Abstract
In the past decade, substantial progress has been made in understanding how Src family kinases regulate the formation and function of invadosomes. Invadosomes are organized actin-rich structures that contain an F-actin core surrounded by an adhesive ring and mediate invasive migration. Src kinases orchestrate, either directly or indirectly, each phase of the invadosome life cycle including invadosome assembly, maturation and matrix degradation and disassembly. Complex arrays of Src effector proteins are involved at different stages of invadosome maturation and their spatiotemporal activity must be tightly regulated to achieve effective invasive migration. In this review, we highlight some recent progress and the challenges of understanding how Src is regulated temporally and spatially to orchestrate the dynamics of invadosomes and mediate cell invasion.
Keywords: Src, invasion, invadosome, invadopodia, podosome, rosette, Rho, Cdc42, FAK, Cortactin, mAbp1, ROS, MT1-MMP
Introduction
Invadosomes are highly dynamic, actin-rich, protrusive structures that promote adhesion to and degradation of the extracellular matrix (ECM), facilitating invasive cell migration. The collective term invadosomes includes podosomes that form in macrophages, dendritic cells, osteoclasts and endothelial cells, and invadopodia that are associated with cancer cells (Saltel et al., 2011). Invadosomes are generally composed of an actin-rich core with actin-nucleating components including cortactin, N-WASP and Arp2/3, surrounded by a ring of adhesion and adaptor proteins such as vinculin, paxillin, and integrins. These protrusive structures promote localized secretion of degradative enzymes including matrix metalloproteinases (MMPs) and can exist independently as dot-like structures or they can be arranged into complex metastructures such as clusters and rosettes (Figure 1). In osteoclasts, podosomes can mature further into a sealing belt that forms a cavity to mediate bone degradation and resorption (Jurdic et al., 2006). The dynamic formation, disassembly and degradation activity of both podosomes and invadopodia have been implicated in invasive cell migration (Linder et al., 2011; Pan et al., 2011; Badowski et al., 2008; Calle et al., 2006; Varon et al., 2006; Seals et al., 2005).
Figure 1. Podosomes and invadopodia from different cell types.
(A) Actin and cortactin co-localize at invadopodia in human MDA-231-MD breast cancer cells. (B) Vinculin forms a ring around the actin cores of podosomes in primary human macrophages. (C) NIH 3T3 cells transformed with constitutively active c-Src527F form both dot (arrowhead) and rosette podosomes (inset). Magnified views of podosomes or invadopodia are shown within insets.
Cell migration and invasion are necessary for a variety of physiological functions including leukocyte trafficking, development, and wound repair. Defective podosome formation can be seen in inherited disorders including Wiskott Aldrich syndrome (Linder et al., 1999; Nusblat et al., 2011), PAPA syndrome (Cortesio et al., 2010), and potentially Frank-Ter-Haar syndrome (Iqbal et al., 2010; Buschman et al., 2009), while defects in osteoclast podosomes are associated with osteopetrosis (Gil-Henn et al., 2007). Moreover, cancer invasion and metastasis have been associated with the formation of dynamic, actin rich invadopodia with the capacity for matrix degradation both in vitro and in vivo (Eckert et al., 2011; Gertler and Condeelis, 2010; Philippar et al., 2008; Packard et al., 2009). Although podosomes and invadopodia are important during invasive migration, it has been suggested that podosome rosettes of smooth muscle cells, vascular endothelial cells, aortic endothelial cells, or fibroblasts may also function in ECM remodeling (Daubon et al., 2011; Rottiers et al., 2009), mechanosensing (Collin et al., 2008) and adhesion to the ECM (Boateng et al., 2012; Kocher et al., 2009; Collin et al., 2006).
Podosomes and invadopodia are highly dynamic and require tight regulation to control their rapid formation and turnover. In contrast to other adhesion structures like focal adhesions, podosomes and invadopodia are primary sites of rapid actin polymerization and are not associated with stabilized actin filament bundles (Destaing et al., 2003; Ochoa et al., 2000). Invadosome cores contain signaling molecules such as Rho GTPases (Bravo-Cordero et al., 2011) and Src family kinases (Gavazzi et al., 1989), as well as actin regulatory proteins including cortactin (Bowden et al., 1999), WASP (Linder et al., 1999), and the actin nucleating Arp 2/3 complex (Yamaguchi et al., 2005). Other components generally concentrate in the surrounding ring structure including integrins and integrin-associated proteins like vinculin, talin and paxillin (Gavazzi et al., 1989; Marchisio et al., 1988; Linder and Aepfelbacher, 2003). As highly dynamic structures, invadosomes can assemble and disassemble within minutes, but in some cases can stabilize and exist for hours.
The invadosome lifetime is divided into stages including assembly, maturation and disassembly (reviewed by Murphy and Courtneidge, 2011). During invadosome precursor formation, signaling proteins such as transmembrane growth factor receptors (Rottiers et al., 2009; Varon et al., 2006) and/or cytoplasmic kinases, Src and protein kinase C (PKC) (Tatin et al., 2006; Gatesman et al., 2004), organize with structural and adaptor proteins including Tks5, Nck1, and cortactin (Gatesman et al., 2004; Stylii et al., 2009; Oser et al., 2009; Crimaldi et al., 2009; Oser et al., 2010) to recruit the Arp2/3 complex and mediate actin polymerization (Yamaguchi et al, 2005). Under some conditions, actin may be organized into metastructures such as clusters and rosettes. Next, the maturation stage includes protrusion mediated by actin bundling or cross-linking proteins (Li et al., 2010; Guiet et al., 2012) and microtubules (Schoumacher et al., 2010), stabilization of actin filaments through cortactin (Oser et al., 2009) and secretion or localization of proteases for ECM degradation (Clark et al., 2007; Chen and Wang, 1999; Nakahara, 2007). Finally, during disassembly, the actin core is dismantled and invadosome components disassociate (Badowski et al., 2008; Cortesio et al., 2008). Understanding the signaling mechanisms and functional components of invadosome formation and turnover has been a key focus for invadosome research and has implications to developing drug targets that control cell invasion.
A major candidate therapeutic target is the non-receptor tyrosine kinase, Src (Wadhawan et al., 2011). Src kinase, often referred to as “the oldest oncogene”, has received considerable attention due to its role in cell transformation and cancer cell invasion. v-Src was initially discovered as the transforming agent of the rous sarcoma virus (David-Pfeuty and Singer, 1980; Tarone et al., 1985), and its cellular counterpart, c-Src, have been the focus of intensive investigation in cancer research. Src is over-expressed or constitutively active in many cancers including breast (Biscardi et al., 1998; Ottenhoff-Kalff et al., 1992), prostate (Nam et al., 2005), and colon cancer (Cartwright et al., 1989; Talamonti et al., 1993), and plays an integral role in regulating each stage of the formation and turnover of invadosomes by targeting distinct substrates. The Src family kinases (SFKs) are composed of nine members: Src, Yes, Fyn, Fgr, Yrk, Hck, Lck, Lyn and Blk (Martin, 2001), with Src, Fyn, and Yes being ubiquitously expressed in non-hematopoietic cells. Src is a non-receptor tyrosine kinase and its mechanism of activation has been well studied over the past several decades (Martin, 2001; Sicheri et al., 1997; Xu et al., 1997; Yeatman, 2004). At the amino terminus, Src has an SH3 and SH2 domain that mediate protein-protein interactions, followed by a linker region and a kinase domain at the C-terminus. During its inactive state, Src is phosphorylated at Y527, which maintains inhibitory intramolecular interactions. When active, the SH2 and SH3 domains are released to initiate intermolecular interactions, and the kinase domain autophosphorylates tyrosine 416 in the activation loop of the catalytic domain for full activity (Kmiecik et al., 1988).
