Abstract
CD8+ T cells have been described as being naïve (TN) or one of four antigen-experienced subtypes representing a continuum of differentiation and maturation: stem cell memory (TSCM), central memory (TCM), effector memory (TEM), and terminally differentiated effector T cells (TEFF). In mice, adoptive cell transfer (ACT) of less differentiated TN, TSCM and TCM subsets have consistently demonstrated superior in vivo expansion, persistence, and antitumor capacities relative to the more differentiated TEM and TEFF cells. Retrospective analyses from human ACT trials have confirmed that transfer of less differentiated T cell subsets is highly correlated with objective clinical responses. These findings, combined with the recent ability to convey de novo antigen reactivity with high efficiency through genetic engineering of exogenous T cell or chimeric antigen receptors, now challenge the field with three important questions: 1) how should less differentiated T cell subsets be isolated for human clinical trials?; 2) what is the best means of expanding T cells ex vivo in such a way as to not corrupt the beneficial traits of the younger subsets?; and 3) is it necessary to physically separate younger subsets from their more differentiated counterparts? Answering these questions will allow for the rational development of the next generation of highly effective and potentially curative T cell therapies for the treatment of cancer.
Introduction
Adoptive cell transfer (ACT), the ex vivo expansion and re-infusion of antigen (Ag)-specific T cells to patients, represents a highly effective and potentially curative systemic therapy for patients with advanced solid and hematologic cancers1,2,3,4,5, recurrent viral diseases6 and post-transplantation lymphoproliferative disease.7 Historically, the most pressing technical issue in ACT therapies has been the generation of a sufficient quantity of Ag-specific T cells for transfer.8,9 For some solid cancers such as melanoma, an elegantly simple solution to this problem was achieved through the discovery that T lymphocytes infiltrating tumor deposits (or TIL cells) frequently possess specific reactivity against autologous or human leukocyte antigen (HLA)-matched tumor lines.10 When TIL cells are obtained from surgically resected tumor masses, expanded non-specifically ex vivo, and subsequently re-infused into patients in conjunction with a lymphocyte depleting pre-conditioning regimen that includes total body irradiation11, cancer regression can be observed in the majority of cases.12,13
Beyond Ag specificity, T cells are heterogeneous with respect to a myriad of other parameters, including anatomic localization, proliferative and engraftment potentials, as well as cytokine secretion, metabolic, and gene expression profiles14,4,15,16. In principle, each of these parameters may independently influence a T cell's ability to mediate cancer regression following ACT. As a matter of course, however, many of these attributes tend to cluster together in discrete, easily discernible populations defined by a characteristic pattern of cell surface markers detectable through the use of fluorescent-activated cell sorting (FACS)17 or, more recently, mass spectrometry18. A straightforward and functionally significant means of classifying T cell subsets can be accomplished by assessing for the co-expression of the lymphoid homing molecules L-selectin (CD62L) and CC-chemokine receptor 7 (CCR7). T cells which display these 2 molecules have a propensity to home to secondary lymphoid structures where they can actively survey professional antigen presenting cells for the presence of cognate Ag. Cells in this category include naïve T cells (TN) as well as two Ag experienced memory T cell populations: the recently identified T memory stem cell (TSCM)19,20,21 population and central memory T cells (TCM)22. Although in humans both TN and TSCM express the RA isoform of CD45, TSCM can be distinguished from TN based on the expression of the IL-2/IL-15β chain receptor (CD122) and Fas (CD95). Central memory T cells, on the other hand, have acquired the expression of the prototypical human Ag experienced T cell marker, CD45RO. In addition to their anatomic localization in lymphoid organs, all three of these T cell subsets possess robust proliferative and engraftment capacities.21
By contrast, effector memory (TEM) and effector T cells (TEFF) are Ag experienced T cells that have strongly down-regulated CD62L and CCR7 and therefore preferentially reside in peripheral rather than lymphoid tissues. Effector memory and TEFF are poised to rapidly execute effector functions upon activation, as evidenced by their capacity to release large amounts of inflammatory cytokines such as interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) and their ability to rapidly lyse Ag-expressing targets. However, these subsets also tend to possess a relatively limited proliferative and engraftment potential compared with their CD62L+ counterparts23,24,25,21,26,27, properties which correlate with their shortened telomere lengths.28,22
In most clinical trials performed to date using TIL cells, the phenotypic and functional attributes of the transferred T cells were subject to natural variation and therefore were largely outside of investigational control. Despite this fact, detailed retrospective analyses from TIL trials have uncovered multiple, cell-intrinsic parameters which significantly correlate with the ability of T cells to mediate objective cancer regression in patients. For example, early studies revealed that properties such as a short duration in culture29,30,31,32 or a relatively rapid doubling time29,30 were correlated with clinical responses. More recently, additional parameters such as clonotypic persistence33,13, telomere length34,13, CD27 expression by CD8+ T cells upon IL-2 withdrawal35,13, and the frequency of cells with a central memory (TCM) phenotype in the infusion product36 have also been correlated with responses. In addition, it has also been noted that highly differentiated tumor Ag-specific effector CD8+ T cell (TEFF) clones generally engraft poorly and are inefficient at mediating cancer regression relative to results seen when a more heterogeneous population of T cells is transferred in TIL trials.37,38,39,40,41 Taken together, these observations in humans have led to the hypothesis that less differentiated T cells may confer superior antitumor efficacy relative to TEM and terminally differentiated TEFF cells.
