Abstract
Fragmentation of multiply-charged peptide ions via interaction with products of gas discharge at atmospheric pressure conditions was studied using ion mobility separation – fragmentation cell - linear ion trap mass spectrometer. The observed fragmentation spectra mainly consisted of c- type ions that are specific to electron capture dissociation. Experiments with different gases flowing through the discharge and different discharge polarities suggested that fragmentation proceeds via capture of free electrons. Fragmentation of a model phosphorylated peptide using this technique produced c- type fragments with an intact phosphorylation group. High field asymmetric waveform ion mobility separation of a peptide mixture prior to the fragmentation cell demonstrated the feasibility of conducting MS/MS-like experiments at atmospheric pressure conditions.
Introduction
Activation and dissociation of gas-phase ions is a central technique used in tandem mass spectrometry (MS/MS) for ion structural characterization. The fragmentation of peptide/protein ions is broadly utilized in proteomics for the identification and characterization of proteins [1] and there is a strong interest in further developing and improving dissociation methods. Collision induced dissociation (CID) is the most commonly used technique to derive structural information about peptide and protein ions through their energetic collisions with neutral gas molecules [2]. The energy acquired in a collision is quickly redistributed over a large number of vibrational degrees of freedom of big biomolecules, causing dissociation of chemical bonds with the lowest activation energy. Peptides primarily fragment to form b- and y- type ions, and relative fragment ion abundances are highly dependent on the peptide amino acid sequence and secondary structure. The drawbacks of CID include the facile loss of labile posttranslational modifications, such as phosphorylation and glycosylation, and incomplete backbone fragmentation in many cases [3]. Therefore, developing alternative peptide fragmentation methods is of considerable interest.
McLafferty and co-workers introduced a new technique, called electron capture dissociation (ECD), which has been shown to complement CID of multiply protonated peptide cations [4]. Capture of a low-energy electron by a protonated peptide is exothermic and causes fragmentation of N-Cα bonds, yielding N-terminal c- and C-terminal z-fragments [5,6]. ECD is believed to be driven by a radical mechanism [7], without randomization of energy prior to fragmentation. As a result, labile modification groups are generally retained during fragmentation, permitting their localization. ECD generally results in cleavage of a wider range of peptide backbone bonds than CID, with weaker dependence on peptide composition [8]. Initially, ECD has been successfully realized only in Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometers, where the electric field is very weak and, additionally, the strong magnetic field confines the electrons. The presence of strong radio frequency (RF) electric fields in 3-D and linear quadrupole ion traps makes introduction of low-energy electrons to the area where the peptide ions are located difficult [9]. Recent improvements in ECD implementation in linear quadrupole ion traps were achieved by superimposing a moderate magnetic field (0.15 T) along the quadrupole axis to confine low-energy electrons and introducing a He buffer gas for ion cooling [10]. In another approach, efficient electron-capture dissociation was demonstrated in a compact, RF-free, hybrid electrostatic/magnetostatic cell, which was placed in-line with the quadrupole ion guide [11,12].
A new fragmentation technique, electron transfer dissociation (ETD), which overcomes the technical challenges of introducing low-energy electrons into strong oscillating RF fields, was recently proposed [13, 14]. In ETD, singly-charged anions transfer an electron to the multiply protonated peptides and induce fragmentation of the peptide backbone along pathways that are similar to those observed in ECD. Simultaneous trapping of cations and anions is readily accomplished by the RF quadrupole field. One of the major drawbacks in ETD is that the peptide structural information that can be obtained is charge-state dependent [15]. The doubly-charged peptide cations give much poorer sequence coverage than triply protonated cations, with fragmentation often limited to bond breakage near one or at both termini of the peptide. It was also demonstrated that the efficiency of electron transfer dissociation decreases with peptide length [16]. Supplementary collisional activation that targets the non-dissociated electron-transfer product ions provided an improvement in sequence coverage of doubly charged peptide ions [17]. Other issues with ETD are the competition between proton transfer to anion with electron transfer [14] and variability of the electron-transfer reaction time, which depends on peptide composition and number density of reacting species in the trap. It was shown recently [18] that even at optimal ETD conditions, the detrimental proton transfer channel constitutes 10% – 30% of cation-anion reaction products.
Electron capture dissociation was also observed at atmospheric conditions. The first demonstration of an atmospheric pressure ECD was reported by Laprévote’s group [19, 20], who observed c- type peptide fragments ions within a PhotoSpray atmospheric pressure photoionization (APPI) source. Authors suggested that fragmentation results from reactions of protonated peptides with electrons released upon photoionization of dopant molecules present in the sample vapor stream. This approach was further refined in [21], where the processes of peptide ionization and dissociation via interaction with electrons were separated in space. In that study, peptide ions were produced by nanospray ionization and electrons were generated by photoionization of an acetone dopant. An alternative approach was proposed in [22], where peptide ions generated in electrospray were subjected to the products of electrical discharge in an atmospheric flow reactor. It was initially assumed that the fragmentation mechanism is based on interaction of protonated peptide ions with hydroxyl radicals [22]. In this study, we demonstrate isolation of ions prior to fragmentation at atmospheric pressure and present data pointing to a different fragmentation mechanism.