Src can be regulated by kinases and phosphatases, or protein-protein interactions with its SH2 and SH3 domains. Negative regulators of Src kinase activity include the non-receptor C-terminal Src kinase, Csk (Ia et al., 2010; Okada and Nakagawa, 1989) and the Csk homologous kinase, Chk (Zrihan-Licht et al., 1997). Csk and Chk phosphorylate Src at Y527 to induce Src folding and autoinhibition. Conversely, phosphatases that activate Src by dephosphorylation of the C-terminal phosphotyrosine, including PTPα (Zheng et al., 1992), SHP-1 (Somani et al., 1997), SHP-2 (Hakak et al., 2000), PTP1B (Bjorge et al., 2000; Cortesio et al., 2008), and PTP-PEST(Chellaiah and Schaller, 2009), release the autoinhibitory configuration of Src, thereby leading to its activation. Both PTP1B and PTP-PEST regulate Src activity at invadopodia and podosomes, respectively (Cortesio et al., 2008; Chellaiah and Schaller, 2009). PTP1B regulates Src phosphorylation at the C-terminal tyrosine during invadopodia formation and proteolysis and activation of PTP1B by calpain-2 can amplify Src activity during invadopodia assembly (Cortesio et al., 2008). PTP-PEST localizes to osteoclast podosomes (Chellaiah et al., 2001) and is important for the control of rosette formation in Src-transformed fibroblasts (Diaz et al., 2009); however, how PTP-PEST regulates Src activity at podosomes is not clear.
In this review, we focus on the challenges of understanding how Src participates in different steps during the invadosome lifecycle, and further, how Src activity can be involved in opposing functions, including roles in assembly and disassembly of invadosomes. Despite substantial progress in understanding the role of Src kinases at invadosomes, fundamental questions remain unanswered. What is the switch that determines the change between Src-induced invadosome formation and disassembly? How does Src target multiple different substrates to mediate invadosome formation, maturation and disassembly? How is Src activity regulated temporally and spatially to control invadosome dynamics and invasion? Here, we focus on several recent examples from the literature that highlight the challenges of understanding the role of Src at invadosomes. Specifically, we discuss mechanisms that regulate the spatiotemporal control of Src activity at invadosomes and how Src phosphorylation of different substrates can orchestrate distinct stages of the invadosome lifecycle. For additional reviews on Src or invadosome regulation we refer the readers to other sources (Destaing et al., 2011; Frame, 2004; Murphy and Courtneidge, 2011).
Spatiotemporal regulation of Src localization and activity at invadosomes
The temporal and spatial localization and activation of Src at podosomes and invadopodia remains poorly understood. However, there has been substantial progress in understanding Src localization to endosomes and in identifying mechanisms that control Src targeting to other related adhesion structures such as focal adhesions. Src localizes to the perinuclear region where it is generally inactive (Rohrschneider, 1979; Welham and Wyke, 1988; Sandilands et al., 2004) and mutation or dephosphorylation of tyrosine 527 can induce the translocation of Src to focal adhesions independent of its catalytic activity (Kaplan et al., 1994; Timpson et al., 2001). Different members of the Rho GTPase family direct the localization of c-Src to distinct intracellular structures. For example, RhoA directs Src targeting to focal adhesions, Rac1 mediates Src localization at lamellipodia, and Cdc42 induces Src translocation to filopodia in Swiss 3T3 fibroblasts (Timpson et al., 2001). By contrast, the mechanisms that direct Src localization to podosomes or invadopodia remain largely unknown.
Trafficking of Src-associated endosomes to focal adhesions at the cell periphery is dependent on a functional actin cytoskeleton, as well as Rho GTPase signaling (Fincham et al., 1996; Timpson et al., 2001; Sandilands et al., 2004). It is likely that Src trafficking to invadosomes is also dependent on the actin cytoskeleton, however the mechanism of endosome delivery to invadosomes may differ, since, in contrast to focal adhesions, stress fiber formation is often disrupted during podosome or invadopodia formation (Berdeaux et al., 2004; Badowski et al., 2008; Albiges-Rizo et al., 2009). Therefore, it is possible that the dynamic actin “cloud” or “comet tail” polymerization associated with RhoB-containing endosomes may provide a mechanism for the transport of Src to invadosomes (Sandilands et al., 2004). In addition to trafficking of Src from the perinuclear region or cytoplasm to the cell periphery during invadosome formation, Src activity must also be tightly regulated at invadosomes. At the onset of invadosome formation, exogenous factors, including the growth factors EGF, TGF-β and PDGF, lead to Src activation (Kypta et al., 1990; Stover et al., 1995) and induce the formation of podosomes or invadopodia to facilitate invasive migration (Eckert et al., 2011; Mader et al., 2011;Luttrell et al., 1994; Marcotte et al., 2009; Varon et al., 2006). Integrin-mediated adhesion can also activate Src at invadosomes (Destaing et al., 2011).
The importance of having tight regulation of the temporal and spatial activation of Src at invadosomes is highlighted by its complex role during different stages of the invadosome lifecycle including formation, degradation and disassembly. For example, v-Src induces the formation of invadopodia precursors but their lifetimes decrease (Oser et al., 2009), indicating the essential role for Src activity in invadopodia formation as well as their disassembly. Accordingly, v-Src expression enhanced overall matrix degradation, but the amount of degradation at individual invadopodia precursors decreased (Oser et al., 2009). Moreover, expression of constitutively active Src in SYF−/− fibroblast cells (c-Src, Yes, and Fyn null cells) is sufficient for invadopodia formation, but not for their maturation and degradation activity (Kelley et al., 2010). Expression of wild type c-Src was sufficient to rescue the defect in invadosome maturation in these cells, supporting the idea that the temporal and spatial activation of endogenous c-Src is critical for distinct stages of the invadosome lifecycle. The challenge is to understand how Src activity is regulated during invadosome formation, maturation and disassembly. Surprisingly few studies have directly addressed how endogenous Src activity is regulated at invadosomes. In large part, progress has been limited due to the lack of tools to reliably detect changes in endogenous Src activity temporally and spatially, but with the development of new biosensors it is anticipated that there will be rapid progress in this area (Gulyani et al., 2011; Welman et al., 2010).
Redox regulation of Src family kinases at invadosomes
In addition to phosphorylation and SH2/SH3 domain interactions, Src family kinases can be regulated by oxidation of specific cysteine residues by reactive oxygen species (ROS), such as hydrogen peroxide (Giannoni et al., 2005; Kemble and Sun, 2009; Yoo et al., 2011). Moreover, recent studies suggest that ROS are generated at invadopodia and NADPH oxidases and ROS are necessary for invadopodia formation (Diaz et al., 2009; Gianni et al., 2010). It is intriguing to speculate that ROS mediate invadosome assembly through the localized activation of Src kinases. Interestingly, the invadosome component Tks5 associates directly with components of the NADPH oxidase complex, including p22phox and promotes its activity (Diaz et al., 2009), providing a potential positive feedback mechanism through ROS, Src and Tks5 that could amplify signaling to mediate invadopodia assembly.
In addition to Src, the serine kinase PKC can be activated by ROS (Gopalakrishna and Anderson, 1989; Gopalakrishna and Anderson, 1991; Gopalakrishna et al., 1995; Wu et al., 2006) and substantial evidence supports the idea that PKC and Src function coordinately to regulate invadosomes (Burger et al., 2011; Li et al., 2010; Wang et al., 2010; Tatin et al., 2006; Gatesman et al., 2004). For example, podosome formation is induced in THP-1 macrophage cells and endothelial cells by PKC-activating PMA treatment, and inhibition of PKC or Src ablates podosome formation (Burger et al., 2011; Wang et al., 2010; Tatin et al., 2006). Further, the interactions between PKC and Src at podosomes may be orchestrated through the adaptor protein, AFAP110, which contains binding domains for both PKC and Src (Guappone et al., 1998; Qian et al., 2002). In support of this idea, AFAP110-deficient cancer cells have impaired Src activation and podosome formation induced by PKC-activating phorbol myristate acetate treatment. By contrast, ectopic expression of an active AFAP-110 enables Src activation and podosome formation (Gatesman et al., 2004). ROS can activate PKC by oxidation of several cysteine residues in the regulatory domain, causing the release of the closed auto-inhibitory conformation of PKC, while oxidation of cysteine residues in the catalytic domain may have an inhibitory effect on enzyme activity (reviewed by Gopalakrishna and Jaken, 2000). The mechanism of PKC function at invadosomes remains unclear, however phosphorylation of fascin at S39 (a PKC site), is required for its actin bundling activity (Yamakita et al., 1996) as well as invadopodia formation and degradation activity in melanoma cells (Li et al., 2010). These reports suggest that redox signaling may play a key role in regulating PKC as well as Src at invadosomes.