Causal relationships between T cell differentiation and antitumor efficacy
The question of which T cell subsets should be targeted for ACT has remained a point of contention and controversy for many years.2,42,43,44 Because the ultimate goal of adoptive immunotherapy is to generate T cells capable of patrolling sites throughout the body in search of metastatic cancer deposits to destroy, it was initially presumed that the most desirable T cells for transfer should possess a natural propensity to infiltrate peripheral tissues and demonstrate strong, immediate, cytolytic capabilities. In effect, this would mean that the CD62L− TEM and TEFF populations would be the preferred cell types for transfer. However, this assumption stood at odds with empiric clinical data suggesting that transfer of less differentiated T cells were correlated with tumor responses. Systematic investigations were therefore undertaken in pre-clinical animal models to determine whether a causal relationship exists between a T cell's differentiation status at the time of infusion and antitumor efficacy (Figure 1).
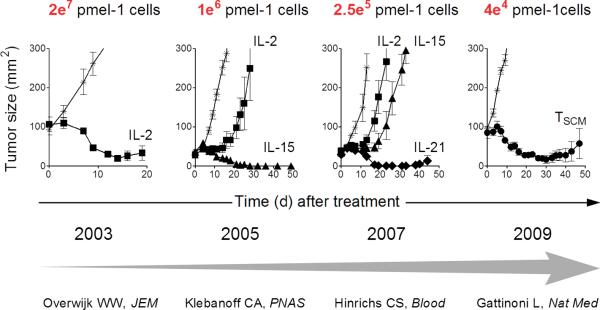
Figure 1. Re-iterative progress in enhancing the efficacy of adoptive CD8+ T cell therapy for the treatment of melanoma using the pmel-1 mouse model.
The use of alternative γc cytokines or less differentiated T cell subsets has resulted in progressive improvements in the therapeutic efficacy of tumor-reactive CD8+ T cells, thus providing for effective tumor destruction with the transfer of limited numbers of cells. IL, interleukin; TSCM, T memory stem cell.
In one set of experiments, murine CD8+ T cells derived from the pmel-1 T cell receptor transgenic mouse45 were re-iteratively stimulated in vitro to generate cells occupying progressively more advanced stages of differentiation termed early, intermediate, or late effectors.25 Consistent with their greater maturational state, intermediate and late effector T cells acquired strong IFNγ-releasing and cytolytic capacities and up-regulated the expression of key transcription factors (TFs) associated with effector-differentiation, including Eomes46 and Id247,48, as well as the replicative senescence marker killer cell lectin-like receptor G1 (KLRG-1).49 Reciprocally, as T cells became more differentiated, they lost the ability to release IL-2 and down-regulated the expression of multiple cell surface markers, including CD62L, CCR7, CD27, and IL-7Rα (CD127). Additionally, re-stimulated T cells reduced the expression of naïve-associated TFs such as Id3.47,48 When the intermediate and late effector T cells were transferred in vivo, these cells expanded and persisted poorly and, most importantly, were significantly impaired in their ability to cause tumor regression relative to naïve and early effector T cells.
In parallel, experiments were also conducted appraising the ability of conventional CD8+ T cell memory subsets to mediate cancer regression following ACT (Figure 2). Initially, the antitumor efficacy of tumor-reactive TCM relative to TEM CD8+ T cells was compared.24 Similar to results observed in viral challenge models23,26,27, TCM exhibited far greater proliferative and survival capacities in vivo following vaccination with cognate tumor Ag relative to TEM cells. While both T cell memory subsets could mediate cancer regression following ACT, only TCM induced complete responses at the cell dose tested while mice receiving TEM ultimately succumbed to uncontrolled tumor growth. Subsequently, the ability of TSCM to mediate cancer regression relative to the TCM and TEM populations was directly compared at limiting cell doses roughly 2 orders of magnitude less than administered in prior experiments.20,50 Consistent with earlier results, TCM mediated superior in vivo expansion, persistence, and antitumor efficacy compared with TEM. However, TSCM CD8+ T cells were even more potent than TCM cells on a per-cell basis. When the potency of tumor regression was evaluated as a function of the input population of T cells, a significant linear correlation between T cell differentiation status and anti-tumor efficacy was found in the order TSCM>TCM>TEM.50 These results were confirmed in a separate, vaccine-independent tumor treatment model system where human T cell subsets genetically engineered to express an anti-mesothelin chimeric antigen receptor (CAR) were used to treat human mesothelioma xenografts in immune-deficient mice.21
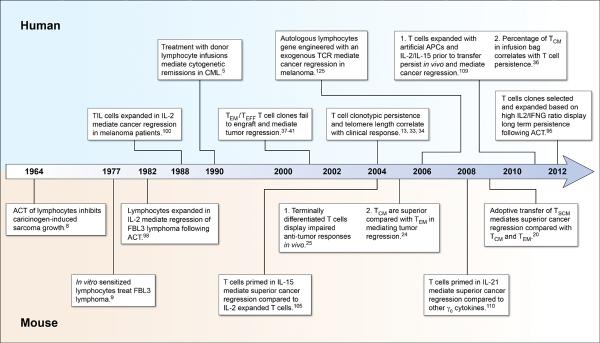
Figure 2. A timeline of progress in the understanding of T cell qualities associated with effective adoptive immunotherapies for the treatment of cancer in mice and humans.