Experimental
Reagents
Solutions of peptides were prepared in molecular biology-grade water (Cambrex Bio Science, Rockland, ME) and reagent-grade methanol (Sigma-Aldrich, St. Lois, MO) (50:50 v/v) at concentrations of 10–50 µM. Substance P, apelin-12, OVA (323–339), CaM kinase II and CaM kinase II phosphorylated peptides were purchased from AnaSpec (San Jose, CA). Nitrogen (ultra high purity grade (UHP)), helium (UHP), oxygen (UHP), air (UHP) and a mixture of nitrogen with 0.05% SO2 were supplied by Airgas (Radnor, PA).
Instrumentation and methods
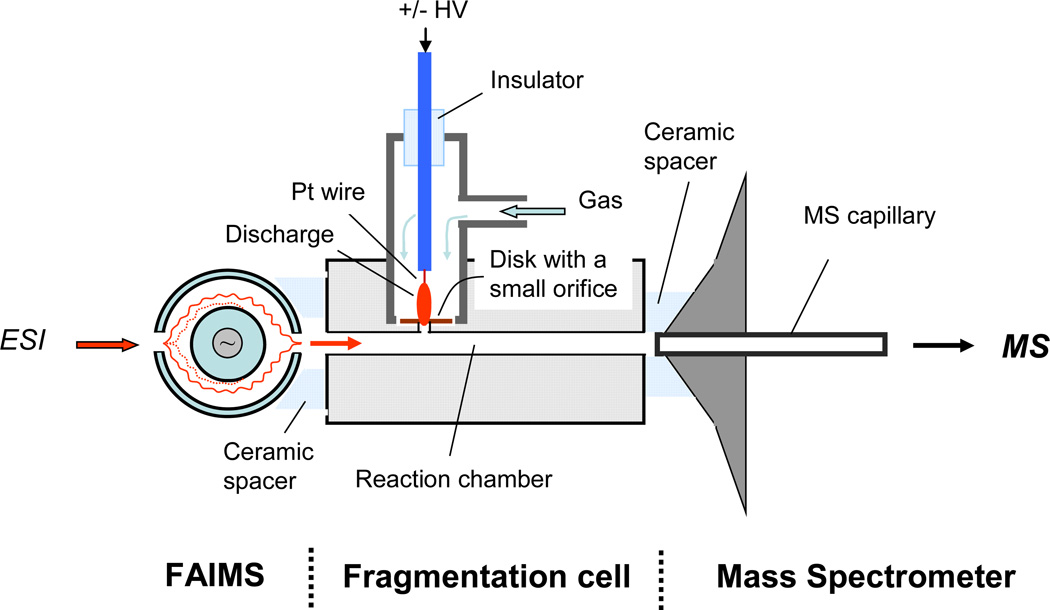
The experimental results were obtained using a linear ion trap mass spectrometer (LTQ, Thermo Scientific, San Jose, CA). The selection of electrospray-produced analyte ions at atmospheric pressure conditions was performed using a high-field asymmetric waveform ion mobility spectrometer (FAIMS, Thermo Fisher Scientific, San Jose, CA). Analyte ions were then transported by a flow of gas created by the mass spectrometer intake (~ 1 L/min) to a reaction chamber, where they interacted with the products of a gas discharge. The LTQ mass spectrometer recorded the ions resulting from these interactions. The schematic illustration of the experimental set-up is shown in Figure 1.
Figure 1.
Schematic drawing of the fragmentation cell interfaced with FAIMS and LTQ mass spectrometer.
The home-made electrospray ion source was mounted in front of FAIMS entrance aperture plate and consisted of a short piece of 40-µm i.d. fused silica capillary emitter connected to a syringe pump via a microtight union and PEEK tubing (Upchurch Scientific, Oak Harbor, WA). The peptide solutions were infused into the ESI source at 0.5 – 1.0 µL/min rate using the syringe pump (Model 11, Harvard Apparatus, Holliston, MA). High voltage has been taken from LTQ output and was applied directly to a stainless steel syringe needle. The potential applied to the ESI source was varied over a 1500–2200 V range.
Pre-selection at atmospheric pressure conditions of ions of interest by high field asymmetric waveform ion mobility separation simplified the study of the fragmentation mechanism. The dispersion voltage of FAIMS was set to −5000V and compensation voltage (CV) was varied to select ions of interest. Electrode temperatures in FAIMS were set to 70° C for the inner electrode and 90° C for the outer electrode. Ultra-high purity nitrogen was flowing through FAIMS at 1.25 – 3.0 L/min flow rate. The linear ion trap mass spectrometer was operated in MS mode with automatic gain control (i.e. ion injection time was automatically adjusted by mass spectrometer software depending on the ion beam intensity).
The stainless steel fragmentation cell with 60 mm length and 2.4 mm i.d. was tightly mounted onto the heated entrance capillary of LTQ using a ceramic spacer. This created an intake flow of about 1 L/min which provided efficient transfer of ions from the exit of FAIMS into the reaction chamber. The reaction time of ~ 15 ms of ions with products of gas discharge was estimated based on the flow rate through the fragmentation cell and the geometrical dimensions. To improve ion transmission from the fragmentation cell into the mass spectrometer, a 50 –100 VDC potential was applied to the cell. The temperatures of the cell and the gas flowing through the discharge chamber were independently controlled. A few kV (positive or negative polarity) potential, applied to a platinum wire (0.125 mm dia) sealed inside the ceramic or stainless steel tube, created the discharge between the platinum wire tip and a stainless steel disk with a small orifice. The diameter of the orifice separating the discharge chamber and reaction cell was 1.25 mm. The distance between the platinum wire and the disk was about 2 mm. The optimal gas flow rates through the discharge were in the 100–200 cc/min range.