In addition to Src or PKC, redox signaling also regulates MMPs (Rajagopalan et al., 1996; Brenneisen et al., 1997; Yoon et al., 2002; Grote et al., 2003), and Rho GTPases (Lander et al., 1995; Savitsky and Finkel, 2002; Heo and Campbell, 2005; Heo et al., 2006) that regulate invadopodia function; however, these mechanisms are less well characterized. ROS can also amplify phospho-tyrosine signals (Denu and Tanner, 1998; Terada, 2006) at invadosomes by inhibiting phosphatases including PTP1B (Chen et al., 2008; Lee et al., 1998), SHP-2 (Meng et al., 2002), and PTP-PEST (Wu et al., 2005; Diaz et al., 2009). While the majority of evidence points to a positive feedback loop between Src and ROS, oxidation of some substrates can reduce Src activity. For example, PTP1B inhibition by ROS (Bogeski et al., 2006) could provide negative feedback to Src and invadopodia formation. A key challenge is to understand how Src and ROS cooperate to regulate invadosomes and how this feedback loop is regulated during different stages of the invadosome life cycle. Phosphatases that regulate Src activity, such as PTP1B and PTP-PEST, are well-positioned to modulate this feedback loop due to their regulation by redox signaling, and thus represent intriguing targets for future studies.
Focal adhesion kinase (FAK): a key spatiotemporal Src regulator
Src activity is necessary for the formation of invadopodia, podosomes and organized rosette structures (Ammer et al., 2009; Pichot et al., 2009; Destaing et al., 2008; Varon et al., 2006), but not for the formation of focal adhesions (McLean et al., 2000; Volberg et al., 2001; Hamadi et al., 2005). On the other hand, Src activity has been implicated in the disassembly and turnover of both focal adhesions and invadosomes (Fincham and Frame, 1998; McLean et al., 2000; Frame et al., 2002; Hamadi et al., 2005). Several lines of evidence suggest that FAK plays a key role in regulating Src activity at both focal adhesions and invadosomes. Integrin-mediated adhesion to the ECM induces the activation of FAK and Src signaling at focal adhesions (Guan et al., 1991; Kornberg et al., 1992; Arias-Salgado et al., 2003). Adhesion induces FAK autophosphorylation on tyrosine 397, which recruits Src to focal adhesions (Schaller et al., 1994). Subsequent Src-mediated phosphorylation of FAK at Y576/577 renders FAK fully active and the FAK-Src complex phosphorylates multiple scaffolding and signaling proteins at focal adhesions that are involved in adhesion dynamics and motility (Huttenlocher and Horwitz, 2011; Mitra and Schlaepfer, 2006; Playford and Schaller, 2004).
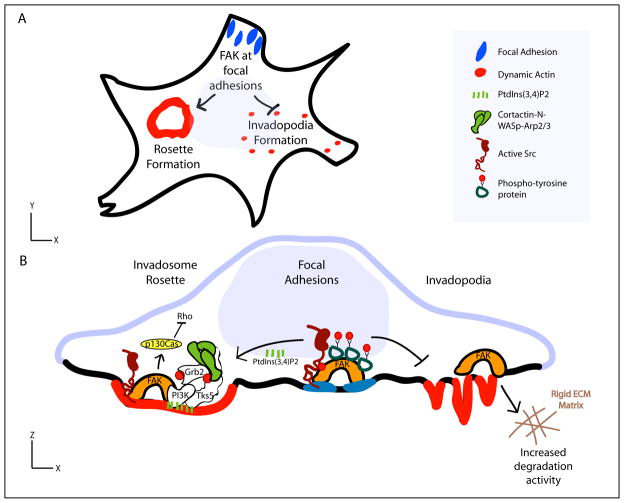
However, how FAK and Src are regulated temporally and spatially to modulate invadopodia or podosomes remains unclear. The SH2 and SH3 domains are important for the localization of Src at adhesions (Destaing et al., 2008), suggesting that specific binding partners, like FAK, may regulate Src localization and activity at invadosomes. Interestingly, in some breast cancer cells, FAK plays an inhibitory role in invadopodia formation by binding and sequestering Src activity at focal adhesions (Figure 2A,B; Chan et al., 2009). Depletion of FAK releases Src from focal adhesions and switches phospho-tyrosine containing proteins from focal adhesions to invadopodia leading to the formation of more invadopodia (Chan et al., 2009). In accordance with an inhibitory role for FAK in invadopodia, α3β1 integrin/laminin-332 sequesters FAK-Src activity at focal adhesions, and FAK inhibition or laminin-332 knockdown enhances invadopodia formation (Liu et al., 2010). These studies suggest that FAK-Src activity is sequestered at focal adhesions and loss of FAK results in redistribution of Src and phospho-tyrosine containing proteins to invadopodia resulting in invadopodia formation. In colon cancer cells and Src-transformed fibroblasts, like breast cancer cells, FAK is not required for invadopodia or dot podosome formation, respectively (Vitale et al., 2008; Pan et al., 2011). It is important to note that although invadopodia produced in FAK-deficient cells are functional, FAK depletion impairs invasive migration of breast cancer cells due to the requirement of FAK for cell migration (Chan et al., 2009). This is in agreement with many studies that have supported a key role for FAK in invasive migration (Hauck et al., 2002; Alexander et al., 2008). However, FAK localization and function at invadopodia seems to be dependent on the mechanical properties of the matrix, since FAK is activated at invadopodia and enhances invadopodia degradation activity on rigid matrices (Figure 2B; Alexander et al., 2008).
Figure 2. FAK regulation of Src activity.
(A) Schematic of FAK regulation of invadosome formation in cells that form both focal adhesions and invadosomes. FAK sequesters Src and phosphotyrosine proteins at focal adhesions to limit invadopodia formation. (B) At focal adhesions, FAK enhances the formation of rosettes. Phosphoinositides and FAK recruit Tks5 and Grb2, which interact with cortactin, N-WASp, and Arp2/3 to mediate dynamic actin polymerization. FAK interacts with p130Cas to inhibit Rho signaling during rosette formation. Although FAK at focal adhesions can sequester phosphotyrosine proteins and impair invadopodia formation, overexpression of FAK can enhance the degradation activity of invadopodia on rigid ECM substrates and promote invasive migration.
Although endogenous FAK is not necessarily required for invadosome formation, recent evidence suggests that FAK plays an important role in the formation of organized podosome rosettes (Pan et al., 2011). FAK-Src signaling induces the assembly of podosome rosettes through FAK-mediated docking of p130Cas and suppression of Rho signaling (Figure 2A,B; Pan et al., 2011). Moreover, FAK-Src signaling at focal adhesions can serve as a platform for rosette formation in Src-transformed fibroblasts (Oikawa et al., 2008). Specifically, Src activation at focal adhesions induces the switch to podosome rosettes through PtdIns(3,4)P2-mediated recruitment of Tks5 and Grb2 (Oikawa et al., 2008). The focal adhesion-PtdIns(3,4)P2-Tks5-Grb2 complex can function as an early precursor before the actin polymerization machinery is recruited to mediate the switch from focal adhesions to podosome rosettes (Figure 2A,B; Oikawa et al., 2008). Collectively, FAK is a key adaptor protein that spatiotemporally regulates Src activity at adhesions to regulate invadosome dynamics; however the effects of FAK on invadosomes are likely context-dependent and can be influenced by the mechanical properties or composition of the matrix.
Src targets multiple substrates to regulate different stages of invadosome dynamics
Src is necessary and sufficient for invadosome formation: ectopic expression of activated Src induces invadopodia and podosomes while Src inhibition impairs their formation. Src promotes invadosome formation by phosphorylating many different substrates including the adaptor protein Tks5 and the actin regulatory proteins cortactin and N-WASP (for a list of Src substrates, see Table 1; Park et al., 2005; Stylli et al., 2009; Tehrani et al., 2007). Src also regulates the degradation activity of invadopodia by affecting MT1-MMP endocytosis through CIP4 (Hu et al., 2011) and endophilin A2 (Wu et al., 2005b), and disassembly through the targeting of specific substrates such as paxillin (Badowski et al., 2008) or indirectly through calpain-mediated substrate proteolysis (Calle et al., 2006; Cortesio et al., 2008). Currently, we do not fully understand how Src targets so many different substrates to orchestrate invadosome formation and turnover during invasive motility. We speculate that Src achieves its specificity at least in part through the temporal and spatial localization of its substrates, in addition to its localized activation. Here we focus on a few specific Src targets that are involved in distinct stages of invadosome formation and turnover.