ACT, adoptive cell transfer; TSCM, T memory stem cell; TCM, T central memory; TEM, T effector memory; TEFF, T effector cell; γc, common γ-chain receptor; CML, chronic myelogenous leukemia; TCR, T cell receptor; APC, antigen presenting cell.
The ability of naturally occurring Ag-specific and genetically engineered TEFF derived from different CD8+ T cell subsets has also been evaluated. Initially, the relative engraftment efficiencies of TEFF derived from conventional memory subsets was investigated (Figure 2). In both an immune-deficient mouse model receiving transfer of human T cells51 as well as in non-human primates52, TEFF derived from TCM precursors demonstrated superior persistence following ACT relative to TEM-derived TEFF. Remarkably, these differences were observed despite the fact that both memory derived TEFF subsets possessed a highly differentiated phenotype at the time of cell transfer, characterized by the low expression of CD62L, CCR7, CD28, and CD127 and high expression of granzyme B and perforin. These data suggest that currently used panels of cell surface markers used to characterize T cell subsets is missing important heterogeneity, possibly as a result of differences in the genetic53, epigenetic54 or metabolic profiles55 of otherwise phenotypically indistinguishable T cells on a single-cell level.
While TCM cells often represent a minor population in humans, TN are generally the predominant population present in the peripheral circulation.28,21,56,57 Moreover, TN possess longer telomeres and therefore have a greater replicative capacity compared with the Ag experienced subsets.28,58 For these reasons, the phenotypic, functional, and anti-tumor capacities of TEFF derived from a naïve rather than TCM population has been evaluated in both mice59 and humans.56 Unlike the results obtained comparing memory-derived TEFF51,52, effector cell derived from TN remained phenotypically and functionally distinguishable from the TCM-derived TEFF. Naive-derived TEFF retained the ability to release IL-2 while withholding the acquisition of the senesce marker KLRG-1. By contrast, TEFF-derived from TCM lost the ability to secrete IL-2 and significantly up-regulated the expression of KLRG-1. Additionally, human naïve-derived CD8+ TEFF exhibited superior retroviral transduction efficiencies for an exogenous T cell receptor (TCR) and maintained significantly longer telomere lengths compared with both TCM- and TEM-derived TEFF following ex vivo expansion.56 When transferred into tumor-bearing mice, TEFF derived from naïve cells exhibited superior in vivo expansion, persistence, and antitumor efficacy relative to TCM-derived TEFF59 Collectively, these data confirm that TEM represent an inferior T cell population for adoptive immunotherapies and demonstrate that among the CD62L+ subsets, the RA+ fraction should be retained rather than focusing solely on isolating, expanding, and re-infusing cells derived exclusively from the TCM subset.
Finally, it should be noted that the conclusion that less differentiated T cells are superior to their more differentiated counterparts in mediating antitumor immunity is not restricted solely to CD8+ T cells but appears to be generalizable to CD4+ T cells as well. Using gene set enrichment analysis based on the gene expression profiles of memory CD8+ T cells which had undergone re-iterative stimulations in vivo using heterologous vaccine constructs60, Th17 cells were found to have a gene expression profile highly enriched in genes associated with primary CD8+ memory T cells.61 By contrast, Th1 cells had an expression profile that was enriched in late memory CD8+ T cells obtained after multiple rounds of in vivo stimulation. Across multiple model systems, adoptively transferred Th17 cells proliferated, persisted, and mediated superior antitumor immunity relative to Th1 cells.61,62,63 In conclusion, findings made in mice, non-human primates, and humans have established a causal inverse relationship between T cell differentiation status and the relative capacities of transferred T cells to engraftment, proliferate, and mediate antitumor immunity. These data strongly support the use of the less differentiated CD62L+ TN, TSCM, and TCM subsets over the CD62L− TEM and TEFF for adoptive immunotherapies.
How should T subsets be isolated for human trials?
Having established in pre-clinical models that younger T cell subsets possess superior traits for adoptive immunotherapy, cellular therapists are now confronted with a practical but critical challenge: generating simple, reproducible, high throughput, and economically feasible means of isolating defined T cell populations under good manufacturing (GMP) conditions for incorporation into human clinical trials. Most approaches to cellular isolation are both conceptually and technically straightforward. Despite this fact, the ability to isolate and expand defined cellular products represents a major regulatory hurdle as evidenced by the fact that only two autologous cell therapies, cultured chonodrocytes (Carticel)64 and a monocyte culture sensitized with the prostate cancer-associated antigen PAP (sipuleucel-T, Provenge)65, have been approved for use by the United States Food and Drug Administration.
Magnetic bead isolation
Magnetic bead isolation has for many years been used for the isolation of defined immune cell populations in animal models66 and increasingly is also being applied for T cell isolation and manipulation protocols in early phase human clinical trials. This system makes use of specific monoclonal antibodies targeting different cell surface associated Ags conjugated to super-paramagentic particles colloquially referred to as beads67 (Figure 3, middle panel). By incubating a bulk population of immune cells with monoclonal antibodies conjugated to beads followed by passage through a magnetic column, T cell populations can be enriched either by directly pulling out labeled cells by positive selection or, alternatively, depleting non-target cells through negative selection. Negative and positive selections can be performed serially to increase the purity of the final cell product. Moreover, with the recent development of reversible antibody conjugated beads, the potential exists to perform serial positive selections, thus allowing for the isolation of rare subsets defined by expression of multiple cell surface markers with high purity68.
