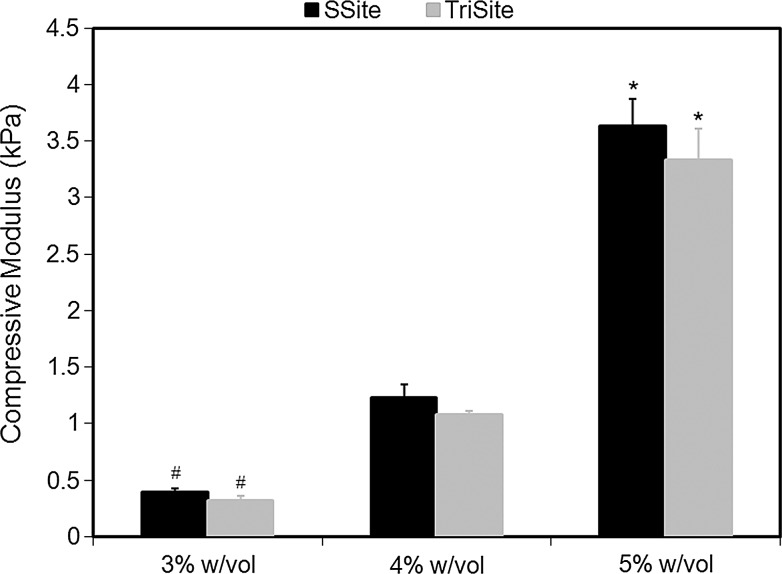
MMP-sensitive peptides cleaved by cell-secreted collagenases were engineered to contain one (GGL↓GPAGGK) (SSite) or three (GGL↓GPAGDGL↓GPAGRGL↓GPAGGK) (TriSite) protease-sensitive cleavage site repeats. Peptides were reacted with acrylate-PEG-SVA (MW=8000 Da) in a single conjugation step to form degradable SSite and TriSite PEGDA macromers, which upon photopolymerization result in a cross linked hydrogel network. To demonstrate that the degradation rate can be tuned independent of variations in mechanical and physical properties of the biomaterials, hydrogels were first characterized by swelling and compression experiments. Comparisons between SSite and TriSite hydrogels showed that these hydrogels exhibited similar swelling ratios (Fig. 1), mesh sizes (Fig. 2), and compressive moduli (Fig. 3) at all PEGDA concentrations investigated when polymerized under the same conditions. Alterations in hydrogel physical and mechanical properties were achieved by changes in the PEGDA concentration. An increase in the PEGDA concentration resulted in a statistically significant decrease in the swelling ratio (Fig. 1) and mesh size (Fig. 2), and a statistically significant increase in the compressive modulus (Fig. 3). Swelling ratios for hydrogels with PEGDA concentrations of 3%, 4%, and 5% w/vol for SSite gels were 43.67±1.46, 38.39±2.02, and 32.29±1.72 and similarly for TriSite gels were 46.60±5.66, 37.77±4.21, and 28.56±1.96 (Fig. 1). The mesh sizes of 3%, 4%, and 5% w/vol hydrogels for SSite gels were 22.07±1.99 nm, 20.37±0.97 nm, and 17.05±0.99 nm and for TriSite gels were 22.57±1.47 nm, 19.97±1.15 nm, and 15.80±1.43 nm (Fig. 2). Compression measurements showed that an increase in the polymer concentration from 3%, 4% to 5% increased the compressive modulus in SSite gels from 0.38±0.04 kPa, 1.23±0.12 kPa to 3.63±0.24 kPa and in TriSite gels from 0.31±0.05 kPa, 1.08±0.03 kPa to 3.33±0.28 kPa (Fig. 3).
FIG. 1.
Swelling ratio as a function of PEGDA concentration in SSite and TriSite hydrogels. *Indicates statistical significance from the 3% and 4% w/vol groups. #Indicates statistical significance from the 4% w/vol group (p<0.05) (n=4). PEGDA, polylethylene glycol diacrylate; w/vol, weight/volume.
FIG. 2.