Results and Discussion
Fragmentation of substance P
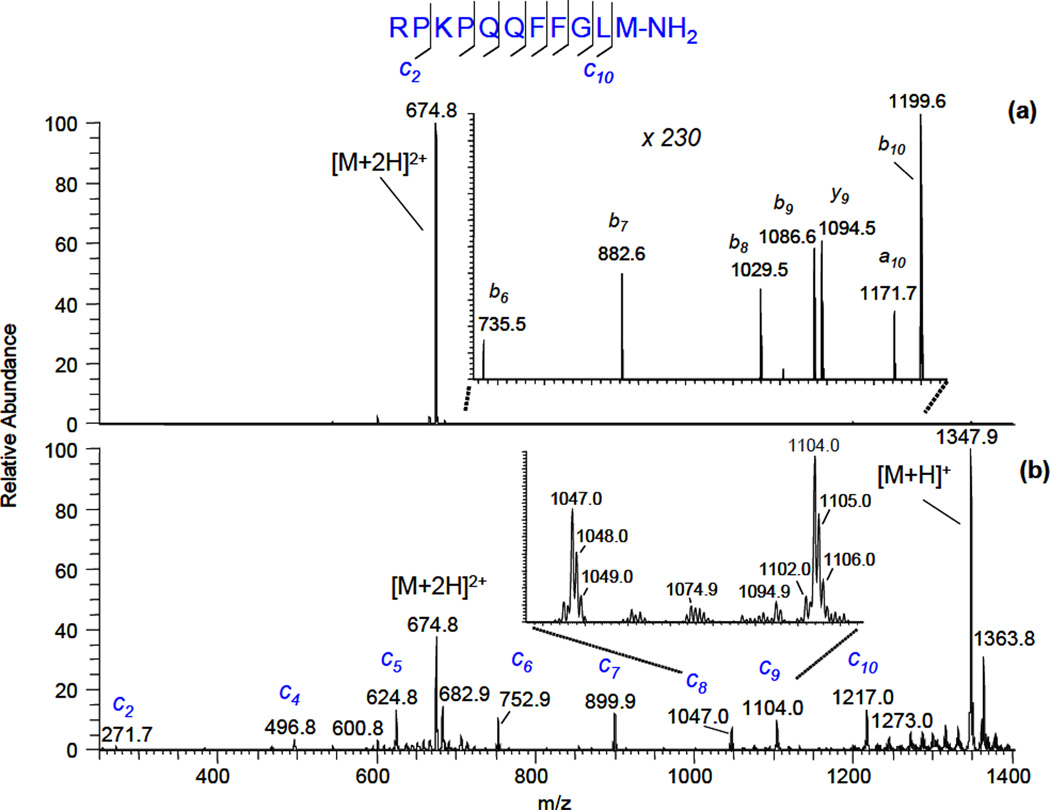
Fragmentation of substance P ions is frequently used as a benchmark test for a new dissociation technique for fragmenting of N-Cα bonds. The solution of substance P in 50:50 (v:v) water:methanol was electrosprayed at the entrance aperture of FAIMS and the compensation voltage was set to select doubly-protonated ions. Single-scan spectra with the discharge off (a) and on (b) (nitrogen was flowing at 100 cc/min through the gas-discharge chamber in both cases) are shown in Figure 2:
Figure 2.
Single-scan fragmentation mass spectra of substance P (2+) ions obtained via interaction with gas discharge species. (a) - nitrogen, discharge is off; (b) - nitrogen, negative polarity discharge (− 40 µA). FAIMS compensation voltage CV was −13.7 V.
In this experiment, the discharge was of negative polarity and a current was −40 µA. The temperature of the fragmentation cell was set at 380° C, since it was found that fragmentation proceeds more efficiently at high temperatures. The data presented in Figure 2 show that ECD-like fragmentation induced by products of nitrogen gas-discharge is quite an efficient process. In Fig. 2b, a complete series of c- type ions was recorded in a single scan. The combined intensity of all c- type ion peaks constituted ~ 2.5% of parent ion peak intensity. Contrary to ECD observed in vacuum [23], z9• ion was not found in the recorded spectrum. Without a discharge, only very weak a-, b- and y- ion peaks were observed, which most likely resulted from thermally induced dissociation [24, 25]. The singly-protonated substance P ion in Fig. 2b was generated via a charge-reduction process with the subsequent elimination of a hydrogen atom. Other two ions present in the spectrum, m/z = 682.9 (2+) and m/z = 1363.8 (1+), differ from the corresponding molecular ions by 8 Da and 16 Da, respectively. These ions most likely originated in oxidation process.