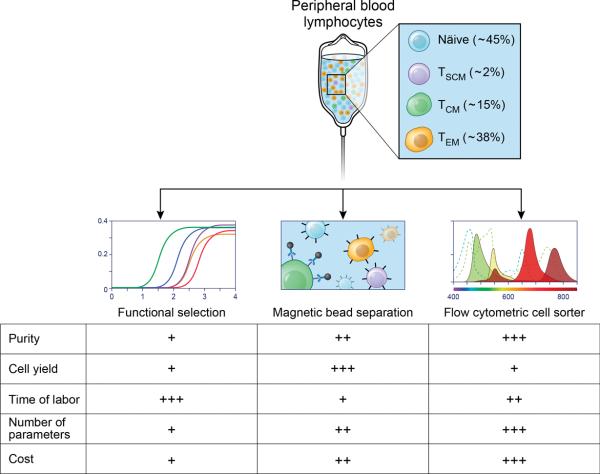
Figure 3. Clinical strategies to isolate specific T cell subsets for adoptive cell transfer.
Peripheral blood lymphocytes can be sorted in specific T cell subsets by employing diverse strategies, including functional isolation by PCR-based screening (left panel), magnetic bead isolation (center panel) and flow cytometric cell-sorting (right panel). Each technique has relative benefits and limitation in terms of cell purity and yield, complexity of the parameters used for isolation, labor and cost which are represented as low (+), intermediate (++) or high (+++).
Using magnetic bead isolation, highly enriched CD8+ T cells have been transferred in melanoma TIL clinical trials69. Similarly, magnetic beads also have been used to isolate CD4+ T cells for transfer to human immunodeficiency virus infected patients70 or patients with hematologic malignancies following allogeneic stem cell transplantation (SCT).71 The isolation and transfer of defined T cell subsets need not be limited simply to the separation of bulk T cell populations based on the expression of either of the CD8 or CD4 co-receptors, however. For example, IL-2Rα (CD25) positive selection has been used alone or in combination with CD8+/CD19+ lymphocyte depletion to isolate and transfer naturally occurring T regulatory cells (Tregs) to patients following allogeneic SCT as graft versus host disease prophylaxis.72,73
More recently, Wang et al. in this issue of the Journal of Immunotherapy and Terakura et al. in Blood have describe optimized protocols for the isolation and genetic modification of human CD8+ TCM under GMP conditions.74 These advances were made possible through the introduction of a new, GMP-quality monoclonal antibody targeting human CD62L. Both of these studies underscore what is currently a limiting factor for magnetic bead isolation strategies: the relative paucity of GMP-quality monoclonal antibodies. It is presently estimated that when all available products from manufacturers are counted together, only a dozen or so GMP-quality antibodies for bead isolation are currently available, including antibodies targeting markers not expressed by T cells. As T cell subsets become defined with increasing precision using multiple cell surface markers, such as the recently described human TSCM population21 rapid translation of promising pre-clinical discoveries will become increasingly bottlenecked by this limitation.
In addition to isolation of T cell subsets based on the expression of surface phenotypic markers, magnetic bead isolation may also be used to isolate Ag-specific T cells. This can be accomplished either directly through the isolation of T cells reactive against HLA-peptide multimer complexes75 or indirectly using a cytokine capture reagent to label and subsequently enrich cytokine-secreting T cells following in vitro stimulation of a bulk population of lymphocytes.76 Proof of principle that labeling of cells using HLA-peptide tetramers followed by enrichment with magnetic beads can be used clinically was demonstrated by Cobbold et al. who successfully isolated and then re-infused CMV-reactive CD8+ T cells.77 Following ACT of cells prepared in this manner, patients in this study had a demonstrable increase in the frequency of CMV-specific T cells in the circulation, exhibited TCR Vβ clonotypic persistence, and contemporaneously exhibited decreases in CMV viral titers. More recently, similar results were obtained when CMV-specific CD8+ T cells were isolated and transferred using GMP-grade reversible binding HLA-peptide streptamers.78
While magnetic bead isolation of HLA-peptide multimer bound T cells allows for the isolation and transfer of Ag-specific T cells, alone this approach does not provide for the selection of specific T cell subsets. The pairing of Ag and subset specificity may be accomplished using cytokine capture. In principle, by pairing capture of cytokines associated with less differentiated CD8+ T cells, such as IL-2, with stimulation with HLA-defined epitopes, one can isolate younger Ag-specific T cells. Pre-clinically, this approach has successfully been used to isolate and expand Ag-specific IFNγ-producing T cells with specificity for adenovirus79,80, cytomegalovirus (CMV)81, Epstein-Bar virus (EBV)82, aspergillus83, as well as Ags associated with certain myeloid and lymphoid leukemias.84 Clinically, the isolation and transfer of viral-specific T cells using cytokine capture has been effective in reducing viral titers in immune-compromised patients with adenovirus85 or CMV86 viremia following SCT. Cytokine capture has also been used to isolate and transfer EBV-specific T cells to patients for the treatment of post-transplant lymphoproliferative disease.87
Preparative FACS-sorting and microfluidic chips
Complimenting magnetic bead isolation strategies are procedures which isolate cells stained for multiple cell surface markers which are carried in a fluidic stream (Figure 3, right panel). These approaches include preparative scale (FACS)-sorting88 and the recently developed use of microelectromechanical systems (MEMS) chips89 in combination with a FACS-based detection system. In both cases, cells can be labeled with multiple markers, allowing for the isolation of well-defined T cell subsets at high purity. FACS-sorting enables the simultaneous use of up to 18 different labels, however this capability comes at the cost of a relatively low throughput and cell yield compared with bead isolation protocols. Moreover, given the limited number of GMP quality antibodies which are currently available, flow- and bead-based isolation protocols suffer from similar limitations with respect to the number of markers which can be used.