Mesh size as a function of PEGDA concentration within SSite and TriSite hydrogels. *Indicates statistical significance from the 3% and 4% w/vol groups (p<0.05) (n=4).
FIG. 3.
Compressive modulus as a function of PEGDA concentration within SSite and TriSite hydrogels. *Indicates statistical significance from the 3% and 4% w/vol groups. #Indicates statistical significance from the 4% w/vol group (p<0.05) (n=4).
Quantification of protease-mediated hydrogel degradation by collagenase enzyme
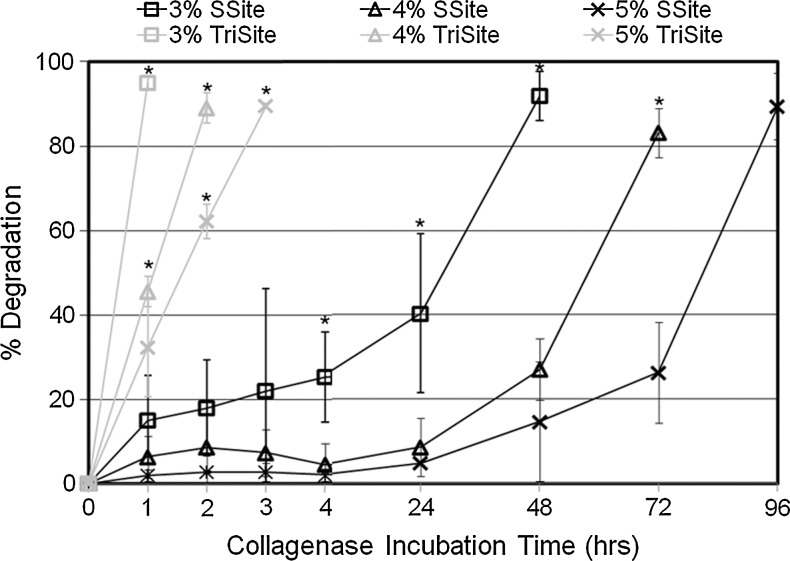
Protease-mediated degradation of MMP-sensitive PEGDA hydrogels was monitored by measuring the change in the wet weight of the hydrogels incubated with collagenase enzyme over time. It was anticipated that TriSite hydrogels compared to SSite hydrogels with increases in the number of protease-sensitive cleavage sites in the PEG backbone would lead to faster degradation times. Hydrogel degradation was therefore controlled by alterations in the number of protease-sensitive cleavage sites within the engineered peptide and through variations in the degradable PEGDA concentration. In both SSite and TriSite hydrogels, an increase in the PEGDA concentration resulted in a statistically significant increase in hydrogel degradation time (Fig. 4). TriSite hydrogels compared to SSite gels, however, exhibited statistically significant decreases in hydrogel degradation times at all PEGDA concentrations investigated. Hydrogels with PEGDA concentrations of 3%, 4%, and 5% w/vol in SSite gels underwent complete degradation within 48, 72, and 96 h, while TriSite gels of the same PEGDA concentration underwent complete hydrogel degradation in 1, 2, and 3 h, respectively.
FIG. 4.
Degradation profiles of SSite and TriSite hydrogels with varying PEGDA concentration. Hydrogels were incubated in collagenase enzyme and the change in the wet weight was monitored over time. *Indicates statistical significance among all groups (p<0.05) (n=4).
Neovascularization within MMP-sensitive PEGDA hydrogels
Neovascularization within MMP-sensitive PEGDA hydrogels was evaluated using a co-culture model of HUVECs and HUASMCs. Hydrogels were formed from macromers containing peptides with one (SSite) or three (TriSite) MMP-sensitive cleavage site repeats between cross links, copolymerized with 15 mg/mL acrylate-PEG3400-YRGDS and investigated as matrices for inducing controlled neovascularization. Co-culture spheroids were encapsulated in the MMP-sensitive PEGDA hydrogels and cultured over a time course of 3 weeks. Vessel invasion was independently investigated as a function of hydrogel degradation time based on the number of MMP-sensitive cleavage sites in the PEGDA macromer and the PEGDA concentration.
