Abstract
Background. Adjuvanted vaccines have the potential to improve influenza pandemic response. AS03 adjuvant has been shown to enhance the immune response to inactivated influenza vaccines.
Methods. This trial was designed to evaluate the immunogenicity and safety of an inactivated 2009 H1N1 influenza vaccine at varying dosages of hemagglutinin with and without extemporaneously mixed AS03 adjuvant system in adults ≥18 years of age. Adults were randomized to receive 2 doses of 1 of 5 vaccine formulations (3.75 µg, 7.5 µg, or 15 µg with AS03 or 7.5 µg or 15 µg without adjuvant).
Results. The study population included 544 persons <65 years of age and 245 persons ≥65 years of age. Local adverse events tended to be more frequent in the adjuvanted vaccine groups, but severe reactions were uncommon. In both age groups, hemagglutination inhibition antibody geometric mean titers after dose one were higher in the adjuvanted groups, compared with the 15 µg unadjuvanted group, and this difference was statistically significant for the comparison of the 15 µg adjuvanted group with the 15 µg unadjuvanted group.
Conclusions. AS03 adjuvant system improves the immune response to inactivated 2009 H1N1 influenza vaccine in both younger and older adults and is generally well tolerated.
ClinicalTrials.gov NCT00963157
(See the editorial commentary by Schuchat and Katzl, on pages 803–5.)
Mass vaccination campaigns are cornerstones of the response to an influenza pandemic. Adjuvants have the potential to improve pandemic response by reducing the amount of antigen required per dose (antigen sparing) or the number of doses needed (dose sparing) and by enhancing the immune response. The latter may be particularly important for groups that do not respond well to standard unadjuvanted influenza vaccines, such as older adults.
AS03 is an adjuvant system containing α-tocopherol and squalene in an oil-in-water emulsion that has been shown to substantially enhance the immune response to inactivated H5 influenza vaccines in children [1], in adults <65 years of age [2–5], and in adults ≥65 years of age [6]. An AS03 adjuvanted H5N1 vaccine containing 3.75 µg of influenza A hemagglutinin (Prepandrix, GlaxoSmithKline) is approved in Europe as a 2-dose series for prepandemic use in adults ≥18 years of age [7]. Inactivated 2009 H1N1 influenza vaccines with AS03 are also highly immunogenic in children [8–10] and adults [11–15], and AS03 adjuvanted 2009 H1N1 vaccines containing 3.75 µg HA (Pandemrix, Arepanrix, GlaxoSmithKline) were authorized for use in numerous countries, including Canada [16], in response to the pandemic. Among adults, immune response to inactivated 2009 H1N1 influenza vaccines with AS03 tends to decrease with increasing age [11, 13, 14], but there are relatively limited data on responses among adults >65 years of age.
In the context of a pandemic, the ability to mix adjuvant and vaccine extemporaneously would allow flexibility in matching vaccine from different sources with available supplies of adjuvant. We conducted a randomized trial to evaluate the immunogenicity and safety of an inactivated 2009 H1N1 vaccine (Sanofi Pasteur) at varying dosages with and without extemporaneously mixed AS03 adjuvant (GlaxoSmithKline Biologicals) in a study population that included both healthy adults (age, 18–64 years) and older adults (age, ≥65 years).
METHODS
Study Design and Participants
This randomized, double-blinded, phase II study was designed to assess the immunogenicity and safety of 2 injections of an inactivated 2009 H1N1 influenza vaccine (Sanofi Pasteur) given with and without AS03 (GlaxoSmithKline). The study evaluated 3 dose levels of hemagglutinin antigen (3.75 µg, 7.5 µg, or 15 µg) combined with AS03 and 2 dose levels (7.5 µg and 15 µg) without adjuvant. Eligible participants were non-pregnant, healthy volunteers or individuals with controlled chronic illness who were ≥18 years of age and provided written informed consent for study participation. Complete eligibility criteria are provided at http://clinicaltrials.gov/ct2/show/NCT00963157. Participants were enrolled from 24 September through 16 November 2009.
Vaccine and Adjuvant
The study vaccine was a monovalent, inactivated, subvirion, preservative-free preparation of the New York Medical College X-179A reassortant of the A/California/07/2009 H1N1 and PR8 strains, manufactured as previously described [17]. The adjuvanted formulations included 0.25 mL of AS03 per 0.5 mL dose. The study vaccine formulations were prepared just prior to administration, all with an injection volume of 0.5 mL.
Study Procedures
Participants in each age stratum (18–64 years and ≥65 years) were randomized with equal probability to 1 of the 5 study groups. Participants received an injection of 0.5 mL of the assigned vaccine formulation intramuscularly in the deltoid on day 0 and received the same vaccine formulation again at the day 21 visit. Vaccines were prepared and administered by unblinded staff members who were not involved with subsequent participant follow-up. Participants attended study clinic visits for screening (days −21 to 0) and on days 0, 8, 21, 29, 42, 201 (6 months after the second vaccination), and 291. At the screening visit, a blood sample was collected for testing for alanine transaminase (ALT) level. Blood samples for antibody assays were collected on days 0, 8, 21, 29, 42, 201, and 291. Blood samples for clinical safety laboratory tests were collected on days 0, 8, 21, and 29 (data not shown). Safety visits were conducted via telephone on days 2, 23, 81, 141, and 386.
Immunogenicity Assays
Microneutralization (MN) and hemagglutination inhibition (HAI) assays were performed using the A/California/07/2009 (H1N1) influenza virus as previously described [17].
Reactogenicity and Safety
At each vaccination visit, participants were provided with a memory aid form to record the presence and severity of local signs (redness and swelling) and symptoms (pain and tenderness), systemic symptoms (feverishness, malaise, myalgia, headache, nausea, chills, arthralgia, and shivering), and oral temperature on the evening of vaccination and for the subsequent 7 days. Local and systemic symptoms were graded as mild if they did not interfere with daily activities, moderate if they resulted in some interference with daily activities, and severe if they prevented participants from engaging in daily activities. Pain that did not interfere with normal activities but required the use of pain medications was defined as moderate. Right and left supraclavicular and axillary lymph nodes were assessed by a study clinician prior to vaccination and at the study visits on days 8 and 21 after each vaccination.