Compared with the use of magnetic bead-based purification strategies, the use of preparative FACS-sorting to isolate T cell subsets for clinical trials is relatively underdeveloped. To date, most studies using FACS-sorted T cell populations have been pre-clinical in nature only. For example, preparative scale FACS-sorting with GMP-adaptable reagents has been used to isolate Tregs from healthy volunteers90 and patients with diseases such as type 1 diabetes.91 To date, only one clinical trial has been published using FACS-sorted Tregs. In this study, naturally occurring Tregs, characterized by the surface expression pattern CD4+CD25+CD127−, were isolated by flow-sorting, expanded ex vivo, and subsequently re-infused into patients with GVHD92. Other clinical studies using flow-sorted Tregs are either underway or planned, and results are anticipated in the near future. In principle, MEMS-based sorting has the potential to dramatically improve sorting speeds compared with conventional FACS-sorters while at the same time minimizing the risk of electrically-mediated cell damage. However, this technology remains very nascent and clinical applications using this technology have yet to receive approval by the F.D.A.
Functional isolation using PCR-based screening
Most recently, the ability to isolate Ag-specific T cell clones with defined functional attributes characteristic of different T cell subsets has been demonstrated using a high throughput PCR-based screening method (Figure 3, left panel).93,94 In this approach, microcultures of patient-derived peripheral blood mononuclear cells undergo in vitro sensitization with Ags of interest, such as the shared melanoma/melanocyte Ags MART-1 or gp100, and cells are subsequently screened for their ability to express specific cytokines. Initially this technique was developed with the goal of isolating CD8+ TEFF with a high capacity to express IFNG following stimulation.94 However, based on pre-clinical data demonstrating the superior ability of CD8+ TCM to engraft and mediate antitumor efficacy relative to TEM24, it has subsequently been adapted to select microcultures enriched in IL-2 producing T cells.95 As noted above, the ability to release IL-2 is a functional characteristic of the less differentiated TCM subset.24,14 In this manner, Wang et al. have recently reported the ability to isolate, expand, and transfer rare human melanoma-specific CD8+ TCM cells. Consistent with their ability to express IL2 following stimulation with an MHC class I-restricted epitope, microcultures with a high IL2:IFNG ratio were enriched in Ag-specific CD8+ T cells with a CD45RO+CD62+ TCM phenotype. By contrast, microcultures with a low IL2:IFNG ratio were enriched in Ag-specific TEM cells. Following subsequent expansion and re-infusion, T cell clones generated in this manner engrafted and persisted at high frequencies in four of the five patients 1 month after transfer. Although objective tumor regression was not observed in this cohort of patients, all five patients developed a cutaneous CD8+ T cell infiltration associated with autoimmune dermatitis. Thus, PCR-based functional screening for Ag-specific T cells provides a valuable new tool for the isolation of specific T cell subsets.
How should T cells be expanded to minimize corruption?
In mice50,96 and humans29,31,32, the absolute number of transferred T cells has often (although not universally) been correlated with tumor responses. However, vigorous ex vivo expansion to generate large numbers of T cells inexorably drives T cell differentiation25,97 and a loss of in vivo anti-tumor efficacy.25,38,40,39 For this reason, culture strategies must be developed which do not corrupt the beneficial attributes of the less differentiated T cell subsets after they have been isolated.
For most pre-clinical and ACT trials, the basic manner in which T cells are propagated to therapeutic levels has not significantly changed in more than 20 years. This methodology combines high doses of IL-2 to establish the initial T cell cultures98,99,100 followed by rapid expansion of cells using potent antigenic stimulation using agonistic antibodies against CD3 in conjunction with allogeneic feeder cells.101 However, it is now recognized that the combination of TCR stimulation and strong IL-2 signaling drives cells to become terminally differentiated TEFF with a compromised ability to successfully enter the long-lived memory pool.102,103 Therefore, efforts have been made to explore whether the use of common gamma chain signaling (γc) cytokines besides IL-2 may produce less differentiated and more therapeutically potent anti-tumor T cells.