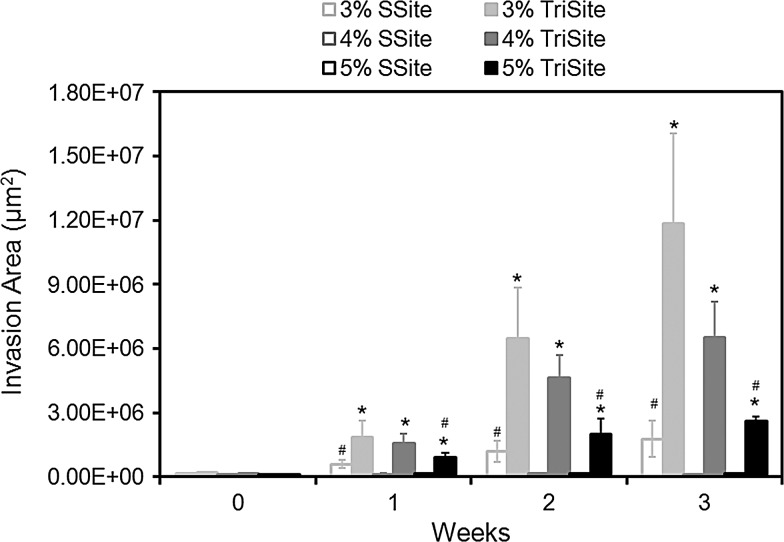
Quantitative analysis of the vessel invasion areas in SSite and TriSite hydrogels made from PEGDA concentrations of 3%, 4%, and 5% w/vol at weeks 0, 1, 2, and 3 is shown in Figure 5. In SSite gels, vessel invasion was only supported at the lowest PEGDA concentration investigated, 3% w/vol, while no invasion resulted in the 4% and 5% w/vol PEGDA concentrations, after a 3-week culture period. In contrast, TriSite gels supported vessel invasion at all PEGDA concentrations investigated with statistically significant increases in invasion compared to SSite gels at weeks 0, 1, 2, and 3. At weeks 1, 2, and 3, TriSite gels had significantly greater invasion areas compared to SSite gels at all three PEGDA concentrations investigated.
FIG. 5.
Time course of vessel invasion areas in SSite and TriSite hydrogels with varying PEGDA concentration. *Indicates statistical significance between SSite and TriSite hydrogels among all PEGDA concentrations. #Indicates statistical significance within a hydrogel group (SSite or TriSite) among all PEGDA concentrations (p<0.05) (n=4–8).
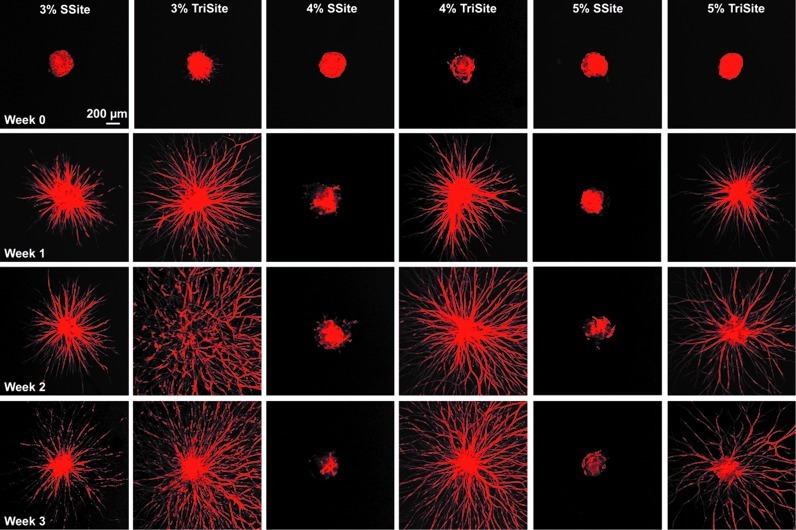
To better visualize the formation of vessel structures within the hydrogels, spheroids within SSite and TriSite hydrogels were stained for F-actin over the 3-week time course (Fig. 6). By week 1, spheroids within the hydrogels formed into extensive vascular structures in gels polymerized with the 3% w/vol PEGDA concentration within the SSite group and at all PEGDA concentrations within the TriSite hydrogel group, which at weeks 2 and 3 formed more dense networks. At weeks 1, 2, and 3, TriSite gels showed more invasion and formation of dense vascular structures as compared to SSite gels formed with the 3% w/vol PEGDA concentration. In addition, vascular sprouts in TriSite gels formed with increasing PEGDA concentration appear to decrease in vascular density, while SSite hydrogels do not support vascular sprout formation in gels cross linked with higher PEGDA concentrations (4% and 5% w/vol) after 3 weeks of culture.