Statistical Analysis
The 2 coprimary immunologic end points, defined at day 21 after the first vaccination, included the proportion of participants who had an HAI titer ≥1:40 and the proportion of participants who met the definition of seroconversion (≥4-fold increase in HAI titer from baseline or a post-vaccination titer ≥1:40 if the baseline titer was <1:10). HAI and MN titers below the limit of detection were assigned a value of 5. Analysis of covariance was used to test for the association of log-transformed titer and prior influenza vaccination, controlling for age, vaccine dose, and adjuvant.
The vaccine and adjuvant manufacturers provided the study products at no cost but had no role in the conduct of the study, analysis of the data, or preparation of this report. The study was approved by the institutional review boards of record of each of the participating study sites.
RESULTS
A total of 789 participants aged 18–91 years received the first dose of study vaccine, and 736 received the second dose (Figure 1). Baseline demographic characteristics of enrolled participants are shown in Table 1. There were no statistically significant differences in demographic characteristics between study groups in each of the 2 age strata.
Figure 1.
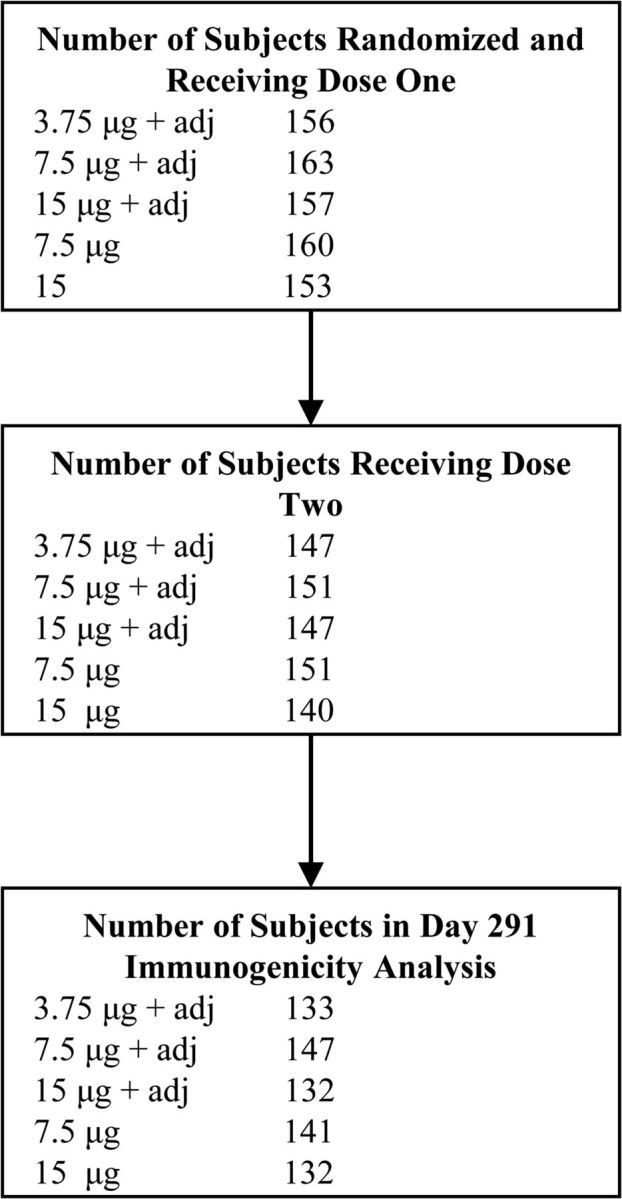
Flowchart of participant enrollment and follow-up.
Table 1.
Demographic Characteristics by Study Group and Age Stratum
| Age Strata | Characteristic | 3.75 µg+ Adjuvant | 7.5 µg+ Adjuvant | 15 µg+ Adjuvant | 7.5 µg | 15 µg |
|---|---|---|---|---|---|---|
| 18–64 y | Mean age (y), (SD) | 42.3 (13.2) | 42.6 (14.2) | 40.9 (14.0) | 42.4 (13.1) | 42.6 (13.3) |
| N = 544 | Female, % | 59 | 50 | 53 | 57 | 50 |
| Race, white, % | 83 | 81 | 80 | 84 | 82 | |
| 65+ y | Mean age (y), (SD) | 71.1 (5.5) | 71.0 (6.0) | 70.9 (5.1) | 72.0 (5.5) | 74.1 (6.7) |
| N = 245 | Female, % | 53 | 54 | 56 | 60 | 41 |
| Race, white, % | 96 | 98 | 88 | 94 | 89 |
Abbreviations: SD, standard deviation.
Safety
Local adverse events tended to be more common in the adjuvanted vaccine groups (Table 2). Elevated oral temperature was infrequent but was only reported by participants who received adjuvanted vaccine. Severe reactions were uncommon.
Table 2.