For example, it has been shown in mice that expansion of naïve CD8+ T cells in the presence of IL-15 generates cells with the phenotypic104,105,106, functional104,105, and metabolic properties107 of naturally occurring TCM cells. Accordingly, when IL-15 expanded TCM-like cells were transferred into tumor-bearing hosts, they exhibited superior proliferative and antitumor responses compared with IL-2 expanded cells which possessed a TEM-like phenotype (Figure 1).105 These findings were recently extended to human patients where IL-15 was combined with a novel artificial antigen presenting cell to generate a polyclonal population of tumor-reactive T cells by in vitro sensitization of peripheral lymphocytes.108 T cells expanded using this system exhibited a TCM phenotype and demonstrated clonotypic persistence and the ability to mediate objective clinical responses following cell transfer.109 Likewise, when the alternative γc cytokine IL-21 is used to expand tumor-reactive CD8+ T cells, the resultant population retains a minimally differentiated phenotypic and functional profile110,111,112,113. In mice, IL-21 expanded tumor-reactive T cells demonstrate augmented proliferative and antitumor capacities relative to cells expanded in either IL-2 or IL-15.110
Complementing the use of alternative γc cytokines, the ability of small molecule modulators of key metabolic and developmental pathways to restrict T cell differentiation has also been evaluated114. It is increasingly being recognized that a T cell's commitment between memory and effector cell fates is governed by transitions between different metabolic states.15,55 These states, in turn, are dictated by inputs from multiple sources, including the TCR, co-stimulatory, and cytokine receptors. Signals from these receptors tend to converge at common developmental and differentiation signal transduction pathways, such as the PI3K-AKT-mTOR and Wnt-β-catenin pathways.4,115 As such, modulation of these pathways can potentially withhold the acquisition of full effector function and maintain T cells in a less differentiated memory-like state. Inhibition of the mTOR pathway using the inhibitor rapamycin, for example, limits the acquisition of key effector associated TFs such as Tbet and promotes memory CD8+ T cell formation.116 Upon ACT, rapamycin-sensitized CD8+ T cells exhibited superior antitumor functions compared with control cells. Similarly, it has also been shown that promotion of the canonical Wnt-β-catenin pathway using the GSK3β inhibitor TWS119 or recombinant Wnt proteins promotes the formation of TSCM and TCM CD8+ T cells while limiting the formation of TEM cells.20 Upon ACT, TWS119 expanded CD8+ T cells are significantly better at mediating tumor regression compared with cells expanded in a vehicle control. In contrast with both mTOR and GSK3β inhibitors, which both limit T cell proliferation while also restraining T cell differentiation, inhibition of the AKT isoforms AKT1 and AKT2 has recently been shown to promote the formation of TCM-like cells without limiting the yield of cells.117 Future experiments will need to be conducted to determine whether direct inhibition of the AKT pathway can successfully uncouple the processes of T cell expansion from cellular differentiation in the generation of therapeutic T cells.
In conclusion, it is now clear that the standard means of expanding T cells to therapeutic levels, namely the use of high dose IL-2 and strong antigenic stimulation, can corrupt the favorable phenotypic and functional attributes of the differentiated T cells. For this reason, the use of alternative γc cytokines alone or in combination small molecules which modulate critical signal transduction pathways will need to be adopted to preserve the benefits of the isolated younger subsets.
Is it necessary to separate younger cells from their mature counterparts?
The ability of subsets of lymphocytes to interact with one another to influence their differentiation status or effector functions has been well characterized. For example, it has been shown that effector and memory CD8+ T cells can kill Ag-bearing dendritic cells, thereby indirectly restricting the differentiation of naïve CD8+ T cells by removing their ability to be primed.118 T cells may also directly regulate the differentiation of other lymphocytes either in a positive or negative direction. The ability of CD4+ Tregs to suppress the differentiation and execution of effector functions by both TEFF CD4+ and CD8+ T cells has been well characterized.119,120,121 Conversely, the ability of T cells to augment the differentiation and effector functions of B cells122 or other T cells123 via the super TNFα-family member CD40L has also been shown. However, whether naïve and memory CD8+ T cell subsets can physically interact with one another to influence one another's differentiation status and ultimately antitumor function remains unknown.124 Resolution of this question remains a critical priority. If older T cell subsets do not have a deleterious impact on the antitumor function of the less differentiated subsets, the need for T cell subset isolation would be less of an imperative than generating strategies to expand and transfer large numbers of cells which contain at least some younger populations. If, on the other hand, older T cells corrupt the beneficial functional attributes of the younger T cell subsets, efficient methods of large scale subset isolation would become a requisite.