FIG. 6.
Time course of vessel invasion within SSite and TriSite hydrogels made with 3%, 4%, and 5% w/vol PEGDA concentrations at weeks 0, 1, 2, and 3. Spheroids were stained for F-actin and imaged at 5× magnification using confocal microscopy. Color images available online at www.liebertpub.com/tea
Investigation of the effect of the PEGDA concentration on vessel invasion showed that SSite gels only supported invasion at the 3% w/vol PEGDA concentration, while no invasion occurred at the 4% and 5% w/vol conditions over a time course of 3 weeks (Figs. 5 and 6). TriSite gels, however, supported invasion at all three PEGDA concentrations investigated. In general, vessel invasion increased with decreasing PEGDA concentration. At weeks 1, 2, and 3, hydrogels with the 3% and 4% w/vol PEGDA concentration had significantly greater invasion areas compared to the 5% w/vol gels, while there were no statistical differences between the 3% and 4% w/vol PEGDA concentrations.
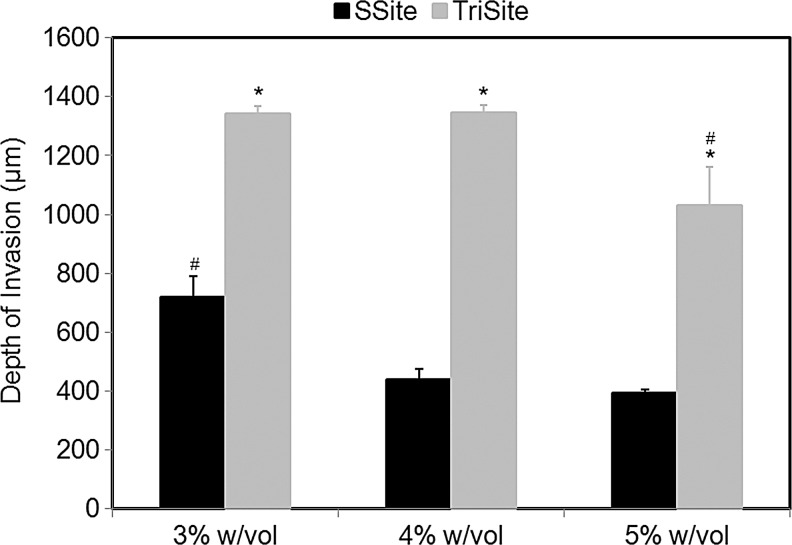
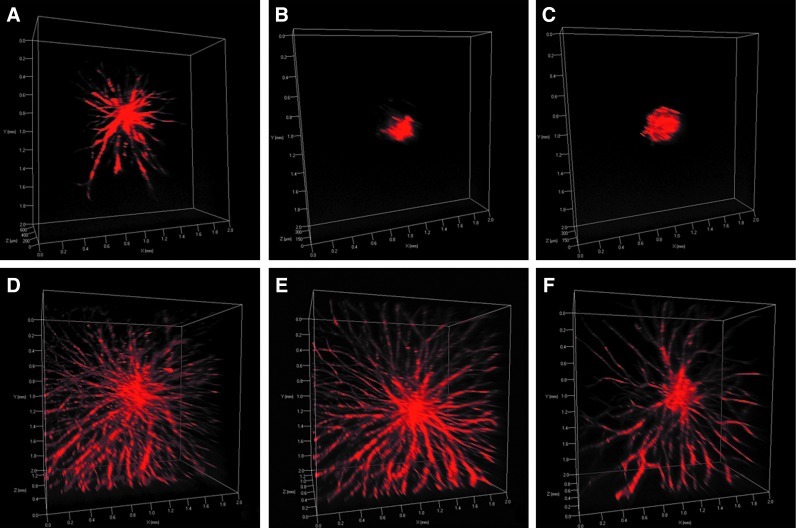
We also investigated the effects of depth of vessel invasion within SSite and TriSite gels. At week 3, all hydrogel samples were fixed and stained for F-actin, and analyzed using confocal microscopy. Figure 7 compares differences in the depth of invasion within SSite and TriSite gels at the various PEGDA concentrations investigated. Consistent with the results of vessel invasion areas, TriSite gels supported a significantly greater depth of invasion as compared to SSite gels at all PEGDA concentrations investigated. Within the TriSite gel group, hydrogels with a PEGDA concentration of 3% and 4% w/vol had significantly greater depth of invasion compared to the 5% w/vol PEGDA concentration. There were no statistical differences noted in the depth of invasion between the 3% and 4% w/vol PEGDA concentrations within the TriSite hydrogel group. These differences can also be seen in the 3D volume rendering of spheroids within the different hydrogel types (Fig. 8).
FIG. 7.