Percentage of Subjects Reporting Solicited Adverse Events Following Vaccination, According to Study Group and Vaccination
| First Vaccination |
Second Vaccination |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reactogenicity | 3.75 µg+ Adjuvant | 7.5 µg+ Adjuvant | 15 µg+ Adjuvant | 7.5 µg | 15 µg | 3.75 µg+ Adjuvant | 7.5 µg+ Adjuvant | 15 µg+ Adjuvant | 7.5 µg | 15 µg |
| Pain | ||||||||||
| Any | 57.7 | 58.9 | 57.3 | 15.6 | 22.9 | 49.7 | 54.3 | 44.9 | 10.6 | 20.0 |
| Mod + severe | 9.6* | 14.1* | 12.7* | 1.3 | 2.0 | 8.8* | 6.0* | 3.4 | 0.7 | 1.4 |
| Severe | 0 | 0 | 0 | 0 | 0.7 | 0 | 0 | 0 | 0 | 0 |
| Tenderness | ||||||||||
| Any | 78.2 | 79.8 | 73.2 | 23.1 | 35.9 | 70.7 | 74.2 | 68.0 | 24.5 | 36.4 |
| Mod + severe | 9.6* | 13.5* | 11.5* | 1.3 | 2.0 | 8.2* | 6.6 | 4.1 | 0 | 2.1 |
| Severe | 0 | 0.6 | 0 | 0 | 0.7 | 0 | 0 | 0 | 0 | 0 |
| Redness | ||||||||||
| Any | 26.3 | 22.1 | 28.0 | 20.6 | 26.1 | 19.0 | 19.2 | 27.2 | 19.9 | 21.4 |
| ≥20 mm | 1.9 | 4.3 | 6.4* | 0 | 1.3 | 5.4 | 2.6 | 4.1 | 2.0 | 1.4 |
| >50 mm | 0.6 | 1.2 | 1.9 | 0 | 0 | 2.7 | 0 | 2.0 | 0 | 0 |
| Swelling | ||||||||||
| Any | 23.1 | 18.4 | 20.4 | 13.8 | 18.3 | 18.4 | 11.3 | 20.4 | 16.6 | 16.4 |
| ≥20 mm | 4.5 | 6.1* | 7.0* | 0 | 0.7 | 6.1* | 2.6 | 2.7 | 0.0 | 0 |
| >50 mm | 1.9 | 4.3 | 3.8 | 0 | 0.7 | 2.7 | 1.3 | 0.7 | 0 | 0 |
| Oral Temperature | ||||||||||
| ≥38°C | 1.3 | 2.5 | 1.9 | 0 | 0 | 5.4 | 2.6 | 4.1 | 0 | 0 |
| ≥38.5°C | 1.3 | 1.2 | 0 | 0 | 0 | 1.4 | 0 | 0.7 | 0 | 0 |
| ≥39°C | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Feverishness | ||||||||||
| Any | 9.6 | 11.0 | 14.0 | 3.1 | 6.5 | 18.4 | 16.6 | 21.8 | 4.0 | 5.7 |
| Mod + severe | 1.9 | 4.9* | 3.8 | 0.0 | 0.7 | 3.4 | 7.3* | 6.1 | 0.7 | 2.1 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.7 | 0 | 0 |
| Malaise | ||||||||||
| Any | 26.9 | 25.2 | 28.0 | 18.1 | 20.9 | 32.0 | 27.2 | 27.2 | 11.3 | 13.6 |
| Mod + severe | 7.7 | 7.4 | 8.9 | 3.8 | 5.9 | 9.5 | 10.6* | 9.5 | 2.6 | 3.6 |
| Severe | 0 | 1.2 | 1.3 | 0 | 0.7 | 0 | 0.7 | 0.7 | 0 | 0.7 |
| Myalgia | ||||||||||
| Any | 20.5 | 26.4 | 28.7 | 8.1 | 15.7 | 25.9 | 25.8 | 23.8 | 6.6 | 10.0 |
| Mod + severe | 4.5 | 3.1 | 5.1 | 1.9 | 3.9 | 5.4 | 9.3* | 10.2* | 2.0 | 2.9 |
| Severe | 0 | 0.6 | 0 | 0 | 0.7 | 0 | 0 | 1.4 | 0 | 0.7 |
| Headache | ||||||||||
| Any | 25.6 | 23.3 | 22.3 | 21.9 | 24.8 | 23.8 | 31.1 | 21.1 | 17.9 | 15.0 |
| Mod + severe | 3.2 | 4.3 | 6.4 | 2.5 | 4.6 | 4.8 | 7.3 | 3.4 | 4.0 | 5.0 |
| Severe | 0 | 0 | 0 | 0 | 1.3 | 0.7 | 0 | 1.4 | 0 | 0 |
| Nausea | ||||||||||
| Any | 7.7 | 5.5 | 7.6 | 4.4 | 6.5 | 10.9 | 6.6 | 6.8 | 3.3 | 3.6 |
| Mod+severe | 1.3 | 1.2 | 1.3 | 0.6 | 2.0 | 4.8 | 1.3 | 1.4 | 0.7 | 0.7 |
| Severe | 0 | 0.6 | 0 | 0 | 0.7 | 0.7 | 0 | 0.7 | 0 | 0 |
| Chills | ||||||||||
| Any | 10.9 | 9.2 | 7.6 | 3.1 | 7.8 | 12.9* | 11.9 | 13.6 | 2.0 | 3.6 |
| Mod + severe | 3.8 | 2.5 | 2.5 | 0 | 0.7 | 6.1 | 4.0 | 2.7 | 0.7 | 0.7 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arthralgia | ||||||||||
| Any | 8.3 | 6.7 | 11.5 | 2.5 | 5.9 | 7.5 | 11.3 | 8.8 | 4.0 | 6.4 |
| Mod + severe | 0.6 | 1.8 | 2.5 | 1.3 | 0.7 | 1.4 | 4.0 | 2.7 | 1.3 | 2.1 |
| Severe | 0 | 0.6 | 0 | 0 | 0.7 | 0 | 0 | 0 | 0 | 0.7 |
| Shivering | ||||||||||
| Any | 4.5 | 5.5 | 4.5 | 1.9 | 3.3 | 9.5 | 6.6 | 10.9 | 2.0 | 1.4 |
| Mod + severe | 0.6 | 1.2 | 1.9 | 0 | 0 | 4.1 | 2.0 | 2.0 | 0.7 | 0.7 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
*P value < .05 in pair-wise comparisons of the proportion with moderate or severe grade events in each of the first four groups with the 15 µg (no adjuvant) group.
Supraclavicular or axillary lymph nodes were detected at the day 8 or day 21 post-vaccination visit in 91 participants. Of those, an axillary lymph node >1 cm in size (range, 1.2–2.0 cm) was detected in 12 participants (7 after adjuvanted vaccine and 5 after unadjuvanted vaccine), and in 6 of those 12, the axillary node was ipsilateral to the vaccination site. One of those 6 participants also had an ipsilateral supraclavicular node >1 cm (1.2 cm) detected; no other participant had a supraclavicular node >1 cm detected. Complications of lymphadenopathy, such as fluctuance, erythema, or ulceration, were not noted.
A total of 125 unsolicited adverse events were judged by the investigator to be associated with vaccination. Of those, 32 were respiratory tract disorders, 27 were related to the injection site, and 20 were musculoskeletal symptoms. Forty-nine serious adverse events were reported, including 8 deaths (4 in recipients of adjuvanted vaccine); none were judged by the investigator to be associated with vaccination.