Conclusions
It is now clear from relevant pre-clinical animal models and detailed retrospective analyses of human clinical trials that infusion of the less differentiated TN, TSCM and TCM subsets is associated with superior T cell engraftment, persistence, and antitumor immunity compared with TEM and TEFF cells. With the recent advent of genetic engineering technology and the ability to reliably confer tumor-Ag reactivity with high efficiency125, it is now possible to generate tumor-reactive CD8+ T cells of any memory subset. In order to facilitate clinical experiments in humans where the functional characteristics of the input population of T cells, beyond tumor-reactivity, can be carefully tested and dissected, robust isolation strategies which efficiently and reliably purify desired T cell subsets will be required. Concurrently, modification of culture conditions in order to prevent the corruption of isolated younger T cell subsets during ex vivo expansion must be routinely introduced into practice. Finally, the question of whether younger and older T cell subsets may interact with one another in a way that influences their differentiation status and ultimately antitumor function must be resolved. Accomplishing these goals will allow for the development of the next generation of highly effective and potentially curative T cell therapies for the treatment of cancer.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts in regards to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;4:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turtle CJ, Hudecek M, Jensen MC, et al. Engineered T cells for anti-cancer therapy. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;6:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012 doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;12:2462–2465. [PubMed] [Google Scholar]
- 6.Sellar RS, Peggs KS. Therapeutic strategies for the prevention and treatment of cytomegalovirus infection. Expert Opin Biol Ther. 2012;9:1161–1172. doi: 10.1517/14712598.2012.693471. [DOI] [PubMed] [Google Scholar]
- 7.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9:510–519. doi: 10.1038/nrclinonc.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DELORME EJ, ALEXANDER P. TREATMENT OF PRIMARY FIBROSARCOMA IN THE RAT WITH IMMUNE LYMPHOCYTES. Lancet. 1964;7351:117–120. doi: 10.1016/s0140-6736(64)90126-6. [DOI] [PubMed] [Google Scholar]
- 9.Cheever MA, Kempf RA, Fefer A. Tumor neutralization, immunotherapy, and chemoimmmunotherapy of a Friend leukemia with cells secondarily sensitized in vitro. J Immunol. 1977;2:714–718. [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;10:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klebanoff CA, Khong HT, Antony PA, et al. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;2:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;32:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;13:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;1:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;4:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay PK, Price DA, Harper TF, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;8:972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 18.Newell EW, Sigal N, Bendall SC, et al. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;1:142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Joe G, Hexner E, et al. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;12:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 20.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;7:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;10:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;6754:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 23.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;3:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 24.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;27:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;6:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmann MF, Wolint P, Schwarz K, et al. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;7:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 27.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;1:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fritsch RD, Shen X, Sims GP, et al. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;10:6489–6497. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzentruber DJ, Hom SS, Dadmarz R, et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994;7:1475–1483. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;15:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 31.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;9:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 32.Itzhaki O, Hovav E, Ziporen Y, et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;2:212–220. doi: 10.1097/CJI.0b013e318209c94c. [DOI] [PubMed] [Google Scholar]
- 33.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;12:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Shen X, Huang J, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;10:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Kerstann KW, Ahmadzadeh M, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;12:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;23:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;11:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;4:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;25:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudley ME, Wunderlich JR, Yang JC, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;3:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapuis AG, Thompson JA, Margolin KA, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci U S A. 2012;12:4592–4597. doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.June C, Rosenberg SA, Sadelain M, et al. T-cell therapy at the threshold. Nat Biotechnol. 2012;7:611–614. doi: 10.1038/nbt.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner MK, Heslop HE. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2010;2:251–257. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu R, Forget MA, Chacon J, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;2:160–175. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;4:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;5647:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 47.Ji Y, Pos Z, Rao M, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang CY, Best JA, Knell J, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henson SM, Franzese O, Macaulay R, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;26:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 50.Klebanoff CA, Gattinoni L, Palmer DC, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;16:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Berger C, Wong CW, et al. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;6:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;1:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flatz L, Roychoudhuri R, Honda M, et al. Single-cell gene-expression profiling reveals qualitatively distinct CD8 T cells elicited by different gene-based vaccines. Proc Natl Acad Sci U S A. 2011;14:5724–5729. doi: 10.1073/pnas.1013084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuddapah S, Barski A, Zhao K. Epigenomics of T cell activation, differentiation, and memory. Curr Opin Immunol. 2010;3:341–347. doi: 10.1016/j.coi.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;10:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 56.Hinrichs CS, Borman ZA, Gattinoni L, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;3:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasi M, Troiano L, Lugli E, et al. Thymic output and functionality of the IL-7/IL-7 receptor system in centenarians: implications for the neolymphogenesis at the limit of human life. Aging Cell. 2006;2:167–175. doi: 10.1111/j.1474-9726.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 58.Weng NP, Levine BL, June CH, et al. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;24:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;41:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wirth TC, Xue HH, Rai D, et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;1:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muranski P, Borman ZA, Kerkar SP, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;6:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;5:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kryczek I, Zhao E, Liu Y, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;104:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterson L, Minas T, Brittberg M, et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 65.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;5:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 66.Battye FL, Shortman K. Flow cytometry and cell-separation procedures. Curr Opin Immunol. 1991;2:238–241. doi: 10.1016/0952-7915(91)90058-9. [DOI] [PubMed] [Google Scholar]