Depth of invasion at week 3 in SSite and TriSite hydrogels with PEGDA concentrations of 3%, 4%, and 5% w/vol. *Indicates statistical significance between SSite and TriSite gels among all PEGDA concentrations. #Indicates statistical significance among all PEGDA concentrations within a hydrogel group (SSite or TriSite) (p<0.05) (n=4).
FIG. 8.
3D volume rendering of vessel invasion at week 3 in SSite (A–C) and TriSite (D–F) hydrogels with PEGDA concentrations of 3% (A, D), 4% (B, E), and 5% (C, F) w/vol. Spheroids were stained for F-actin and imaged at 5× magnification using confocal microscopy. Color images available online at www.liebertpub.com/tea
Discussion
Vascularization of engineered scaffolds still remains a significant challenge in tissue engineering. Designing an ideal scaffold for tissue replacement requires that vascularization occurs before complete material degradation. Therefore, the ability to control scaffold degradation is critical for the vascularization of engineered tissues. PEG hydrogels have received significant attention as scaffolding materials because they can be easily modified to mimic key elements of the ECM. The incorporation of protease-sensitive substrates into the backbone of PEG render these hydrogels degradable by cell-secreted proteases and allow for localized degradation, while maintaining overall structural integrity. However, the degradation rates of these biomaterials may be too slow limiting in vivo cell invasion, vascularization, and neotissue formation. Therefore, approaches to enhance and control degradation are necessary to induce rapid vascularization. In this study, we present a novel approach in engineering protease-sensitive peptides containing increased numbers of cleavage sites that allow for controlled study of hydrogel degradation, while maintaining the physical and mechanical properties of the hydrogel network. The engineered peptides were cross linked into the backbone of PEGDA hydrogels and evaluated for their ability to induce vascularization.