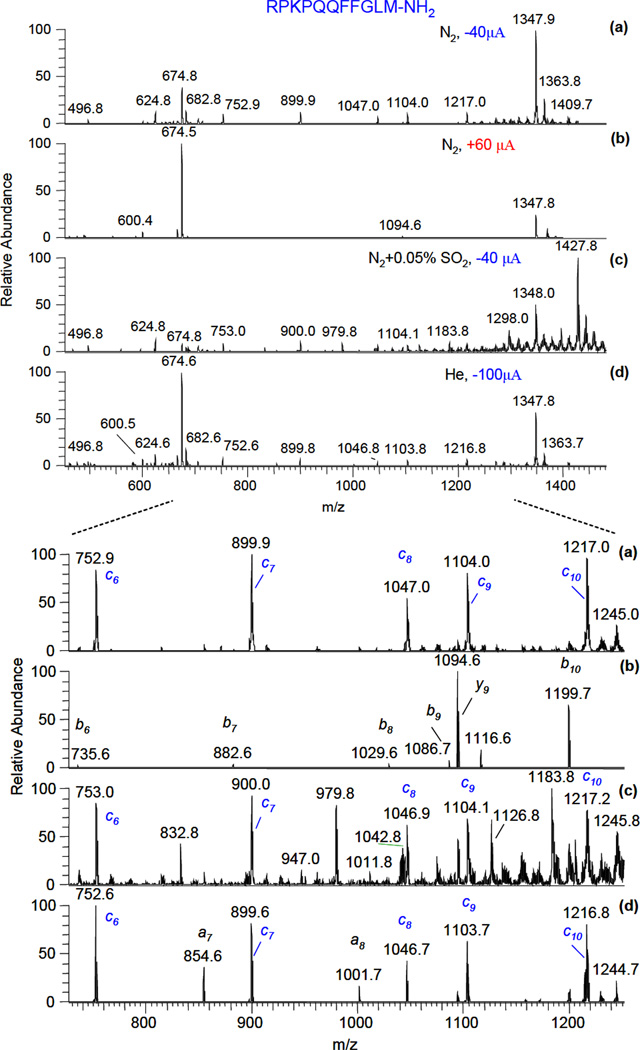
To get insight into the fragmentation mechanism, experiments with different gases flowing through the gas-discharge chamber and different discharge polarities were conducted. The recorded spectra are shown in Figure 3 (typically, MS spectra were averaged over 10 s):
Figure 3.
The effect of gas and discharge polarity on fragmentation of substance P (2+) ions: (a) - nitrogen, negative polarity discharge (−40 µA); (b) – nitrogen, positive polarity discharge (60 µA); (c) – nitrogen + 0.05% SO2, negative polarity discharge (− 40 µA); (d) – helium, negative polarity discharge (−100 µA). FAIMS compensation voltage CV was −13.7 V. Zoomed ion peaks in the mass range m/z = 700–1250 are shown in the corresponding spectra (see bottom of Fig.3).
The results for nitrogen with positive polarity discharge (Fig. 3b) show, that contrary to negative polarity discharge (Fig. 3a), no c- type ions are present in the spectrum. Only weak b- and y- ion peaks of substance P were observed in this case, similarly to a situation when the discharge was turned off. The addition of a small amount (500 ppm) of an electron scavenger (SO2) to the nitrogen flow with negative polarity discharge (Fig. 3c) resulted in a significant reduction of c-type ions intensities (~ 40 times). Another feature of this spectrum is the series of ions which differ from c-type ions by 80 Da (m/z = 832.8, 979.8, 1126.8 and 1183.8). These ions most likely appear from reactions with SO3−. The ion related to a singly-charged molecular substance P ion at m/z = 1427.8 is also formed in a similar reaction. Substantially reduced c-type fragmentation was observed for air or oxygen flowing through the discharge chamber (results are not shown). The free-electron concentration in the discharge is also substantially decreased in these cases because of formation of long-lived molecular negative ions, such as O2− for oxygen flow and additionally NO2−, NO3− for air flow [26]. Intense c-type ion peaks were observed when helium was flowing through negative polarity discharge (Figure 3 (d)). In this case, a-type ions were also observed, which indicates on a possible contribution of electronically-excited metastable states of helium to the fragmentation process [27].
Fragmentation of different charge states
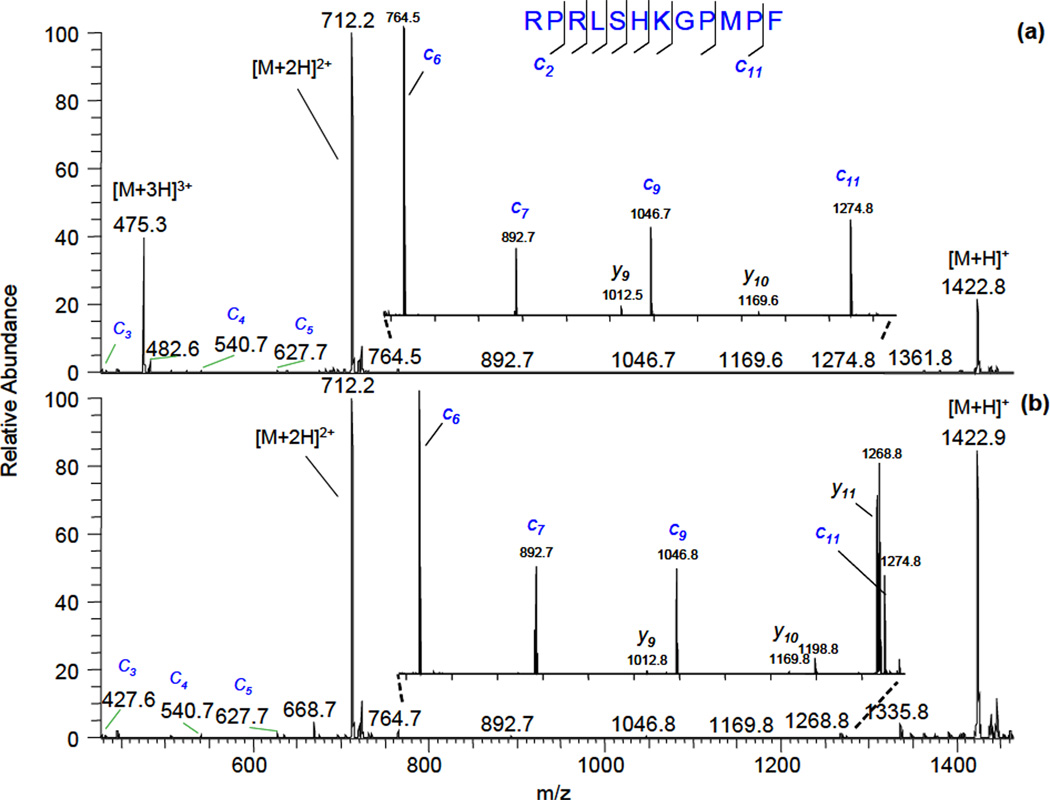
It was shown that for ECD taking place in vacuum, the electron-capture cross section is proportional to a square of ionic charge [6]. To test if there is a similar charge-state dependence at atmospheric pressure conditions, we selected two different charge states of the same peptide by using high field asymmetric waveform ion mobility separation and subjected them to species flowing from a helium discharge. The helium flow rate was 100 cc/min and the cell temperature was 340° C in these experiments. The resulting fragmentation spectra of different charge states of apelin-12 peptide are shown in Figure 4:
Figure 4.