Immunogenicity
At baseline, HAI GMTs were generally low (Table 3). In the 18–64 year age group, HAI GMTs at day 21 after dose one were higher in the adjuvanted groups, compared with 15 µg unadjuvanted group, and this difference was statistically significant for the comparison of the 15 µg + AS03 group with the 15 µg unadjuvanted group. In the adjuvanted groups, there was a further increase in HAI GMT after the second vaccination, and at this time point, the GMT in each of the adjuvanted groups and the proportion with an HAI titer ≥40 was significantly higher than in the 15 µg unadjuvanted group. There were no statistically significant differences in GMT among the 3 adjuvanted vaccine study groups at 21 days after dose one or at 21 days after dose two.
Table 3.
Hemagglutinin Inhibition Antibody End Points, by Study Group and Age Stratum
| Immunogenicity End Point | 3.75 + AS03 | 7.5 + AS03 | 15 + AS03 | 7.5 | 15 |
|---|---|---|---|---|---|
| Age 18 through 64 y | |||||
| Titer ≥ 1:40 – % (95% CI) | |||||
| Baseline | 11 (6–19) | 10 (5–17) | 9 (5–16) | 10 (5–17) | 10 (5–18) |
| 21 d after dose one | 90 (83–95) | 89 (82–94) | 95 (89–98) | 74 (65–82) | 83 (74–90) |
| 21 d after dose two | 98 (93–100)a | 97 (92–99)a | 98 (93–100) | 79 (70–87) | 82 (73–89) |
| 6 mo after dose two | 92 (85–96) | 84 (76–91) | 86 (78–93) | 67 (56–76) | 71 (61–80) |
| 9 mo after dose two | 79 (69–87) | 71 (61–80) | 77 (67–85) | 59 (49–69) | 65 (54–74) |
| Seroconversion – % (95% CI) | |||||
| 21 d after dose one | 86 (78–92) | 88 (80–93) | 92 (85–97) | 69 (59–77) | 82 (73–89) |
| 21 d after dose two | 95 (89–98)a | 96 (90–99)a | 96 (90–99) | 75 (66–83) | 81 (72–88) |
| Geometric mean titer – value (95% CI) | |||||
| Baseline | 8 (6–9) | 7 (6–9) | 7 (6–9) | 7 (6–9) | 7 (6–8) |
| 21 d after dose one | 266 (200–354) | 270 (199–366) | 341 (262–444) | 120 (82–175) | 185 (132–259) |
| 21 d after dose two | 483 (396–590)a | 402 (316–512)a | 465 (373–581) | 124 (87–175) | 181 (131–249) |
| 6 mo after dose two | 132 (105–167) | 107 (82–140) | 133 (104–172) | 59 (43–81) | 69 (51–95) |
| 9 mo after dose two | 82 (62–111) | 72 (52–101) | 79 (59–106) | 41 (29–57) | 46 (33–65) |
| Age ≥65 y | |||||
| Titer ≥ 1:40 – % (95% CI) | |||||
| Baseline | 8 (2–20) | 19 (10–33) | 23 (12–38) | 14 (6–27) | 9 (2–21) |
| 21 d after dose one | 71 (56–83)b | 81 (67–90) | 89 (76–96) | 56 (41–71)b | 61 (45–76)b |
| 21 d after dose two | 82 (67–92)b | 94 (82–99) | 95 (83–99) | 63 (48–77)b | 68 (50–82) |
| 6 mo after dose two | 66 (50–80) | 77 (62–88) | 90 (76–97) | 57 (41–71) | 50 (33–67) |
| 9 mo after dose two | 49 (33–65) | 64 (49–77) | 75 (59–87) | 38 (24–54) | 36 (21–54) |
| Seroconversion – % (95% CI) | |||||
| 21 d after dose one | 67 (52–80)b | 71 (57–83)b | 82 (68–92) | 40 (26–55)b | 56 (39–70)b |
| 21 d after dose two | 75 (60–87)b | 87 (74–95) | 90 (76–97) | 50 (35–65)b | 62 (45–78)b |
| Geometric mean titer – value (95% CI) | |||||
| Baseline | 7 (6–9) | 11 (7–15) | 10 (7–15) | 8 (6–11) | 7 (6–10) |
| 21 d after dose one | 82 (50–136)b | 124 (80–193)b | 161 (102–254)b | 48 (28–81)b | 55 (31–100)b |
| 21 d after dose two | 125 (79–200)b | 195 (135–283)b | 222 (154–320)b | 61 (37–98)b | 67 (36–124)b |
| 6 mo after dose two | 51 (36–72) | 80 (53–122) | 95 (68–133) | 35 (23–55) | 35 (20–62) |
| 9 mo after dose two | 32 (22–48) | 51 (32–79) | 54 (35–83) | 19 (12–32) | 21 (12–35) |
Bold indicates P < .05 for comparisons of each of the first four listed study groups with the “standard” 15 mcg group, by time point, and within each age stratum (eg, geometric mean titer in the 15 + AS03 group vs the 15 group, at 21 days after dose one, in the 18 through 64 year age group [341 vs 185]). Only comparisons of values reported at the time points of 21 days after dose one and 21 days after dose two are reported in the table.
aP < .05 in comparisons of 21 days after dose one and 21 days after dose two, within each study group and age stratum (eg, comparison of % with titer ≥40 21 days after dose one and 21 days after dose two in the 3.75 + AS03 study group among the 18 through 64 years age stratum [90% vs 98%]).
bP < .05 in comparisons of the younger vs the older age strata, at 21 days post dose one and at 21 days post dose two, within each study group (eg, comparison of % seroconversion in the 18 through 64 year old age group compared with the ≥65 year old age group, at 21 days after dose one, among those who received 3.75 + AS03 [86% vs 67%]).