We first characterized the physical and mechanical properties of hydrogels containing peptides with one (SSite) or three (TriSite) protease-sensitive cleavage sites. Hydrogels polymerized under the same conditions, but cross linked with either SSite or TriSite peptide-containing PEGDA macromers did not result in alterations in swelling (Fig. 1), mesh size (Fig. 2), or compressive modulus (Fig. 3) at all conditions investigated. Furthermore, tunable hydrogel properties were achieved by varying the PEGDA concentration. Increases in the PEGDA concentration resulted in decreases in hydrogel swelling, mesh size, and increases in the compressive modulus. The resultant range of hydrogel properties obtained included swelling ratios between 29 and 47, mesh sizes between 16 and 23 nm, and compressive moduli between 0.3 and 3.6 kPa, which agree with previously published studies.21,30
The incorporation of peptides with increased numbers of protease-sensitive cleavage sites between cross links increased proteolytically mediated gel degradation independent of alterations in the physical (swelling ratio and mesh size) and mechanical (compressive modulus) properties of the hydrogels. We characterized the degradation profiles of hydrogels made from SSite and TriSite peptides by incubation with collagenase enzyme over time. Hydrogels made with peptides containing three protease-sensitive cleavage sites resulted in significantly faster degradation times compared to hydrogels with peptides containing one protease-sensitive cleavage site. By increasing the number of protease-sensitive cleavage sites from one to three within the peptide, the resultant hydrogel degradation times decreased from 2–4 days to 1–3 h (Fig. 4). We also demonstrate that hydrogel degradation can be tuned through alterations in the PEGDA concentration with increases in concentration resulting in increased degradation time, which correspond to increases in the compressive modulus and decreases in the mesh size and swelling ratio.
In a recently published study,19 we used a different approach to synthesize peptide-containing PEGDA macromers with multiple proteolytic cleavage sites. These macromers were synthesized using a multistep conjugation process with combinations of acrylate-PEG-SVA and SVA-PEG-SVA to generate (PEG-peptide)3 diacrylate macromers. We showed that hydrogels made from macromers with multiple proteolytic cleavage sites result in enhanced degradation and fibroblast invasion. This multistep conjugation approach, however, results in the generation of multiple reaction products that may directly affect scaffold properties. Therefore, without extensive purification to obtain the macromer of interest, this approach makes it difficult to obtain PEGDA macromers with controllable proteolytic cleavage site presentation.
In the present study, we use an alternative methodology for generating PEGDA macromers with multiple proteolytic cleavage sites that can be conjugated to PEGDA using a one-step process. By synthesizing and designing peptide sequences with multiple proteolytic cleavage sites that are subsequently reacted with acrylate-PEG-SVA, we are able to form PEGDA cross linking macromers with a controllable number of proteolytically degradable cleavage sites between the terminal acrylate groups. The presented approach results in more robust differences in degradation and cellular invasion in vitro as compared to the previously published approach.19 For example, hydrogel degradation times between SSite and TriSite hydrogels using our previously published method were 3.5 versus 3 h, respectively, while those obtained in the present study yielded degradation times of 48 h for SSite versus 2 h for TriSite hydrogels with a 10-fold reduction in enzyme concentration levels.
Other approaches targeted toward enhancing degradation of PEG-based hydrogels have looked at creating high MW PEG-peptide macromers using step-growth conjugation mechanisms.15 These hydrogels support sprout formation from embedded embryonic chick aortic arches; however, they result in simultaneous increases in swelling and degradation and do not investigate the role of matrix properties on inducing vascularization. In another study, PEG hydrogels formed by Michael-type addition reactions were engineered with peptides that possess an increased catalytic activity and substrate cleavage by MMP-1 and MMP-2 enzymes, which lead to enhanced degradation, cell spreading, and proliferation in fibroblasts and sprout formation from implanted aortic ring segments.18 Matrix properties, however, were not characterized and correlated with degradation times.