Table I.
Src substrates at invadosomes
| Protein | Phosphosite(s) | Invadosome Function | Notes | References |
|---|---|---|---|---|
| AFAP-110 | Y93, Y94, Y451, Y453 | Podosome formation | Amplifies Src activity | Guappone et al., 1998; Gatesman et al., 2004 |
| ASAP-1 | Y782 | Podosome and invadopodia formation | pY782 is required for podosome formation | Bharti et al., 2007 |
| Caveolin | Y14 | ND | pY14 involved in podosome rosette-like formation | Colonna and Podesta, 2005 |
| CIP4 | Y471 | Degradation | p471 inhibits endocytosis of MT1-MMP at invadopodia | Hu et al., 2011 |
| Cortactin | Y421, Y466 | Actin polymerization | pY421 and pY466 interact with Nck1 to facilitate actin polymerization | Oser et al., 2010; Tehrani et al., 2007; Head et al., 2003 |
| Y482 | Degradation | Y421D enhances degradation; 3YF inhibits degradation | Ayala et al., 2008 | |
| Dynamin | Y231, Y597 | ND | Function of Y231 or Y597 unknown at invadosomes | Ahn et al., 1999; Ochoa et al., 2000 |
| ND | Degradation | Dominant-negative mutant blocks degradation | Baldassarre et al., 2003 | |
| Endophilin A2 | Y315 | Degradation | pY315 inhibits endocytosis of MT1-MMP at podosomes | Wu et al., 2005b |
| Ezrin | Y477 | ND | pY477 is crucial for 3D invasion | Heiska et al., 2011 |
| ND | Podosome formation | Localizes with cortactin to form rosettes | Kocher et al., 2009 | |
| FAK | Y576, Y577 | Podosome formation | Necessary for rosette formation | Pan et al., 2011 |
| Y925 | ND | pY925 interacts with Grb2 | Schlaepfer et al., 1994 | |
| Y861 | ND | pY861 interacts with p130Cas | Lim et al., 2004 | |
| ND | Inhibits invadopodia | Sequesters pY proteins at focal adhesions | Chan et al., 2009 | |
| HS1 | ND | Podosome organization | Important for podosome array organization, function of tyrosine sites unknown. | Dehring et al., 2011 |
| mAbp1 | Y337, Y347 | Actin polymerization | pY337 and pY347 Inhibit dot podosome formation | Boateng et al., 2012 |
| Actin organization | pY347 necessary for rosette formation | Boateng et al., 2012 | ||
| MT1-MMP | Y573 | Degradation | Phosphorylation may have a role in regulating MT1-MMP endocytosis | Nyalendo et al., 2007; Uekita et al., 2001 |
| Nox-A1 | Y110 | Invadopodia formation | pY110 interacts with Tks4 at invadopodia | Gianni et al., 2010 |
| N-WASP | Y253 | Actin polymerization | Interacts with Arp2/3 | Park et al., 2005 |
| p130Cas | Y165, Y249, Y410 | ND | Src phosphorylation sites reported at focal adhesions, and p130Cas localizes to some invadopodia | Meenderink et al., 2010; Chan et al., 2009 |
| ND | Podosome formation | FAK-p130Cas complex mediates rosette formation | Wang and McNiven, 2012 | |
| p190RhoGAP | ND | Formation/Degradation | Antibodies against p190 inhibit matrix degradation | Nakahara et al., 1998 |
| Paxillin | Y31, Y118 | Disassembly | pY31 and pY118 important for disassembly of inner ring of podosome rosettes | Badowsky et al., 2008; Vindis et al., 2004 |
| Pyk2 | Y579, Y580, Y881 | Podosome formation | Phosphorylation function unknown at invadosomes. Y881 associates with Grb2 | Lakkakorpi et al., 2003; Duong et al., 1998; Schlaepfer et al., 1999 |
| RhoGDI | Y1105 | ND | pY1105 associates with p120RasGAP, mediates disassembly of stress fibers | Roof et al., 1998; Fincham et al., 1999 |
| Y156 | ND | pY156 induces release of RhoA, Cdc42 and Rac1 from RhoGDI inhibition | DerMardirossian et al., 2006 | |
| Tensin | Y1173, Y1206, Y1256 | ND | Src phosphorylation sites identified at focal adhesions. Tensin localizes to podosomes in osteoclasts | Hiura et al., 1995; Qian et al., 2009 |
| Tks4 | Y25, Y373 | Podosome formation | 3YF has defect in podosome rosette formation | Buschman et al., 2008 |
| Y508 | Maturation | pY508 interacts with NoxA1 to generate ROS at invadopodia | Gianni et al., 2010 | |
| Y508 | Degradation | 3YF cannot degrade matrix at podosomes | Buschman et al., 2008 | |
| Tks5 | Y557, Y619 | Podosome and Invadopodia formation | pY557 and pY619 interact with N-WASp to promote actin polymerization | Stylli et al., 2009 |
| Y557 | Degradation | pY557 interacts with Nck1/2 to promote ECM degradation at invadopodia |
Src regulates Rho GTPase signaling during invadosome formation
Rho GTPases are required for podosome and invadopodia formation, and Rho GTPase activity is tightly regulated, in part by Src-mediated phosphorylation of both GTPase activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs). For example, Src phosphorylates Fgd1, a GEF for Cdc42 (Miyamoto et al., 2003) and active Cdc42 works in concert with N-WASP to facilitate actin polymerization during invadopodia formation (Ayala et al., 2009). In addition, Src can activate both Cdc42 and RhoA by activating the Rho GTPase GEF, Arhgef5, to mediate podosome and invadopodia formation (Kuroiwa et al., 2011).
Several lines of evidence suggest that RhoA activity is important for podosome rosette formation, however, RhoA activity must be tightly regulated (Berdeaux et al., 2004; Pan et al., 2011; Schramp et al., 2008; Chellaiah et al., 2000). Src activates RhoA at rosettes by phosphorylating RhoGDI, to limit its inhibitory effects on RhoA (DerMardirossian et al., 2006). By contrast, mechanisms that limit RhoA activity during rosette formation include Src-mediated activation of FAK (Pan et al., 2011) and ERK5 (Schramp et al., 2008), and possibly through p190RhoGAP (Bravo-Cordero et al., 2011; Fincham et al., 1999). The targeting of both GAPs and GEFs by Src provides an additional challenge for understanding the spatiotemporal control of downstream effectors during podosome and invadopodia formation and turnover. Recent work provides an example of strict spatiotemporal organization of active RhoC at invadopodia. Within the invadopodia core, p190RhoGAP can inhibit RhoC activity which results in cofilin-mediated generation of barbed ends and actin polymerization (Bravo-Cordero et al., 2011). Conversely, surrounding the core, p190RhoGEF can activate RhoC, and cofilin activity is sequestered (Bravo-Cordero et al., 2011). It is an intriguing idea that Src may regulate the spatiotemporal activation of RhoC at invadopodia through the opposing functions of temporally and spatially restricted GAPs and GEFs.