- 67.Miltenyi S, Muller W, Weichel W, et al. High gradient magnetic cell separation with MACS. Cytometry. 1990;2:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 68.Stemberger C, Dreher S, Tschulik C, et al. Novel serial positive enrichment technology enables clinical multiparameter cell sorting. PLoS One. 2012;4:e35798. doi: 10.1371/journal.pone.0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dudley ME, Gross CA, Langhan MM, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;24:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levine BL, Bernstein WB, Aronson NE, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;1:47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 71.Fowler DH, Odom J, Steinberg SM, et al. Phase I clinical trial of costimulated, IL-4 polarized donor CD4+ T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;11:1150–1160. doi: 10.1016/j.bbmt.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;3:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di IM, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;14:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 74.Terakura S, Yamamoto TN, Gardner RA, et al. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;1:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keenan RD, Ainsworth J, Khan N, et al. Purification of cytomegalovirus-specific CD8 T cells from peripheral blood using HLA-peptide tetramers. Br J Haematol. 2001;2:428–434. doi: 10.1046/j.1365-2141.2001.03106.x. [DOI] [PubMed] [Google Scholar]
- 76.Brosterhus H, Brings S, Leyendeckers H, et al. Enrichment and detection of live antigen-specific CD4(+) and CD8(+) T cells based on cytokine secretion. Eur J Immunol. 1999;12:4053–4059. doi: 10.1002/(SICI)1521-4141(199912)29:12<4053::AID-IMMU4053>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 77.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;3:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmitt A, Tonn T, Busch DH, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;3:591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 79.Feuchtinger T, Lang P, Hamprecht K, et al. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp Hematol. 2004;3:282–289. doi: 10.1016/j.exphem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 80.Feuchtinger T, Richard C, Joachim S, et al. Clinical grade generation of hexon-specific T cells for adoptive T-cell transfer as a treatment of adenovirus infection after allogeneic stem cell transplantation. J Immunother. 2008;2:199–206. doi: 10.1097/CJI.0b013e31815ef862. [DOI] [PubMed] [Google Scholar]
- 81.Rauser G, Einsele H, Sinzger C, et al. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood. 2004;9:3565–3572. doi: 10.1182/blood-2003-09-3056. [DOI] [PubMed] [Google Scholar]
- 82.Hammer MH, Brestrich G, Mittenzweig A, et al. Generation of EBV-specific T cells for adoptive immunotherapy: a novel protocol using formalin-fixed stimulator cells to increase biosafety. J Immunother. 2007;8:817–824. doi: 10.1097/CJI.0b013e318155a11c. [DOI] [PubMed] [Google Scholar]
- 83.Beck O, Topp MS, Koehl U, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;6:2562–2569. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- 84.Jedema I, Meij P, Steeneveld E, et al. Early detection and rapid isolation of leukemia-reactive donor T cells for adoptive transfer using the IFN-gamma secretion assay. Clin Cancer Res. 2007;2(Pt 1):636–643. doi: 10.1158/1078-0432.CCR-06-2093. [DOI] [PubMed] [Google Scholar]
- 85.Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;1:64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 86.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;20:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 87.Moosmann A, Bigalke I, Tischer J, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;14:2960–2970. doi: 10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- 88.Basu S, Campbell HM, Dittel BN, et al. Purification of specific cell population by fluorescence activated cell sorting (FACS) J Vis Exp. 2010;41 doi: 10.3791/1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shoji S, Kawai K. Flow control methods and devices in micrometer scale channels. Top Curr Chem. 2011:1–25. doi: 10.1007/128_2011_146. [DOI] [PubMed] [Google Scholar]
- 90.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;83:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Putnam AL, Brusko TM, Lee MR, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;3:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+C. Clin Immunol. 2009;1:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Kammula US, Lee KH, Riker AI, et al. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;12:6867–6875. [PubMed] [Google Scholar]
- 94.Kammula US, Serrano OK. Use of high throughput qPCR screening to rapidly clone low frequency tumour specific T-cells from peripheral blood for adoptive immunotherapy. J Transl Med. 2008;60 doi: 10.1186/1479-5876-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang A, Chandran S, Shah SA, et al. The Stoichiometric Production of IL-2 and IFN-gamma mRNA Defines Memory T Cells That Can Self-Renew After Adoptive Transfer in Humans. Sci Transl Med. 2012;149:149ra120. doi: 10.1126/scitranslmed.3004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Witte MA, Jorritsma A, Kaiser A, et al. Requirements for effective antitumor responses of TCR transduced T cells. J Immunol. 2008;7:5128–5136. doi: 10.4049/jimmunol.181.7.5128. [DOI] [PubMed] [Google Scholar]
- 97.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;8:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eberlein TJ, Rosenstein M, Rosenberg SA. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med. 1982;2:385–397. doi: 10.1084/jem.156.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Topalian SL, Muul LM, Solomon D, et al. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987;1:127–141. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 100.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;25:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 101.Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;5067:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 102.Kalia V, Sarkar S, Subramaniam S, et al. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;1:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 103.Pipkin ME, Sacks JA, Cruz-Guilloty F, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;1:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weninger W, Crowley MA, Manjunath N, et al. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;7:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;7:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sung JH, Zhang H, Moseman EA, et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;6:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van der Windt GJ, Everts B, Chang CH, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;1:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Butler MO, Lee JS, Ansen S, et al. Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin Cancer Res. 2007;6:1857–1867. doi: 10.1158/1078-0432.CCR-06-1905. [DOI] [PubMed] [Google Scholar]
- 109.Butler MO, Friedlander P, Milstein MI, et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Transl Med. 2011;80:80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;11:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;4:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;1:229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Albrecht J, Frey M, Teschner D, et al. IL-21-treated naive CD45RA+ CD8+ T cells represent a reliable source for producing leukemia-reactive cytotoxic T lymphocytes with high proliferative potential and early differentiation phenotype. Cancer Immunol Immunother. 2011;2:235–248. doi: 10.1007/s00262-010-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med. 2009;11:11ps12. doi: 10.1126/scitranslmed.3000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;19:4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rao RR, Li Q, Odunsi K, et al. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;1:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Macintyre AN, Finlay D, Preston G, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;2:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guarda G, Hons M, Soriano SF, et al. L-selectin-negative. Nat Immunol. 2007;7:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;12:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 120.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;2:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;5:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 123.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;5589:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 124.Linnemann C, Schumacher TN, Bendle GM. T-cell receptor gene therapy: critical parameters for clinical success. J Invest Dermatol. 2011;9:1806–1816. doi: 10.1038/jid.2011.160. [DOI] [PubMed] [Google Scholar]
- 125.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;5796:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]