Fragmentation mass spectra of different charge states of apelin-12 peptide: (a) – triply-charged, discharge current was −40 µA; (b) – doubly-charged, discharge current was −200 µA.
In Fig. 4, the most intense product ion peaks are lower charge state protonated molecular ions. To produce the same rate of parent ion conversion into lower charge states, the discharge current needed to be 5 times bigger in the case of doubly-charged apelin-12 ions (Fig 4a) compared to triply-charged ions (Fig. 4b). Note that the electron density is increasing when the discharge current is raised [26]. This effect is another proof that free electrons play an important role in the fragmentation process. A complete series of c-type ions was observed in both cases (c8 and c10 ions are not present due to absence of cleavage on the N-terminal side of proline; this phenomena is also observed in vacuum ECD fragmentation [7]). Some y- type ions, present in the spectra, were also observed in the absence of discharge and most likely resulted from thermally-induced dissociation.
Fragmentation of phosphorylated peptide
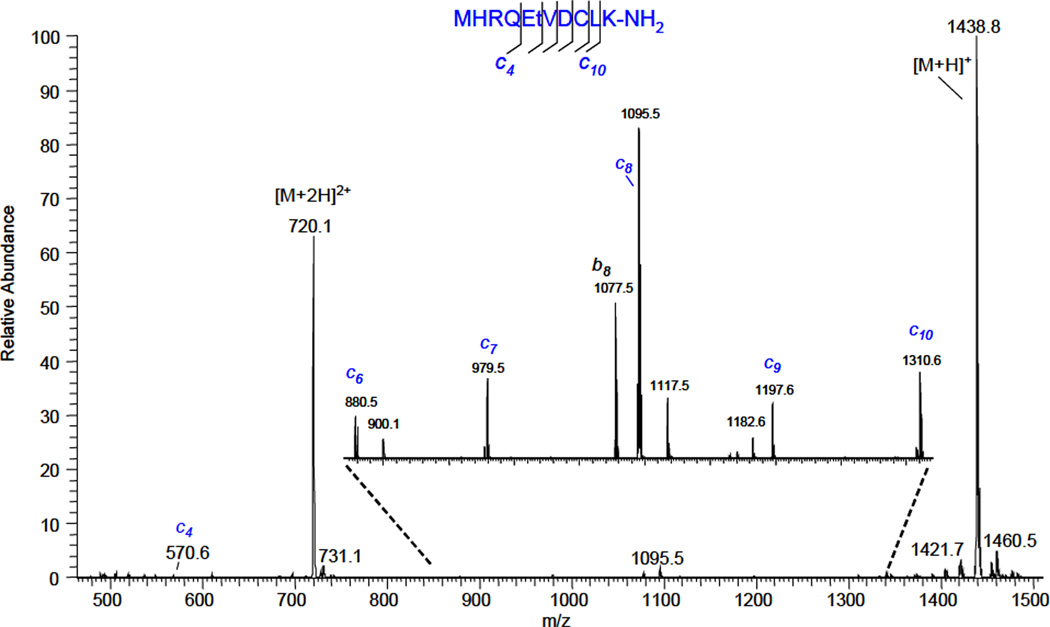
An essential feature of vacuum ECD is the preservation of labile modification groups during fragmentation. To study the dissociation of modified peptides, doubly-charged molecular ions of phosphorylated CaM kinase II were selected by FAIMS and fragmented in the reaction chamber:
Helium was flowing through the gas-discharge chamber at flow rate of about 100 cc/min. The cell temperature was set at 340° C. Fragmentation spectrum showed a near complete series of c-type ions, along with some b- type ions. A few ions, such as m/z=900.1 and m/z=1117.5, coincide with c7-HPO3 and c9-HPO3, respectively, but they were also found in the spectrum when the discharge was turned off. Otherwise, no loss of phosphorylation was observed in the fragmentation spectrum.