Src targets actin regulatory proteins with different roles at invadosomes
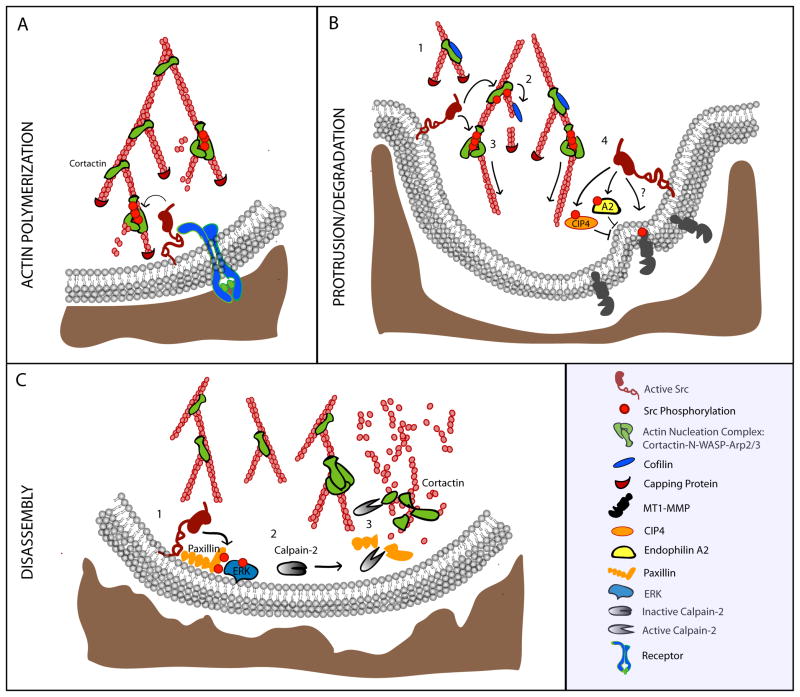
Cortactin and mammalian actin-binding protein-1 (mAbp1) are actin regulatory proteins with high structural homology, and both have been implicated in regulating sites of dynamic F-actin such as lamellipodia and invadosomes. Cortactin is a multi-functional Src substrate that is involved in both the assembly and maturation of invadosomes and is important for invasive cell migration (Bowden et al., 1999; Clark et al., 2007; Oser et al., 2009; Desmarais et al., 2009). Src phosphorylates cortactin at three sites, Y421, Y466 and Y482 (Huang et al., 1998), and cortactin phosphorylation is important for barbed end formation at invadopodia precursors (Figure 3A), but not for precursor formation itself (Oser et al., 2009). For example, phosphorylated cortactin releases its inhibitory grip on cofilin to allow for cofilin-mediated actin severing and barbed end formation at invadopodia (Figure 3B; Oser et al., 2009). In addition to its effects on the actin-severing activity of cofilin, phosphorylated cortactin facilitates actin polymerization through its interactions with N-WASp and Arp2/3 (Figure 3B; Oser et al., 2009; Tehrani et al., 2007; Martinez-Quiles et al., 2004), therefore, cofilin and the Nck1-cortactin-N-WASp-Arp2/3 complex, work synergistically to create barbed ends and mediate actin polymerization at invadopodia (DesMarais et al., 2009; Ichetovkin et al., 2002).
Figure 3. Src regulation of invadosomes.
(A) Src becomes activated downstream of receptor and/or integrin-mediated signaling and phosphorylates multiple substrates to induce actin polymerization. Cortactin is phosphorylated at three tyrosine residues and interacts with WASP and Arp2/3 to nucleate new actin branches. (B) 1: New actin branches are stabilized by dephosphorylated cortactin which binds cofilin in an inactive state. 2: During later stages, cortactin is phosphorylated and cofilin is released. Cofilin cleaves actin filaments resulting in the formation of new barbed ends. 3: Cortactin interacts with the N-WASP/Arp2/3 complex to nucleate de novo actin branches. 4: Src can regulate MT1-MMP matrix degradation activity by phosphorylating the cytoplasmic tail at Y573 which may regulate its trafficking. Alternatively, Src can phosphorylate endophilin A2 or the Cdc42 interacting protein (CIP4) to inhibit endocytosis of MT1-MMP thereby increasing surface expression. (C) During disassembly, Src phosphorylates paxillin which activates Erk signaling. Erk leads to the activation of the protease Calpain-2 which cleaves multiple invadosome proteins including paxillin and cortactin to mediate invadosome disassembly.
mAbp1, in contrast to cortactin, impairs invadopodia formation and invasive cell migration (Boateng et al., 2012). mAbp1 is phosphorylated by Src at two sites, Y337 and Y347 (Larbolette, 1999; Lock et al., 1998), and phosphorylation of these sites is important for mAbp1-mediated inhibition of podosome dot formation and invasion (Boateng et al., 2012). Since these two actin-binding Src substrates appear to have opposing roles during invadopodia/podosome dot formation, then the spatiotemporal phosphorylation of these substrates will help to elucidate how they work together to regulate invadosome dynamics. Live imaging experiments suggest that cortactin is present at invadopodia during early assembly, stabilization, and early degradation, but not during late degradation activity (Artym et al., 2006), while mAbp1 appears to arrive at mature podosome dots and facilitates the formation of podosome rosettes (Boateng et al., 2012). These results suggest that Src may target different actin-binding proteins during distinct stages of invadosome formation and maturation to regulate actin dynamics and invadosome function.
Src regulates degradation activity and MT1-MMP function
MT1-MMP is a transmembrane matrix metalloproteinase that is critical for the degradative functions of invadopodia (Poincloux et al., 2009; Williams and Coppolino, 2011) and its trafficking to invadopodia is a growing area of interest. MT1-MMP is a target of Src-mediated phosphorylation and Src appears to modulate the function of MT1-MMP by regulating its endocytosis rather than directly controlling its protease activity (Nyalendo et al., 2007; Wu et al., 2005b). Src can directly phosphorylate MT1-MMP on Y573 at the cytoplasmic tail, downstream of EGF signaling (Figure 3B). This phosphorylated tyrosine is important for tumor cell proliferation and 3D invasion of collagen matrices (Nyalendo et al., 2008) and inhibition of MTI-MMP tyrosine phosphorylation at Y573 in mice impairs tumor progression (Nyalendo et al., 2010). Interestingly, this Src-phosphorylated tyrosine is part of a critical sequence (LLY573) that is required for clathrin-mediated uptake of MT1-MMP, which has been implicated in cell invasion (Uekita et al., 2001). Whether Src-phosphorylation of MT1-MMP specifically promotes or inhibits clathrin-mediated endocytosis remains to be determined. On the other hand, Src phosphorylates endophilin A2 to inhibit MT1-MMP endocytosis and enhance matrix degradation at podosome rosettes (Wu et al., 2005b). Further, Src phosphorylates the Cdc42 interacting protein (CIP4) to inhibit CIP4-mediated endocytosis of MTI-MMP and enhance degradation activity at invadopodia (Hu et al., 2011). It is likely that Src-effects on MT1-MMP trafficking and activity are complex, but this regulation provides critical control of the late stages of invadosome function and matrix degradation.
Src activity during invadosome disassembly
Src activity is necessary not only for assembly of invadosomes, but also for their disassembly (Badowski et al., 2008; Destaing et al., 2008). Osteoclasts from Src−/− mice form fewer podosomes that are longer-lived and have impaired podosome turnover compared to osteoclasts from control mice (Destaing et al., 2008). Conversely, overexpression of v-Src enhances the turnover of invadopodia in MTLn3 cells leading to shorter-lived invadopodia (Oser et al., 2009). Targeting of specific substrates can mediate invadosome turnover. For example, Src-mediated paxillin phosphorylation on Y31 and Y118 is necessary for the disassembly and turnover of invadosomes (Badowski et al., 2008). Specifically, phosphorylated paxillin can activate calpain through Erk signaling to mediate disassembly (Figure 3C; Badowski et al., 2008; Glading et al., 2001). Calpain-2 is a cysteine protease that cleaves multiple adaptor proteins, such as talin, and paxillin, that are components of invadosomes (Calle et al., 2006). Calpain-2 also cleaves cortactin (Cortesio et al., 2008), WASP (Macpherson et al., 2012) and Pyk2 (Calle et al., 2006) to mediate invadosome disassembly. Accordingly, expression of a calpain-resistant cortactin decreases the rate of invadopodia disassembly (Cortesio et al., 2008). Finally, calpain-2 can positively regulate Src activity through the proteolysis and activation of PTP1B (Cortesio et al., 2008), a phosphatase that activates Src, thereby potentially creating a feedback loop between Src and calpain-2 through PTP1B. Collectively, these studies suggest that Src and calpain-2 coordinately regulate invadosome turnover. The remaining challenge is to determine the spatiotemporal mechanisms by which Src mediates the switch to invadosome disassembly.