In the present study, the engineered hydrogels were further evaluated for their ability to induce neovascularization in vitro using a 3D cell culture model of sprouting angiogenesis. Since the interaction between endothelial cells and smooth muscle cells (SMC) is important during the remodeling and stabilization stage of angiogenesis, a co-culture of HUVECs and HUASMCs was used. The angiogenesis model recapitulates many of the steps that occur during neovascularization in vivo, including vessel sprouting and the formation of a branched vascular network.31 We show that hydrogels made from TriSite peptides in the presence of acrylate-PEG-YRGDS resulted in significant increases in invasion compared to hydrogels made from SSite peptides with sevenfold increases in invasion areas and twofold increases in depth of invasion into the hydrogels by week 3 (Figs. 5 and 7). We also investigated the range of hydrogel properties that support vessel invasion by tuning the PEGDA concentration. We show that hydrogels made from SSite peptides with compressive moduli greater than 0.4 kPa act as a barrier to cells, inhibiting invasion, and vascularization within hydrogels, while hydrogels made from TriSite peptides support vessel invasion under all conditions investigated and over a wider range of compressive moduli (0.3–3.6 kPa). This suggests that TriSite hydrogels can be used to stimulate neovascularization in a wider range of tissues.32
Recent studies using MMP-sensitive PEG hydrogels have explored these systems for their vascular inductive capabilities. Common cross-linking reaction techniques to render the hydrogels include Michael-type addition33 and cross linking by free-radical polymerization.23 These hydrogel systems have been functionalized with RGD as the cell adhesion ligand and tethered with growth factors (such as vascular endothelial growth factor)14,23,34 or growth factor combinations35 and have been shown to promote vascularization both in vitro and in vivo. In these studies, however, the role of matrix properties on vascularization is either not explored or is controlled through simultaneous alterations in hydrogel degradation and mechanical properties. Furthermore, PEG hydrogel systems have not been widely investigated for their ability to promote vascularization in the absence of growth factors. In this study, we demonstrate that in vitro vascularization can be induced and controlled via alterations in matrix properties without the incorporation of growth factors. In addition, through the inclusion of peptides that contain multiple protease-sensitive cleavage sites in the cross linked network, we show that vascularization can be induced in PEGDA hydrogels over a wide range of mechanical properties, showing promise of the presented approach in targeting vascularization in stiffer tissues.
Conclusion
In this study, we present a novel approach in engineering protease-sensitive peptides with multiple proteolytic cleavage sites that can be covalently incorporated in the backbone of PEG without compromising the hydrogel physical and mechanical properties. We show that hydrogels formed with the engineered peptides lead to enhanced degradation and vascularization. In addition, hydrogels with enhanced susceptibility to degradation promote vascularization over a wider range of matrix properties. The presented approach allows for systematic tuning of hydrogel degradation as well as the physical and mechanical properties of PEG hydrogels, leading to enhanced localized and cell-mediated degradation and neovascularization, and can be tailored toward the design of tissues that require rapid vascularization and regeneration.
Acknowledgments
This research was supported by Award Number R21HL094916 from the National Heart, Lung, and Blood Institute and the Illinois Institute of Technology Educational Research Initiative Fund. The authors would like to thank Professor David Venerus and Jef Larson for their assistance with the compression studies and Michael Turturro for his assistance with peptide synthesis and purification. For peptide characterization and purification, proteomics and informatics services were provided by the CBC-UIC Research Resources Center Mass spectrometry, Metabolomics and Proteomics Facility, which was established in part by a grant from The Searle Funds at the Chicago Community Trust to the Chicago Biomedical Consortium.
Disclosure Statement
No competing financial interests exist.