In the ≥65 year age group, post-vaccination HAI GMTs were consistently lower than in the 18–64 year age group. There was evidence of a dose response to the adjuvanted vaccine formulations, and at 21 days after dose one and 21 days after dose two, the GMTs in the 7.5 µg + AS03 group and the 15 µg + AS03 group were significantly higher than in the 15 µg unadjuvanted group. Only 68% of participants achieved an HAI titer ≥1:40 after 2 doses of the 15 µg unadjuvanted formulation, whereas 81% achieved that titer after a single dose of the 7.5 µg + AS03 formulation, and the proportion increased to 94% after a second dose of that formulation.
In both age groups, antibody levels decreased during the 9 months after dose two. However, in the 18–64 year age group, 92% of participants in the 3.75 µg + AS03 group maintained a titer ≥1:40 at 6 months after dose two, compared with 71% in the 15 µg unadjuvanted group. In the ≥65 year age group, 90% of participants in the 15 µg + AS03 group maintained a titer ≥1:40 at 6 months after dose two, compared with 50% in the 15 µg unadjuvanted group.
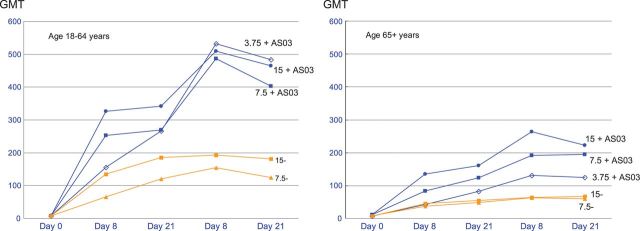
In evaluations of the kinetics of the immune response to each vaccination, much of the increase in HAI GMT seen at 21 days after dose one occurred by 8 days after vaccination (Figure 2). After the second vaccination, the HAI GMTs peaked at day 8 and then tended to decrease slightly by day 21 in the adjuvanted vaccine groups.
Figure 2.
Hemagglutination inhibition antibody geometric mean titers at days 0, 8, and 21 after dose one and days 8 and 21 after dose two among participants 18–64 and ≥65 years of age.
In analyses stratified into 5 age groups (18–35, 36–50, 51–64, 65–69, and ≥70 years), the highest HAI GMTs in each vaccine study group were in the 18–35 year age group (Table 4). The GMTs were generally similar between the 36–50 and 51–64 year age groups and were lower in the ≥65 year age groups. In comparison of the 7.5 µg and 15 µg doses of unadjuvanted vaccine, among the 3 younger age groups, there was some evidence of a dose response, but those differences were not statistically significant. In the 2 oldest age groups, responses to the 7.5 µg and 15 µg formulations were similar.
Table 4.
Hemagglutinin Inhibition Antibody Geometric Mean Titers and 95% Confidence Intervals, After Dose One and Dose Two, by Study Group and by Age Groups within the Age Strata
| Age Group | Study Group |
||||
|---|---|---|---|---|---|
| 3.75 + AS03 | 7.5 + AS03 | 15 + AS03 | 7.5 | 15 | |
| 21 d after dose one | |||||
| 18–35 y | 403 (261–622) | 409 (252–665) | 604 (468–780) | 199 (112–354) | 351 (218–563) |
| 36–50 y | 195 (116–327)* | 237 (135–413) | 294 (189–459)* | 85 (38–188) | 141 (72–277)* |
| 51–64 y | 226 (130–393) | 200 (114–351) | 144 (72–288)* | 90 (46–175) | 120 (66–217)* |
| 65–69 y | 84 (42–168)* | 88 (45–175)* | 164 (93–290)* | 48 (23–101)* | 53 (13–225)* |
| ≥70 y | 80 (36–179)* | 185 (108–315)* | 157 (69–359)* | 48 (21–108)* | 56 (28–111)* |
| 21 d after dose two | |||||
| 18–35 y | 567 (400–803) | 671 (515–874) | 684 (559–837) | 237 (147–380) | 351 (227–541) |
| 36–50 y | 427 (293–623) | 320 (184–556)* | 435 (292–649)* | 92 (43–195)* | 131 (69–249)* |
| 51–64 y | 458 (339–621) | 289 (189–441)* | 243 (130–451)* | 77 (42–140)* | 122 (68–216)* |
| 65–69 y | 104 (52–207)* | 189 (113–317)* | 299 (204–437)* | 52 (27–102)* | 48 (14–165)* |
| ≥70 y | 157 (81–306)* | 204 (115–362)* | 136 (65–283)* | 70 (33–148)* | 74 (35–157)* |
*P < .05 in comparisons with the 18–35 year old age group.
The association of prior receipt of seasonal influenza vaccine and response to the study vaccine (at 21 days after dose two) among the 18–64 year ohort was evaluated in an analysis of covariance model including age group (18–35, 36–50, and 51–64), study group, and prior receipt of seasonal vaccine status (categorized as receipt of neither or both of the 2008/2009 and 2009/2010 seasonal influenza vaccines [the category of receipt of 2009/2010 vaccine but not 2008/2009 vaccine was not evaluated because of small sample size]). The analyses were restricted to the 18–64 year cohort because very few participants ≥65 years of age had not previously received seasonal influenza vaccine. In the model, older age and prior receipt of both seasonal vaccines were independently associated with significantly lower HAI GMT responses (P < .01 after adjustment for multiple comparisons).
The absolute values of titers detected by the MN assay were higher than those detected by the HAI assay, but the patterns of response — by age, study group, and over time — were generally similar to those found in analyses of the HAI titers (Table 5).
Table 5.