Concluding Remarks
There has been substantial progress in understanding how Src regulates invadosomes and invasive migration. However, fundamental questions about how Src functions to mediate the different stages of invadosome formation, maturation and turnover remain unanswered. Challenges include understanding how Src is spatiotemporally regulated during invasive migration. The recent development of biosensors that detect Src activity have suggested that membrane tension can induce localized Src activity (Wang et al., 2005) and the future application of Src biosensors to understand how Src is regulated temporally and spatially will advance the field (Gulyani et al., 2011; Welman et al., 2010). Moreover, tools to read out activation of different Src substrates—such as the phosphorylation of cortactin versus mAbp1 at different stages of invadosome formation and turnover would provide further understanding about how the targeting of different substrates can have such diverse effects. These types of advances will allow us to identify strategies to target Src activity or specific Src substrates to aid in the development of new targeted therapies to modulate invasive cell migration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- Alexander NR, Branch KM, Parakh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammer AG, Kelley LC, Hayes KE, Evans JV, Lopez-Skinner LA, Martin KH, Weed SA. Saracatinib impairs head and neck squamous cell carcinoma invasion by disrupting invadopodia function. J Cancer Sci Ther. 2009;1:52–61. doi: 10.4172/1948-5956.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc Natl Acad Sci. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Meuller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggio S, Tete S, Polishchuk R, Castronovo V, Buccione R. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 2009;69:747–752. doi: 10.1158/0008-5472.CAN-08-1980. [DOI] [PubMed] [Google Scholar]
- Badowski C, Pawlak G, Grichine A, Chabadel A, Oddou C, Jurdic P, Pfaff M, Albiges-Rizo C, Block MR. Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol Biol Cell. 2008;19:633–645. doi: 10.1091/mbc.E06-01-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdeaux RL, Diaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166:317–323. doi: 10.1083/jcb.200312168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KM, Mueller SC, Barr VA, Randazzo PA. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol. 2007;27:8271–8283. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi JS, Belsches AP, Parsons SJ. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog. 1998;21:261–272. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J Biol Chem. 2000;275:41439–41446. doi: 10.1074/jbc.M004852200. [DOI] [PubMed] [Google Scholar]
- Boateng LR, Cortesio CL, Huttenlocher A. Src-mediated phosphorylation of mammalian Abp1 (DBNL) regulates podosome rosette formation in transformed fibroblasts. J Cell Sci. 2012;125:1329–1341. doi: 10.1242/jcs.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogeski I, Bozem M, Sternfeld L, Hofer HW, Schulz I. Inhibition of protein tyrosine phosphatase 1B by reactive oxygen species leads to maintenance of Ca2+ influx following store depletion in HEK 293 cells. Cell Calcium. 2006;40:1–10. doi: 10.1016/j.ceca.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic Biol Med. 1997;22:515–524. doi: 10.1016/s0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- Burger KL, Davis AL, Isom S, Mishra N, Seals DF. The podosome marker protein Tks5 regulates macrophage invasive behavior. Cytoskeleton. 2011;68:694–711. doi: 10.1002/cm.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci. 2006;119:2375–2385. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989;83:2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–370. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah M, Kizer N, Silva M, Alvarez U, Kwiatkowski D, Hruska KA. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol. 2000;148:665–678. doi: 10.1083/jcb.148.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah MA, Biswas RS, Yuen D, Alvarez UM, Hruska KA. Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J Biol Chem. 2001;276:47434–47444. doi: 10.1074/jbc.M107494200. [DOI] [PubMed] [Google Scholar]
- Chellaiah MA, Schaller MD. Activation of Src kinase by protein-tyrosine phosphatase-PEST in osteoclasts: comparative analysis of the effects of bisphosphonate and protein-tyrosine phosphatase inhibitor on Src activation in vitro. J Cell Physiol. 2009;220:382–393. doi: 10.1002/jcp.21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann N Y Acad Sci. 1999;878:361–371. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- Chen YY, Chu HM, Pan KT, Teng CH, Wang DL, Wang AH, Khoo KH, Meng TC. Cysteine S-nitrosylation protects protein-tyrosine phosphatase 1B against oxidation-induced permanent inactivation. J Biol Chem. 2008;283:35265–35272. doi: 10.1074/jbc.M805287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-organized podosomes are dynamic mechanosensors. Curr Biol. 2008;18:1288–1294. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin O, Tracqui P, Stephanou A, Usson Y, Clement-Lacroix J, Planus E. Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility. J Cell Sci. 2006;119:1914–1925. doi: 10.1242/jcs.02838. [DOI] [PubMed] [Google Scholar]
- Colonna C, Podesta EJ. ACTH-induced caveolin-1 tyrosine phosphorylation is related to podosome assembly in Y1 adrenal cells. Exp Cell Res. 2005;304:432–442. doi: 10.1016/j.yexcr.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesio CL, Wernimont SA, Kastner DL, Cooper KM, Huttenlocher A. Impaired podosome formation and invasive migration of macrophages from patients with a PSTPIP1 mutation and PAPA syndrome. Arthritis Rheum. 2010;62:2556–2558. doi: 10.1002/art.27521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimaldi L, Courtneidge SA, Gimona M. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp Cell Res. 2009;315:2581–2592. doi: 10.1016/j.yexcr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Daubon T, Buccione R, Genot E. The Aarskog-Scott syndrome protein Fgd1 regulates podosome formation and extracellular matrix remodeling in transforming growth factor beta-stimulated aortic endothelial cells. Mol Cell Biol. 2011;31:4430–4441. doi: 10.1128/MCB.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980;77:6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehring DA, Clarke F, Ricart BG, Huang Y, Gomez TS, Williamson EK, Hammer DA, Billadeau DD, Argon Y, Burkhardt JK. Hematopoietic lineage cell-specific protein 1 functions in concert with the Wiskott-Aldrich syndrome protein to promote podosome array organization and chemotaxis in dendritic cells. J Immunol. 2011;186:4805–4818. doi: 10.4049/jimmunol.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell. 2006;17:4760–4768. doi: 10.1091/mbc.E06-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesMarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton. 2009;66:303–316. doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Curr Opin Cell Biol. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J Clin Invest. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, Unlu M, Brunton VG, Pitts JD, Wyke JA, Frame MC. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J Cell Biol. 1996;135:1551–1564. doi: 10.1083/jcb.135.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, Frame MC. The catalytic activity of Src is dispensible for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 1998;17:81–92. doi: 10.1093/emboj/17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112 (Pt 6):947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src’s hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–245. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117:989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC. Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol Cell Biol. 2004;24:7578–7597. doi: 10.1128/MCB.24.17.7578-7597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I, Nermut MV, Marchisio PC. Ultrastructure and gold-immunolabelling of cell-substratum adhesions (podosomes) in RSV-transformed BHK cells. J Cell Sci. 1989;94:85–99. doi: 10.1242/jcs.94.1.85. [DOI] [PubMed] [Google Scholar]