References
- 1.Patel Z.S. Mikos A.G. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J Biomater Sci Polym Ed. 2004;15:701. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 2.Temenoff J.S. Mikos A.G. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21:431. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 3.Atala A. Bauer S.B. Soker S. Yoo J.J. Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 4.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 5.Jain R.K. Munn L.L. Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer. 2002;2:266. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 6.Papavasiliou G. Cheng M.H. Brey E.M. Strategies for vascularization of polymer scaffolds. J Investig Med. 2010;58:838. doi: 10.231/JIM.0b013e3181f18e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanjaya-Putra D. Wanjare M. Gerecht S. Biomaterials for vascular engineering applications: a review of the past and future trends. In: Burdick J.A., editor; Mauck R.L., editor. Biomaterials Approaches in Vascular Engineering: A Review of the Past and Future Trends. New York: Springer; 2011. pp. 457–487. [Google Scholar]
- 9.Davis G.E. Senger D.R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 10.Moses M.A. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15:180. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- 11.Lutolf M.P. Raeber G.P. Zisch A.H. Tirelli N. Hubbell J.A. Cell-responsive synthetic hydrogels. Adv Mater. 2003;15:888. [Google Scholar]
- 12.Lutolf M.P. Lauer-Fields J.L. Schmoekel H.G. Metters A.T. Weber F.E. Fields G.B., et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West J.L. Hubbell J.A. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1998;32:241. [Google Scholar]
- 14.Phelps E.A. Landazuri N. Thule P.M. Taylor W.R. Garcia A.J. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107:3323. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller J.S. Shen C.J. Legant W.R. Baranski J.D. Blakely B.L. Chen C.S. Bioactive hydrogels made from step-growth derived PEG-peptide macromers. Biomaterials. 2010;31:3736. doi: 10.1016/j.biomaterials.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seliktar D. Zisch A.H. Lutolf M.P. Wrana J.L. Hubbell J.A. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68:704. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 17.Nagase H. Fields G.B. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers. 1996;40:399. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C399::AID-BIP5%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Patterson J. Hubbell J.A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 19.Sokic S. Papavasiliou G. FGF-1 and proteolytically mediated cleavage site presentation influence three-dimensional fibroblast invasion in biomimetic PEGDA hydrogels. Acta Biomater. 2012;8:2213. doi: 10.1016/j.actbio.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.H. Moon J.J. Miller J.S. West J.L. Poly(ethylene glycol) hydrogels conjugated with a collagenase-sensitive fluorogenic substrate to visualize collagenase activity during three-dimensional cell migration. Biomaterials. 2007;28:3163. doi: 10.1016/j.biomaterials.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Raeber G.P. Lutolf M.P. Hubbell J.A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canal T. Peppas N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res. 1989;23:1183. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- 23.Moon J.J. Saik J.E. Poché R.A. Leslie-Barbick J.E. Lee S.-H. Smith A.A., et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant S.J. Chowdhury T.T. Lee D.A. Bader D.L. Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 25.DeKosky B.J. Dormer N.H. Ingavle G.C. Roatch C.H. Lomakin J. Detamore M.S., et al. Hierarchically designed agarose and poly(ethylene glycol) interpenetrating network hydrogels for cartilage tissue engineering. Tissue Eng Part C Methods. 2010;16:1533. doi: 10.1089/ten.tec.2009.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korff T. Kimmina S. Martiny-Baron G. Augustin H.G. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- 27.Moya M.L. Cheng M.H. Huang J.J. Francis-Sedlak M.E. Kao S.W. Opara E.C., et al. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31:2816. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis-Sedlak M.E. Moya M.L. Huang J.J. Lucas S.A. Chandrasekharan N. Larson J.C., et al. Collagen glycation alters neovascularization in vitro and in vivo. Microvasc Res. 2010;80:3. doi: 10.1016/j.mvr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Chiu Y.-C. Cheng M.-H. Engel H. Kao S.-W. Larson J.C. Gupta S., et al. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials. 2011;32:6045. doi: 10.1016/j.biomaterials.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 30.Bott K. Upton Z. Schrobback K. Ehrbar M. Hubbell J.A. Lutolf M.P., et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31:8454. doi: 10.1016/j.biomaterials.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Brey E.M. Uriel S. Greisler H.P. McIntire L.V. Therapeutic neovascularization: contributions from bioengineering. Tissue Eng. 2005;11:567. doi: 10.1089/ten.2005.11.567. [DOI] [PubMed] [Google Scholar]
- 32.Levental I. Georges P.C. Janmey P.A. Soft biological materials and their impact on cell function. Soft Matter. 2007;3 doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 33.Kraehenbuehl T.P. Ferreira L.S. Zammaretti P. Hubbell J.A. Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30:4318. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leslie-Barbick J.E. Moon J.J. West J.L. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed. 2009;20:1763. doi: 10.1163/156856208X386381. [DOI] [PubMed] [Google Scholar]
- 35.Saik J.E. Gould D.J. Watkins E.M. Dickinson M.E. West J.L. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly(ethylene glycol) hydrogels. Acta Biomater. 2011;7:133. doi: 10.1016/j.actbio.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]