Microneutralization Antibody End Points, by Study Group and Age Stratum
| Immunogenicity End Point | 3.75 + AS03 | 7.5 + AS03 | 15 + AS03 | 7.5 | 15 |
|---|---|---|---|---|---|
| Age 18 through 64 years | |||||
| Titer ≥ 1:40 – % (95% CI) | |||||
| Baseline | 17 (10–25) | 15 (9–23) | 17 (10–25) | 13 (7–20) | 14 (8–22) |
| 21 d after dose one | 93 (87–97) | 90 (83–95) | 96 (90–99) | 85 (76–91) | 88 (80–93) |
| 21 d after dose two | 99 (95–100) | 99 (95–100)a | 95 (95–100) | 90 (83–95) | 92 (84–96) |
| 6 mo after dose two | 98 (93–100) | 97 (92–99) | 99 (94–100) | 89 (81–94) | 92 (84–96) |
| 9 mo after dose two | 92 (85–97) | 85 (76–91) | 90 (82–95) | 80 (70–87) | 78 (69–86) |
| Seroconversion – % (95% CI) | |||||
| 21 d after dose one | 90 (82–95) | 89 (82–94) | 92 (85–97) | 75 (66–83) | 86 (78–92) |
| 21 d after dose two | 96 (90–99) | 98 (93–100)a | 95 (89–98) | 81 (72–88) | 89 (80–94) |
| Geometric mean titer – value (95% CI) | |||||
| Baseline | 11 (9–13) | 10 (8–12) | 12 (9–15) | 10 (8–12) | 10 (8–12) |
| 21 d after dose one | 371 (282–489) | 370 (282–484) | 486 (383–616) | 208 (150–289) | 272 (201–369) |
| 21 d after dose two | 599 (495–725)a | 509 (422–615) | 607 (502–734) | 228 (172–302) | 282 (215–370) |
| 6 mo after dose two | 350 (282–434) | 342 (271– 432) | 358 (288– 445) | 215 (163– 285) | 243 (183– 322) |
| 9 mo after dose two | 180 (139–233) | 165 (124–220) | 185 (144–238) | 108 (79–146) | 123 (91–168) |
| Age ≥65 y | |||||
| Titer ≥ 1:40 – % (95% CI) | |||||
| Baseline | 16 (7–30) | 31 (19–45) | 21 (11–36) | 18 (9–31) | 15 (6–29) |
| 21 d after dose one | 85 (72–94) | 88 (77–96) | 89 (76–96) | 58 (43–72)b | 70 (55–83)b |
| 21 d after dose two | 91 (78–97)b | 98 (89–100) | 95 (83–99) | 74 (59–86)b | 68 (50–82)b |
| 6 mo after dose two | 95 (85–99) | 91 (80–98) | 98 (87–100) | 74 (59–86) | 72 (55–86) |
| 9 mo after dose two | 79 (64–90) | 83 (69–92) | 85 (70–94) | 63 (47–77) | 56 (38–72) |
| Seroconversion – % (95% CI) | |||||
| 21 d after dose one | 75 (60–85)b | 73 (59–84)b | 84 (71–94) | 46 (31–61)b | 64 (48–78)b |
| 21 d after dose two | 75 (60–87)b | 83 (69–92)b | 90 (76–97) | 61 (45–75)b | 69 (52–84)b |
| Geometric mean titer – value (95% CI) | |||||
| Baseline | 12 (9–16) | 20 (13–31) | 15 (10–22) | 13 (10–18) | 12 (9–17) |
| 21 d after dose one | 147 (98–220)b | 248 (169–366) | 298 (194–456)b | 86 (53–139)b | 87 (53–144)b |
| 21 d after dose two | 242 (164–357)b | 264 (185–377)b | 314 (215–460)b | 111 (70–175)b | 101 (60–171)b |
| 6 mo after dose two | 209 (144–304) | 264 (184–378) | 301 (218–416) | 96 (64–143) | 109 (62–190) |
| 9 mo after dose two | 71 (51–100) | 113 (77–165) | 109 (74–161) | 62 (39–97) | 51 (30–87) |
Bold indicates P < .05 for comparisons of each of the first four listed study groups with the “standard” 15 mcg group, by time point, and within each age stratum. Only comparisons of values reported at the time points of 21 days after dose one and 21 days after dose two are reported in the table.
aP < .05 in comparisons of 21 days after dose one and 21 days after dose two, within each study group and age stratum.
bP < .05 in comparisons of the younger vs the older age strata, at 21 days post dose one and at 21 days post dose two, within each study group.
DISCUSSION
In this randomized trial of adults ≥18 years of age, a single dose of AS03 adjuvanted vaccine containing 15 µg of hemagglutinin induced a significantly higher HAI GMT than did the standard 15 µg dose of unadjuvanted vaccine in both younger adults and those ≥65 years of age In addition, the HAI GMTs were higher (but not significantly different) in the 3.75 µg + AS03 group than in the 15 µg unadjuvanted group, indicating that addition of the AS03 adjuvant was dose sparing. In both age groups, there were further increases in titer after a second dose of adjuvanted vaccine. In contrast, the relatively poor response to a first dose of the unadjuvanted vaccine formulations was not substantially enhanced by the second vaccination in either age group.
We also evaluated the immune response at an early time point, 8 days after each dose of vaccine, in addition to the more typical evaluation at 21 days after vaccination. In most of the groups, much of the increase in HAI titer achieved by day 21 after the first vaccination was present at day 8. These results suggest that at least partial clinical protection may be achieved within a week after a first vaccination.
In all of the study groups, the postvaccination GMTs were considerably lower in adults ≥65 years of age than in younger adults, as has been reported in other evaluations of unadjuvanted and AS03 adjuvanted 2009 H1N1 vaccines [13, 14, 18–21]. However, in our analyses of more finely stratified age groups (18–35, 36–50, 51–64, 65–69, and ≥70 years), we also found evidence of age-related differences in HAI response among adults <65 years of age, with trends toward higher GMTs in those 18–35 years of age, compared with the older age groups, for both adjuvanted and unadjuvanted vaccines. Similar differences by age have been noted in other studies of AS03 adjuvanted and unadjuvanted 2009 H1N1 vaccines [11, 13, 22]. The difference in responses between the 18–35 year age group and the 36–50 and 51–64 year age groups suggests that age-related decreases in immunogenicity occur before age 65 and that homogeneity of vaccine responses among adults <65 years of age should not be assumed.
As with several other evaluations of 2009 H1N1 vaccines [11, 18, 23–25], our results suggest that prior receipt of seasonal influenza vaccine is associated with a lower response to 2009 H1N1 vaccine. Prior receipt of seasonal influenza vaccine has also been associated with lower responses to H5N1 influenza vaccines [26]. Mechanisms for this immunologic interference have been postulated [24, 26, 27], but its occurrence and impact, if any, on protective efficacy requires further investigation.