- Gertler F, Condeelis J. Metastasis: tumor cells becoming MENAcing. Trends Cell Biol. 2010;21:81–90. doi: 10.1016/j.tcb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol. 2010;5:981–993. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Uberall F, Keyse SM, Lauffenburger DA, Wells A. Membrane proximal ERK signaling is required for M-calpain activation downstream of epidermal growth factor receptor signaling. J Biol Chem. 2001;276:23341–23348. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Anderson WB. Ca2+ and phospholipid-independent activation of protein kinase C by selective oxidative modification of regulatory domain. Proc Natl Acad Sci. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R, Anderson WB. Reversible oxidative activation and inactivation of PKC by the mitogen/tumor promoter periodate. Arch Biochem Biophys. 1991;285:380–387. doi: 10.1016/0003-9861(91)90377-u. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Chen ZH, Gundimeda U. Modification of cysteine-rich regions in protien kinase C induced by oxidant tumor promoters and the enzyme specific inhibitors. Meth Enzymol. 1995;252:134–148. doi: 10.1016/0076-6879(95)52016-3. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- Guan JL, Trevethick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guappone AC, Weimer T, Flynn DC. Formation of a stable src-AFAP-110 complex through either an amino-terminal or a carboxy-terminal SH2-binding motif. Mol Carcinog. 1998;22:110–119. doi: 10.1002/(sici)1098-2744(199806)22:2<110::aid-mc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Guiet R, Verollet C, Lamsoul I, Cougoule C, Poincloux R, Labrousse A, Calderwood DA, Glogauer M, Lutz PG, Maridonneau-Parini I. Macrophage mesenchymal migration requires podosome stabilization by filamin A. J Biol Chem. 2012;287:13051–13062. doi: 10.1074/jbc.M111.307124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyani A, Vitriol E, Allen R, Wu J, Gremyachinskiy D, Lewis S, Dewar B, Graves LM, Kay BK, Kuhlman B, Elston T, Hahn KM. A biosensor generated via high-throughput screening quantifies cell edge Src dynamics. Nat Chem Biol. 2011;7:437–444. doi: 10.1038/nchembio.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Hsu YS, Martin GS. Shp-2 mediates v-Src-induced morphological changes and activation of the anti-apoptotic protein kinase Akt. Oncogene. 2000;19:3164–3171. doi: 10.1038/sj.onc.1203655. [DOI] [PubMed] [Google Scholar]
- Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Ronde P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci. 2005;118:4415–4425. doi: 10.1242/jcs.02565. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Ilic D, Sclaepfer DD. v-Src SH3-enhanced interaction with focal adhesion kinase ast B1 integrin-containing invadopodia promotes cell invasion. J Biol Chem. 2002;277:12487–12490. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, Weed SA. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14:3216–3229. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiska L, Melikova M, Zhao F, Saotome I, McClatchey AI, Carpen O. Ezrin is key regulator of Src-induced malignant phenotype in three-dimensional environment. Oncogene. 2011;30:4953–4962. doi: 10.1038/onc.2011.207. [DOI] [PubMed] [Google Scholar]
- Heo J, Campbell SL. Mechanism of redox-mediated guanine nucleotide exhange on redox-active Rho GTPases. J Biol Chem. 2005;280:31003–31010. doi: 10.1074/jbc.M504768200. [DOI] [PubMed] [Google Scholar]
- Heo J, Raines KW, Mocanu V, Campbell SL. Redox regulation of RhoA. Biochem. 2006;45:14481–14489. doi: 10.1021/bi0610101. [DOI] [PubMed] [Google Scholar]
- Hiura K, Lim SS, Little SP, Lin S, Sato M. Differentiation dependent expression of tensin and cortactin in chicken osteoclasts. Cell Motil Cytoskeleton. 1995;30:272–284. doi: 10.1002/cm.970300405. [DOI] [PubMed] [Google Scholar]
- Hu J, Mukhopadhyay A, Truesdell P, Chander H, Mukhopadhyay UK, Mak AS, Craig AW. Cdc42-interacting protein 4 is a Src substrate that regulates invadopodia and invasiveness of breast tumors by promoting MT1-MMP endocytosis. J Cell Sci. 2011;124:1739–1751. doi: 10.1242/jcs.078014. [DOI] [PubMed] [Google Scholar]
- Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ia KK, Mills RD, Hossain MI, Chan KC, Jarasrassamee B, Jorissen RN, Cheng HC. Structural elements and allosteric mechanisms governing regulation and catalysis of CSK-family kinases and their inhibition of Src-family kinases. Growth Factors. 2010;28:329–350. doi: 10.3109/08977194.2010.484424. [DOI] [PubMed] [Google Scholar]
- Ichetovkin I, Grant W, Condeelis J. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr Biol. 2002;12:79–84. doi: 10.1016/s0960-9822(01)00629-7. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Cejudo-Martin P, de Brouwer A, van der Zwaag B, Ruiz-Lozano P, Scimia MC, Lindsey JD, Weinreb R, Albrecht B, Megarbane A, Alanay Y, Ben-Neriah Z, Amenduni M, Artuso R, Veltman JA, van Beusekom E, Oudakker A, Millan JL, Hennekam R, Hamel B, Courtneidge SA, van Bokhoven H. Disruption of the podosome adaptor protein TKS4 (SH3PXD2B) causes the skeletal dysplasia, eye, and cardiac abnormalities of Frank-Ter Haar Syndrome. Am J Hum Genet. 2010;86:254–261. doi: 10.1016/j.ajhg.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Bibbins KB, Swedlow JR, Arnaud M, Morgan DO, Varmus HE. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J. 1994;13:4745–4756. doi: 10.1002/j.1460-2075.1994.tb06800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LC, Ammer AG, Hayes KE, Martin KH, Machida K, Jia L, Mayer BJ, Weed SA. Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J Cell Sci. 2010;123:3923–3932. doi: 10.1242/jcs.075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble DJ, Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc Natl Acad Sci U S A. 2009;106:5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik TE, Johnson PJ, Shalloway D. Regulation by the autophosphorylation site in overexpressed pp60c-src. Mol Cell Biol. 1988;8:4541–4546. doi: 10.1128/mcb.8.10.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher HM, Sandle J, Mirza TA, Li NF, Hart IR. Ezrin interacts with cortactin to form podosomal rosettes in pancreatic cancer cells. Gut. 2009;58:271–284. doi: 10.1136/gut.2008.159871. [DOI] [PubMed] [Google Scholar]
- Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- Kuroiwa M, Oneyama C, Nada S, Okada M. The guanine nucleotide exchange factor Arhgef5 plays crucial roles in Src-induced podosome formation. J Cell Sci. 2011;124:1726–1738. doi: 10.1242/jcs.080291. [DOI] [PubMed] [Google Scholar]
- Kypta RM, Goldberg Y, Ulug ET, Courtneidge SA. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990;62:481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi PT, Bett AJ, Lipfert L, Rodan GA, Duong le T. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J Biol Chem. 2003;278:11502–11512. doi: 10.1074/jbc.M206579200. [DOI] [PubMed] [Google Scholar]
- Lander HM, Ogiste JS, Teng KK, Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- Larbolette O, Wollscheid B, Schweikert J, Nielsen P, Wienards J. SH3P7 is a cytoskeletal adapter protein and is coupled to signal transduction from lymphocyte antigen receptors. Mol Cell Biol. 1999;19:1539–1546. doi: 10.1128/mcb.19.2.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, Anderson K, Machesky LM. The actin bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:399–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Han I, Jeon J, Park H, Bahk YY, Oh ES. Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for Ras transformation of fibroblasts. J Biol Chem. 2004;279:29060–29065. doi: 10.1074/jbc.M401183200. [DOI] [PubMed] [Google Scholar]
- Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends. 2003;7:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- Liu S, Yamashita H, Weidow B, Weaver AM, Quaranta V. Laminin-332-beta1 integrin interactions negatively regulate invadopodia. J Cell Physiol. 2010;223:134–142. doi: 10.1002/jcp.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock P, Abram CL, Gibson T, Cortneidge SA. A new method for isolating tyrosine kinase substrates used to identify Fish, and an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L, Monypenny J, Blundell MP, Cory GO, Tome’-Garcia J, Thrasher AJ, Jones GE, Calle Y. Tyrosine phosphorylation of WASP promotes calpain-mediated podosome disassembly. Haematologica. 2012;97:687–691. doi: 10.3324/haematol.2011.048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio PC, Bergui L, Corbascio GC, Cremona O, D’Urso N, Schena M, Tesio L, Caligaris-Cappio F. Vinculin, talin, and integrins are localized at specific adhesion sites of malignant B lymphocytes. Blood. 1988;72:830–833. [PubMed] [Google Scholar]
- Marcotte R, Zhou L, Kim H, Roskelly CD, Muller WJ. c-Src associates with ErbB2 through an interaction between catalytic domains and confers enhanced transforming potential. Mol Cell Biol. 2009;29:5858–5871. doi: 10.1128/MCB.01731-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean GW, Fincham VJ, Frame MC. v-Src induces tyrosine phosphorylation of focal adhesion kinase independenty of tyrosine 397 and formation of a complex with Src. J Biol Chem. 2000;275:23333–23339. doi: 10.1074/jbc.M909322199. [DOI] [PubMed] [Google Scholar]