MS/MS-like experiments at atmospheric pressure
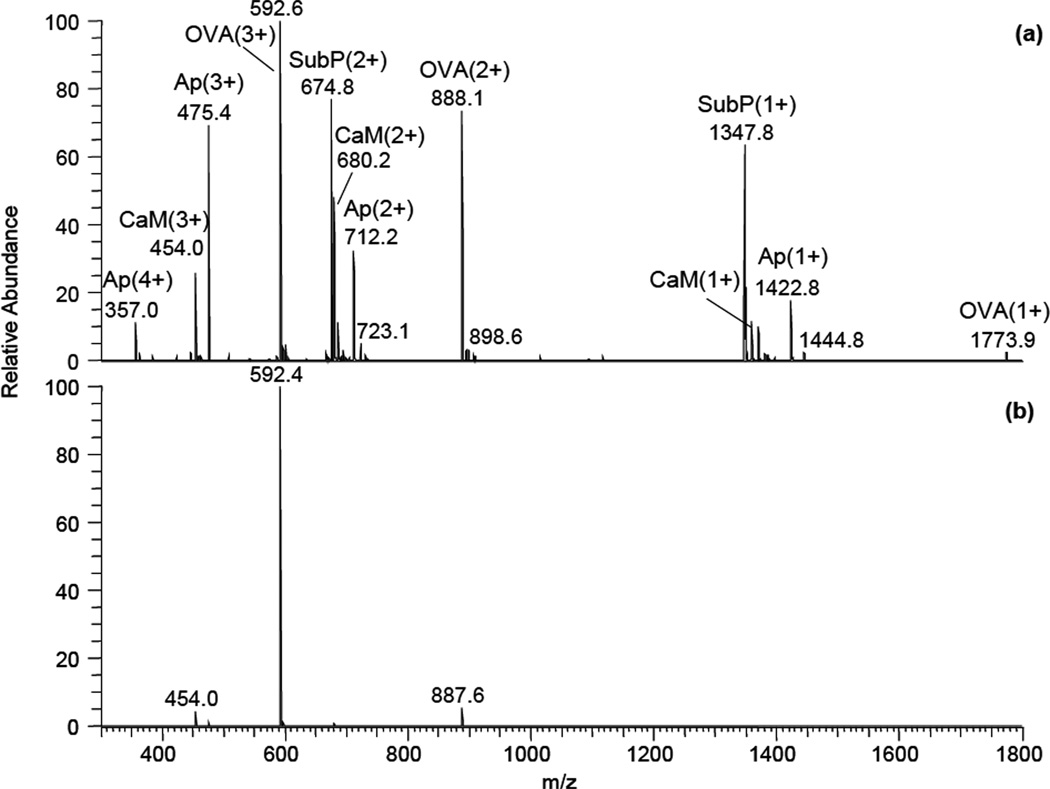
Ion mobility separation combined with a new fragmentation technique opens a possibility of conducting MS/MS-like experiments at atmospheric pressure. The selection of triply-charged OVA (323–339) peptide ions from a mixture is shown in Figure 6:
Figure 6.
Demonstration of peptide ion selection using high-field asymmetric waveform ion mobility spectrometer from a mixture containing different charge states of apelin-12, CaM kinase II, OVA(323–339) and substance P: (a) – no selection, (b) – selection of triply-charged OVA(323–339) peptide ion with FAIMS compensation voltage −23.2 V.
Multiply protonated peptide ions were produced by electrospray ionization from a mixture of four peptides in 50:50 (v:v) water:methanol solution. Singly-, doubly- and triply-charged molecular ions were observed for each peptide (Fig. 6a), along with a 4+ molecular ion for apelin-12 and some weak sodiated ions. As an example, high-field asymmetric waveform ion mobility separation was used to select triply-charged OVA (323–339) ions before the fragmentation cell (Fig. 6b). Weak CaM kinase II (3+) and OVA (323–339) (3+) ion peaks, remained in the spectrum due to incomplete FAIMS separation. Fragmentation was induced by turning the discharge voltage on. The resulting fragmentation spectrum is shown in Fig. 7:
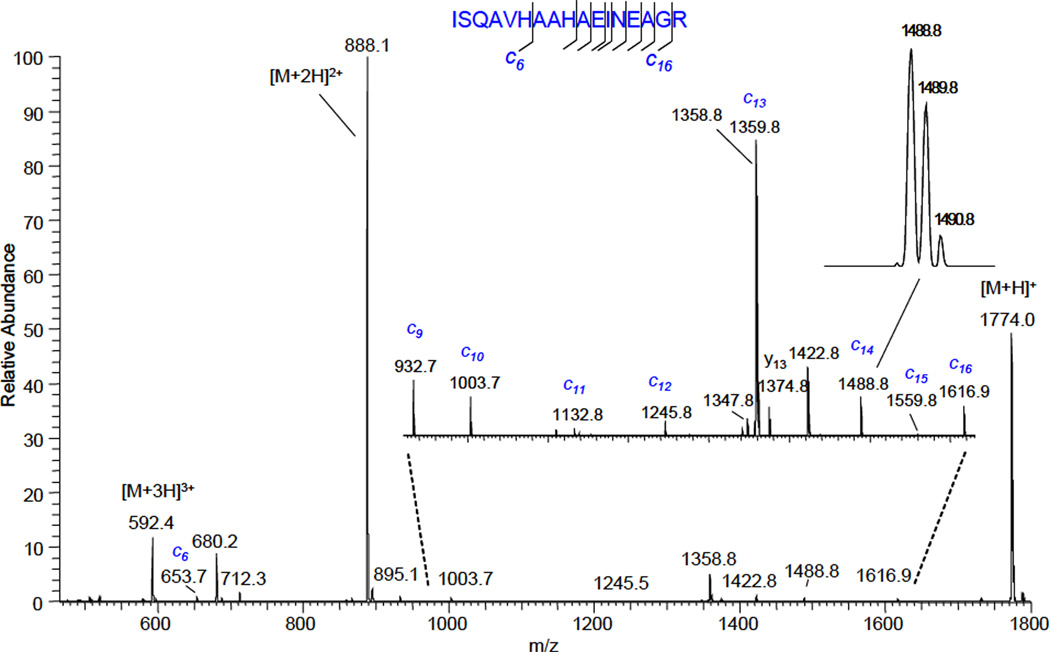
Figure 7.