We evaluated the responses to a half dose (7.5 µg) and full dose (15 µg) of unadjuvanted vaccine and found that, among participants ≥65 years, there was little difference in the immune response to the half dose and full dose, whereas among younger participants, GMTs were somewhat higher after administration of the 15 µg unadjuvanted vaccine; however, the differences were not statistically significant. Our findings are consistent with other evaluations of intramuscular administration of full or reduced doses of trivalent inactivated influenza vaccines in both young and older adults that found either no significant dose-related differences in immune response or only modest differences by dose [28–33]. This suggests that generally comparable levels of protection may be obtained by administration of a half dose or full dose unadjuvanted 2009 H1N1 vaccine in both younger and older adults.
Axillary or supraclavicular lymphadenopathy has been reported in a minority of participants after receipt of AS03 adjuvanted vaccines. In a study of an AS03 adjuvanted H5N1 influenza vaccine, lymphadenopathy was reported as an unsolicited adverse event in 7 (3.5%) of 200 participants given the adjuvanted vaccine formulation but was not identified in participants who received unadjuvanted vaccine [3]. In a study of another AS03 adjuvanted H5N1 vaccine, lymph node enlargement or tenderness was solicited by physical examination per protocol and was detected in 4.6% of participants after a first or second dose of an AS03 adjuvanted vaccine formulation, compared with 3.8% of participants after a first or second dose of unadjuvanted vaccine [2]. In a placebo-controlled trial of an AS03 adjuvanted H5N1 vaccine, lymphadenopathy was rarely identified and was no more common in the vaccine than in the placebo group [34]. In our study, axillary and supraclavicular lymphadenopathy were solicited adverse events assessed at days 8 and 21 after each vaccination, but lymph nodes >1 cm in size were detected in <2% of (3.5%) in either the adjuvanted or unadjuvanted vaccine groups.
Consistent with previous studies of AS03 adjuvanted influenza vaccines in adults, we found that solicited local adverse events and some solicited systemic adverse events tended to be more commonly reported in groups given an adjuvanted formulation but that most of the adverse events were mild or moderate in severity, and they were self limited [2–4, 11]. The inactivated 2009 H1N1 study vaccine with extemporaneously mixed AS03 adjuvant was well tolerated in the study population of adults ≥18 years of age and allowed dose sparing, suggesting the feasibility of matching vaccine from different sources with available supplies of adjuvant in the context of an influenza pandemic.
Notes
Acknowledgments. We thank the many members of the study teams and other individuals who contributed to the conduct of this clinical trial, including Tom Archer, Connie Baum, Joyce Benoit, Patti Benson, Lora Bounds, Shannon Byler, Barbara Carste, Carol Dean, Nancy Dorn, Maya Dunstan, Farah Hawasli, Michelle Hill, Joanne Greene, Edd Keudell, Abraham Le, Bill Lee, Angel Mathis, Hallie Phillips, Anita Ross, Marie Schwartz, Theresa Shea, Pat Starkovich, Janice Suyehira, Amena Wardak (Group Health); Sally Mackey, Sue Swope, Anthony Trela, Barbara Sullivan, Martha Hamilton, Elizabeth Farman-Farmaian, Allison Akana, Sandra O'Neal, Dianne Christopherson, Sara Sherman-Levine, Raquel Fleischmann, Kyrsten Spann, Thu Quan, Michele Ugur, Sri Srivasatava, Ashima Goel, Brian Tse, Alicia Gutierrez, Dianne Vierra (Stanford University); Joshua Schiffer, Nicholas Moss, Negusse Ocbamichael, Claire Stevens, Steven Kuntz, Anne Jo-Nes, Meredith Potochnic, Robert Santucci (University of Washington); Melissa Billington, Joel Chua, Giovanna Yanez, Paul Kamate, Panagiota Komninou, Jeffrey Barbers, and Maria Johnson (University of Maryland); Amy Cline, Tara Foltz, Stacy Harman, Karen Cossins, Amy Hoeper, Vicki Smith, Sally McCartney, Tammy Lewis-McCauley, Michelle Dickey, Jesse LePage, Kristie Price, Joanna Quattrone, Sherry Haubert, and Heather Frommeyer (Cincinnati Children's Hospital Medical Center); Dan Zhao, Nancy Wagner, Kathryn Flanders, Mary Reidy, Ellen Segar, Deborah Pearson, Geri Dull, Michelle Rodenburg, Nicole Gerot, and Heather Grieser-Yoder (University of Iowa); Jane Skvarich, Vickie Grimes, Ellen DeStefano, Nayoka Rimann, Rebecca Gerkin, Ildefonso Tellez, Ming Liu, Beverly Weaver, Kai Ying, Paula Frew, Brooke Hartwell, Alexis Daugherty, Kathleen Stephens, Paul Spearman, Joseph Hilinski, Allison Ross, Andrea Shane, Jianguo Xu, Eileen Osinski, Theron Stuart, Susan Rogers, Eric Strait, Talib Sirajud-Deen (Emory University); Barbara Taggart and the technical staff at the Southern Research Institute; Ashley Vena (EMMES); Robin Mason, Katherine Muth, Soju Chang, Robert Johnson, Angelita Ray, Richard Gorman, Wendy Buchanan, Linda Lambert, Shy Shorer, and Suzanne Murray (National Institute of Allergy and Infectious Diseases, National Institutes of Health [NIH]), and the members of the Safety Monitoring Committee (Robert Salata [Chair], David Kimberlin, Margo Schilling, Donald Stablein, Jeanne Sheffield, David Rimland, Mitchell Cohen, and Sam Kocoshis) for their valuable input.
Financial support. This work was primarily supported by Vaccine and Treatment Evaluation Unit contracts from the National Institutes of Health (contracts HHSN272200800004C [Group Health], HHSN27220080000C [Vanderbilt], HHSN272200800001C [Maryland], HHSN272200800005C [Emory], HHSN272200800008C [Iowa], and HHSN272200800006C [Cincinnati]), and by contract N01-AI-30063 to Southern Research Institute. At Emory University, Stanford University, the University of Washington, the University of Maryland, and the University of Iowa, partial support was also provided by additional funding sources, including: the Atlanta Clinical and Translational Science Institute, Supported by Public Health Service (PHS) Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Center for Research Resources (NCRR), NIH; the Clinical and Translational Science Award 5UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the NCRR, NIH; the University of Washington Institute of Translational Health Science funded by UL1 RR025014, KL2 RR025015, and TL1 RR025016 from the NCRR and by PHS contract NO1-AI-80001 from NIAID, NIH; the University of Maryland General Clinical Research Center grant M01-RR-016500 from the NCRR, and by NCRR grant K12-RR-023250; and the Clinical and Translational Science Award 5UL1 RR025744 for the University of Iowa Institute for Clinical and Translational Science.