- Meenderink LM, Ryzhova LM, Donato DM, Gochberg DF, Kaverina I, Hanks SK. P130Cas Src-binding and substrate domains have distinct roles in sustaining focal adhesion disassembly and promoting cell migration. PLoS One. 2010;5:e13412. doi: 10.1371/journal.pone.0013412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Itoh H. Src kinase regulates the activation of a novel FGD-1-related Cdc42 guanine nucleotide exchange factor in the signaling pathway from the endothelin A receptor to JNK. J Biol Chem. 2003;278:29890–29900. doi: 10.1074/jbc.M301559200. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, Chen WT. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci. 2007;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- Nusblat LM, Dovas A, Cox D. The non-redundant role of N-WASP in podosome-mediated matrix degradation in macrophages. Eur J Cell Biol. 2011;90:205–212. doi: 10.1016/j.ejcb.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, Beliveau R. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J Biol Chem. 2007;282:15690–15699. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]
- Nyalendo C, Beaulieu E, Sartelet H, Michaud M, Fontaine N, Gingras D, Beliveau R. Impaired tyrosine phosphorylation of membrane type 1-matrix metalloproteinase reduces tumor cell proliferation in three-dimensional matrices and abrogates tumor growth in mice. Carcinogenesis. 2008;29:1655–1664. doi: 10.1093/carcin/bgn159. [DOI] [PubMed] [Google Scholar]
- Nyalendo C, Sartelet H, Gingras D, Beliveau R. Inhibition of membrane-type 1 matrix metalloproteinase tyrosine phosphorylation blocks tumor progression in mice. Anticancer Res. 2010;30:1887–1895. [PubMed] [Google Scholar]
- Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, Kim W, Cao H, McNiven M, Baron R, De Camilli P. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Nakagawa H. A protein tyrosine kinase involved in regulation of pp60c-src function. J Biol Chem. 1989;264:20886–20893. [PubMed] [Google Scholar]
- Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, Condeelis J. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff-Kalff AE, Rijksen G, van Beurden EA, Hennipman A, Michels AA, Staal GE. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 1992;52:4773–4778. [PubMed] [Google Scholar]
- Packard BZ, Artym VV, Komoriya A, Yamada KM. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol. 2009;28:3–10. doi: 10.1016/j.matbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YR, Chen CL, Chen HC. FAK is required for the assembly of podosome rosettes. J Cell Biol. 2011;195:113–129. doi: 10.1083/jcb.201103016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Suetsugu S, Takenawa T. Interaction of HSP90 to N-WASP leads to activation and protection from proteasome-dependent degradation. EMBO J. 2005;24:1557–1570. doi: 10.1038/sj.emboj.7600586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, Gertler FB. A Mena invasion isoform p. otentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot CS, Hartig SM, Xia L, Arvanitis C, Monisvais D, Lee FY, Frost JA, Corey SJ. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer. 2009;101:38–47. doi: 10.1038/sj.bjc.6605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- Qian Y, Baisden JM, Cherezova L, Summy JM, Guappone-Koay A, Shi X, Mast T, Pustula J, Zot HG, Mazloum N, Lee MY, Flynn DC. PC Phosphorylation increases the ability of AFAP-110 to cross-link actin filaments. Mol Biol Cell. 2002;13:2311–2322. doi: 10.1091/mbc.E01-12-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Li G, Vass WC, Papageorge A, Walker RC, Asnaghi L, Steinbach PJ, Tosato G, Hunter K, Lowy DR. The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell. 2009;16:246–258. doi: 10.1016/j.ccr.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider LR. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979;16:11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Roof RW, Haskell MD, Dukes BD, Sherman N, Kinter M, Parsons SJ. Phosphotyrosine (p-Tyr)-dependent and -independent mechanisms of p190 RhoGAP-p120 RasGAP interaction: Tyr 1105 of p190, a substrate for c-Src, is the sole p-Tyr mediator of complex formation. Mol Cell Biol. 1998;18:7052–7063. doi: 10.1128/mcb.18.12.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers P, Saltel F, Daubon T, Chaigne-Delalande B, Tridon V, Billottet C, Reuzeau E, Génot E. TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J Cell Sci. 2009;122:4311–4318. doi: 10.1242/jcs.057448. [DOI] [PubMed] [Google Scholar]
- Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Genot E. Invadosomes: intriguing structures with promise. Eur J Cell Biol. 2011;90:100–107. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell. 2004;7:855–869. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Savitsky PA, Finkel T. Redox regulation of Cdc25C. J Biol Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramp M, Ying O, Kim TY, Martin GS. ERK5 promotes Src-induced podosome formation by limiting Rho activation. J Cell Biol. 2008;181:1195–1210. doi: 10.1083/jcb.200801078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- Somani AK, Bignon JS, Mills GB, Siminovitch KA, Branch DR. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 1997;272:21113–21119. doi: 10.1074/jbc.272.34.21113. [DOI] [PubMed] [Google Scholar]
- Stover DR, Becker M, Liebetanz J, Lydon NB. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J Biol Chem. 1995;270:15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- Stylii SS, Stacey TT, Verhagen AM, Xu SS, Pass I, Courtneidge SA, Lock P. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J Cell Sci. 2009;122:2727–2740. doi: 10.1242/jcs.046680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Tatin F, Varon C, Genot E, Moreau V. A signaling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- Uekita T, Itoh Y, Yana I, Ohno H, Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon C, Tatin F, Moreau V, Van Obberghen-Schilling E, Fernandez-Sauze S, Reuzeau E, Kramer I, Genot E. Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol. 2006;26:3582–3594. doi: 10.1128/MCB.26.9.3582-3594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindis C, Teli T, Cerretti DP, Turner CE, Huynh-Do U. EphB1-mediated cell migration requires the phosphorylation of paxillin at Tyr-31/Tyr-118. J Biol Chem. 2004;279:27965–27970. doi: 10.1074/jbc.M401295200. [DOI] [PubMed] [Google Scholar]
- Vitale S, Avizienyte E, Brunton VG, Frame MC. Focal adhesion kinase is not required for Src-induced formation of invadopodia in KM12C colon cancer cells and can interfere with their assembly. Eur J Cell Biol. 2008;87:569–579. doi: 10.1016/j.ejcb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Volberg T, Romer L, Zamir E, Geiger B. pp60c-src and related kinases: a role in the assembly and reorganization of matrix adhesions. J Cell Sci. 114:2279–2289. doi: 10.1242/jcs.114.12.2279. [DOI] [PubMed] [Google Scholar]
- Wadhawan A, Smith C, Nicholson RI, Barrett-Lee P, Hiscox S. Src-mediated regulation of homotypic cell adhesion: implications for cancer progression and opportunities for therapeutic intervention. Cancer Treat Rev. 2011;37:234–241. doi: 10.1016/j.ctrv.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Wang J, Yin G, Menon P, Pang J, Smolock EM, Yan C, Berk BC. Phosphorylation of G protein-coupled receptor kinase 2-interacting protein 1 tyrosine 392 is required for phospholipase C-gamma activation and podosome formation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010;30:1976–1982. doi: 10.1161/ATVBAHA.110.212415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham MJ, Wyke JA. A single point mutation has pleiotrophic effects on pp60v-src function. J Virol. 1988;62:1898–1906. doi: 10.1128/jvi.62.6.1898-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welman A, Serrels A, Brunton VG, Ditzel M, Frame MC. Two-color photoactivatable probe for selective tracking of proteins and cells. J Biol Chem. 2010;285:11607–11616. doi: 10.1074/jbc.M110.102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Coppolino MG. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J Biol Chem. 2011;286:43405–43416. doi: 10.1074/jbc.M111.297069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gan B, Yoo Y, Guan JL. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell. 2005b;9:185–196. doi: 10.1016/j.devcel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Wu WS, Tsai RK, Chang CH, Wang S, Wu JR, Chang YX. Reactive oxygen species mediated sustained activation of protein kinase C alpha and extracellular signal-regulated kinase for migration of human hepatoma cell Hepg2. Mol Cancer Res. 2006;4:747–758. doi: 10.1158/1541-7786.MCR-06-0096. [DOI] [PubMed] [Google Scholar]
- Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakita Y, Ono S, Matsumura F, Yamashiro S. Phosphorylation of human facsin inhibits its actin binding and bundling activities. J Biol Chem. 1996;271:12632–12638. doi: 10.1074/jbc.271.21.12632. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Park SJ, Yoon CH, Chung AS. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- Zheng XM, Wang Y, Pallen CJ. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992;359:336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
- Zrihan-Licht S, Lim J, Keydar I, Sliwkowski MX, Groopman JE, Avraham H. Association of csk-homologous kinase (CHK) (formerly MATK) with HER-2/ErbB-2 in breast cancer cells. J Biol Chem. 1997;272:1856–1863. doi: 10.1074/jbc.272.3.1856. [DOI] [PubMed] [Google Scholar]