The fragmentation mass spectrum of selected by FAIMS triply-charged OVA(323–339) peptide ion obtained via interaction with products of helium gas-discharge. The discharge current was −100 µA.
A nearly complete series of c-type ions (weak peaks of c7 and c8 ions are not visible on chosen scale) was observed in the fragmentation mass spectrum, as well as lower charge states of the OVA (323–339) peptide molecular ion. Ions at m/z = 680.2, m/z = 712.3 and m/z = 1347.8 are doubly-charged CaM kinase II, doubly-charged apelin-12 and singly-charged substance P molecular ions, respectively. Their presence is due to incomplete ion mobility separation. Ions at m/z = 1358.8 and m/z = 1422.8 are a singly-charged CaM kinase II and apelin-12, respectively. They resulted from reaction of corresponding doubly-charged ions with products of the gas discharge. Some y- type ions were present in the spectrum in the absence of discharge and resulted from thermally-induced dissociation. Subtracting the spectrum obtained when the discharge was turned off from the fragmentation spectrum (discharge is on) will allow viewing c-type ions in isolation, thus facilitating data interpretation.
Discussion
The ECD-like fragmentation via interaction of multiply-charged peptide molecular ions with products of a gas discharge was only observed for discharge of negative polarity. This indicates that negatively charged species are causing fragmentation because in the case of positive voltage on the platinum wire they are prevented from entering the reaction chamber by a strong electric field. Experiments with ultra-high pure grade of nitrogen and helium show that these species are most likely free electrons because gas molecules in the discharge capable of forming long-lived negative ions are present in only trace amounts. Additionally, when a small amount (500 ppm) of sulfur dioxide (electron scavenger) was added to nitrogen, a significant reduction of c- type ion intensities was observed. This reduction is most likely associated with decreased electron density in the gas flow entering the reaction chamber. All these data point to a fragmentation proceeding via interaction with free electrons generated in the gas discharge:
Contrary to data where photoelectrons were used for inducing fragmentation at atmospheric pressure [21], no [M+nH](n−1)+• or z• type ions were observed for the studied peptides. This is most likely related to the presence of highly reactive species in gas discharge products, which quickly interact with peptide ion radical intermediates. In our experimental conditions, [M+nH](n−1)+• ions are converted either to [M+(n−1)H](n−1)+ ions or to c- type fragments prior to mass analysis.
Conclusions
The design (electrode configuration, separating aperture diameter and the reaction channel diameter and length) of the atmospheric pressure fragmentation cell based on interaction of multiply-charged peptide ions with products of gas discharge was optimized, which allowed ECD-like fragmentation spectra to be obtained in a single MS scan. Experiments with different gases flowing through the discharge and different discharge polarities demonstrated that fragmentation proceeds via capture of free electrons produced in the gas discharge by peptide ions. Mostly c- type ions and lower charge states of molecular ions are observed in the fragmentation spectra. Similar to vacuum ECD, this technique allows localization of the phosphorylation group because it remains intact during fragmentation. Coupling of this fragmentation cell with an ion mobility separation allows conducting MS/MS-like experiments at atmospheric pressure.
Figure 5.
The fragmentation mass spectrum of doubly-charged phosphorylated CaM kinase II. The discharge current was −200 µA.
The atmospheric pressure fragmentation cell, based on interactions between multiply-charged peptide ions and products of gas discharge, allowed ECD-like fragmentation spectra to be obtained in a single MS scan.
Experiments with different gases flowing in the discharge and different discharge polarities demonstrated that fragmentation proceeds via capture of free electrons produced in the gas discharge by peptide ions.
c- type ions and lower charge state molecular ions are mainly observed in the fragmentation spectra.
Similar to vacuum ECD, this technique allows localization of the phosphorylation group because it remains intact during fragmentation.
Coupling this fragmentation cell with an ion mobility separation allows conducting MS/MS-like experiments at atmospheric pressure.