Potential conflicts of interest. L. A. J. has received research support (paid to institution) from GSK, Sanofi Pasteur, and Novartis Vaccines. J. T. S. has received grant support (paid to institution) for vaccine and HIV-related research from GSK and past grant support from Sanofi Pasteur. C. L. D. is on the scientific advisory board of Pharmajet. All other authors report no potential conflict. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Diez-Domingo J, Garces-Sanchez M, Baldo JM, et al. Immunogenicity and Safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase ii, randomized, open, controlled study. Pediatr Infect Dis J. 2010;29:e35–46. doi: 10.1097/INF.0b013e3181daf921. [DOI] [PubMed] [Google Scholar]
- 2.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201:1644–53. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 3.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–9. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 4.Chu DW, Hwang SJ, Lim FS, et al. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009;27:7428–35. doi: 10.1016/j.vaccine.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz TF, Horacek T, Knuf M, et al. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009;27:6284–90. doi: 10.1016/j.vaccine.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Heijmans S, De Meulemeester M, Reynders P, et al. Immunogenicity Profile of a 3.75-{micro}g hemagglutinin pandemic rH5N1 split virion AS03A-Adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis. 2011;203:1054–62. doi: 10.1093/infdis/jiq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Committee for medicinal products for human use post-authorisation summary for positive opinion for prepandrix. http://www.ema.europa.eu/pdfs/human/opinion/Prepandrix_33405509en.pdf. Accessed 12 May 2010. [Google Scholar]
- 8.Waddington CS, Walker WT, Oeser C, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. doi: 10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmona A, Omenaca F, Tejedor JC, et al. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6–35 months. Vaccine. 2010;28:5837–44. doi: 10.1016/j.vaccine.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Sicilia J, Gillard P, Carmona A, et al. Immunogenicity and safety of AS03-adjuvanted H1N1 pandemic vaccines in children and adolescents. Vaccine. 2011;29:4353–61. doi: 10.1016/j.vaccine.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03(A)-adjuvant: Preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–5. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Study 113456 ( NCT00968539): safety and immunogenicity study of GSK Biologicals’ Pandemic influenza vaccine comprising A/California/7/2009 (H1N1)v-like strain with adjuvant in adults aged 18 to 60 years. Accessed 15 July 2010. [Google Scholar]
- 13.Nicholson KG, Abrams KR, Batham S, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis. 2011;11:91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 14.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–77. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 15.Madhun AS, Akselsen PE, Sjursen H, et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine. 2010;29:266–73. doi: 10.1016/j.vaccine.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Health Canada. Notice of Decision for AREPANRIX™ H1N1. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/phase1-decision/drug-med/nd_ad_2009_arepanrix_h1n1_132070-eng.php. Accessed 15 July 2010. [Google Scholar]
- 17.Jackson LA, Patel SM, Swamy GK, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis. 2011;6:854–63. doi: 10.1093/infdis/jir440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 19.Liang XF, Wang HQ, Wang JZ, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 21.Talaat KR, Greenberg ME, Lai MH, et al. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J Infect Dis. 2010;202:1327–37. doi: 10.1086/656601. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 23.Andrews NJ, Walker WT, Finn A, et al. Predictors of immune response and reactogenicity to AS03B-adjuvanted split virion and non-adjuvanted whole virion H1N1 (2009) pandemic influenza vaccines. Vaccine. 2011;29:7913–9. doi: 10.1016/j.vaccine.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 24.Ohfuji S, Fukushima W, Deguchi M, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis. 2011;203:1301–8. doi: 10.1093/infdis/jir026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uno S, Kimachi K, Kei J, et al. Effect of prior vaccination with a seasonal trivalent influenza vaccine on the antibody response to the influenza pandemic H1N1 2009 vaccine: a randomized controlled trial. Microbiol Immunol. 2011;55:783–9. doi: 10.1111/j.1348-0421.2011.00381.x. [DOI] [PubMed] [Google Scholar]
- 26.Nolan T, Richmond PC, Formica NT, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–91. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 27.Leroux-Roels I, Roman F, Forgus S, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine. 2010;28:849–57. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010;50:1331–8. doi: 10.1086/652144. [DOI] [PubMed] [Google Scholar]
- 29.Jackson LA, Austin G, Chen RT, et al. Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine. 2001;19:4703–9. doi: 10.1016/s0264-410x(01)00225-0. [DOI] [PubMed] [Google Scholar]
- 30.Engler RJ, Nelson MR, Klote MM, et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med. 2008;168:2405–14. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]
- 31.Belshe RB, Newman FK, Wilkins K, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–63. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atmar RL, Keitel WA, Cate TR, Munoz FM, Ruben F, Couch RB. A dose-response evaluation of inactivated influenza vaccine given intranasally and intramuscularly to healthy young adults. Vaccine. 2007;25:5367–73. doi: 10.1016/j.vaccine.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treanor J, Keitel W, Belshe R, et al. Evaluation of a single dose of half strength inactivated influenza vaccine in healthy adults. Vaccine. 2002;20:1099–105. doi: 10.1016/s0264-410x(01)00440-6. [DOI] [PubMed] [Google Scholar]
- 34.Langley JM, Risi G, Caldwell M, et al. Dose-Sparing H5N1 A/Indonesia/05/2005 Pre-pandemic Influenza Vaccine in Adults and Elderly Adults: a Phase III, Placebo-Controlled, Randomized Study. J Infect Dis. 2011;203:1729–38. doi: 10.1093/infdis/jir172. [DOI] [PMC free article] [PubMed] [Google Scholar]