Acknowledgment
The authors gratefully acknowledge Dr. T.D. Saul for help in conducting experiments and the National Institutes of Health for financial support (NIH SBIR grant 5R44RR023224-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Aebersold R, Mann M. Mass spectrometry based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 2.Lane CS. Mass spectrometry-based protomics in life sciences. Cellular and Molecular Life Sciences. 2005;62:848–869. doi: 10.1007/s00018-005-5006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson RJ, Connoly LM, Eddes JS, Pereira JJ, Moritz RL, Reid GE. Proteomic analysis of the human colon carcinoma cell line (LIM 1215): Development of a membrane protein database. Electrophoresis. 2000;21:1707–1732. doi: 10.1002/(SICI)1522-2683(20000501)21:9<1707::AID-ELPS1707>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J. Am. Chem. Soc. 1998;120:3265–3266. [Google Scholar]
- 5.Zubarev RA, Kruger NA, Fridriksson EK, Lewis MA, Horn DM, Carpenter BK, McLafferty FW. Electron capture dissociation of gaseous multiply-charged proteins is favored at disulfide bonds and other sites of high hydrogen atom affinity. J. Am. Chem. Soc. 1999;121:2857–2862. [Google Scholar]
- 6.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 7.Simons J. Mechanisms for S-S and N-Cα bond cleavage in peptide ECD and ETD mass spectrometry. Chem. Phys. Lett. 2010;484:81–95. [Google Scholar]
- 8.Zubarev RA. Reactions of polypeptide ions with electrons in the gas phase. Mass Spectrom. Rev. 2003;22:57–77. doi: 10.1002/mas.10042. [DOI] [PubMed] [Google Scholar]
- 9.Silivra OA, Kjeldsen F, Ivonin IA, Zubarev RA. Electron capture dissociation of polypeptides in a three-dimensional quadrupole ion trap: Implementation and first results. J. Am. Soc. Mass Spectrom. 2005;16:22–27. doi: 10.1016/j.jasms.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Baba T, Hashimoto Y, Hasegawa H, Hirabayashi A, Waki I. Fast multiple electron capture dissociation in an linear radio frequency quadrupole ion trap. Anal. Chem. 2007;79:8755–8761. doi: 10.1021/ac071462z. [DOI] [PubMed] [Google Scholar]
- 11.Voinov VG, Deinzer ML, Barofsky DF. Electron-capture dissociation (ECD), collision-induced dissociation (CID) and ECD/CID in a linear radiofrequency-free magnetic cell. Rapid Commun. Mass Spectrom. 2009;23:3028–3030. doi: 10.1002/rcm.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voinov VG, Beckman JS, Deinzer ML, Barofsky DF. Radio-frequency-free cell for electron capture dissociation in tandem mass spectrometry. Anal. Chem. 2009;81:1238–1243. doi: 10.1021/ac802084w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl Acad. Sci. USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coon JJ, Syka JEP, Schwartz JC, Shabanowitz J, Hunt DF. Anion dependence in the partitioning between proton and electron transfer in ion/ion reactions. Int. J Mass Spectrom. 2004;236:33–42. [Google Scholar]
- 15.Pitteri SJ, Chrisman PA, Hogan JM, McLuckey SA. Electron transfer ion/ion reactions in a three-dimensional quadrupole ion trap: reactions of doubly and triply protonated peptides with SO2•−. Anal. Chem. 2005;77:1831–1839. doi: 10.1021/ac0483872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitteri SJ, Chrisman PA, McLuckey SA. Electron transfer ion/ion reactions of doubly protonated peptides: effect of elevated bath gas temperature. Anal. Chem. 2005;77:5662–5669. doi: 10.1021/ac050666h. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swaney D, McAlister G, Wirtala M, Schwartz J, Syka J, Coon J. Supplemental activation method for high-efficiency electron-transfer dissociation of doubly protonated peptide precursors. Anal. Chem. 2007;79:477–485. doi: 10.1021/ac061457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compton PD, Strukl JV, Bai DL, Shabanowitz J, Hunt DF. Optimization of electron transfer dissociation via informed selection of reagents and operating parameters. Anal. Chem. 2012;84:1781–1785. doi: 10.1021/ac202807h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delobel A, Halgand F, Laffranchise-Gosse B, Snijders H, Laprévote O. Characterization of hydrophobic peptides by atmospheric pressure photoionization-mass spectrometry and tandem mass spectrometry. Anal. Chem. 2003;75:5961–5968. doi: 10.1021/ac034532k. [DOI] [PubMed] [Google Scholar]
- 20.Debois D, Giuliani A, Laprévote O. Fragmentation induced in atmospheric pressure photoionization of peptides. J. Mass Spectrom. 2006;41:1554–1560. doi: 10.1002/jms.1122. [DOI] [PubMed] [Google Scholar]
- 21.Robb DB, Rogalski JC, Kast J, Blades MW. A new ion source and procedures for atmospheric pressure-electron capture dissociation of peptides. J. Am. Soc. Mass Spectrom. 2011;22:1699–1706. doi: 10.1007/s13361-011-0202-0. [DOI] [PubMed] [Google Scholar]
- 22.Vilkov AN, Laiko VV, Doroshenko VM. Peptide fragmentation induced by radicals at atmospheric pressure. J. Mass Spectrom. 2009;44:477–484. doi: 10.1002/jms.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsybin YO, Haselmann KF, Emmett MR, Hendrikson CL, Marshall AG. Charge location directs electron capture dissociation of peptide dications. J. Am. Soc. Mass Spectrom. 2006;17:1704–1711. doi: 10.1016/j.jasms.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood AL, Busman M, Udseth HR, Smith RD. Thermally induced dissociation of ions from electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 1991;5:582–585. [Google Scholar]
- 25.Chen H, Eberlin LS, Cooks RG. Neutral fragment mass spectra via ambient thermal dissociation of peptide and protein ions. J. Am. Chem. Soc. 2007;129:5880–5886. doi: 10.1021/ja067712v. [DOI] [PubMed] [Google Scholar]
- 26.Raizer YP. Gas discharge physics. Berlin: Springer-Verlag; 1991. [Google Scholar]
- 27.Berkout VD, Doroshenko VM. Fragmentation of phosphorylated and singly charged peptide ions via interaction with metastable atoms. Int. J Mass Spectrom. 2008;278:150–157. doi: 10.1016/j.ijms.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]