Abstract
Pertussis is a contagious, acute respiratory illness caused by the bacterial pathogen Bordetella pertussis. Although it is widely believed that transmission of B. pertussis occurs via aerosolized respiratory droplets, no controlled study has ever documented airborne transmission of pertussis. We set out to determine if airborne transmission occurs between infected and naive animals, utilizing the baboon model of pertussis. Our results showed that 100% of exposed naive animals became infected even when physical contact was prevented, demonstrating that pertussis transmission occurs via aerosolized respiratory droplets.
(See the editorial commentary by Fernandez, on pages 808–10.)
Whooping cough is a highly contagious, acute respiratory illness caused by the bacterial pathogen Bordetella pertussis (for review, see: [1]). The introduction of pertussis vaccines in the 1940s and nationwide coverage in children in excess of 95% led to a dramatic decrease in the disease. However, for unexplained reasons, pertussis rates in the United States have been steadily rising over the last 20 years in infants, children, and adolescents [2]. With greater than 27 000 reported cases in the United States in 2010, the highest number since the 1950s, pertussis is the most commonly occurring vaccine-preventable disease [2]. This resurgence is occurring throughout the industrial world despite similar high rates of vaccination [3].
In humans, infection with B. pertussis results in a wide spectrum of clinical manifestations, depending on the age and immune status of the host, and ranges from mild respiratory symptoms to a severe cough illness, which may be accompanied by the hallmark inspiratory whoop and post-tussive emesis [1]. Clinical signs include high leukocytosis, hypoglycemia, and reduced pulmonary capacity. Because of the acute nature of pertussis infections, and because B. pertussis is a strict human pathogen with no known animal or environmental reservoir, maintenance of the organism within the population is thought to require continuous transmission of the disease from infected to naive hosts.
Pertussis is often described as being highly infectious. In previous reports of household contact studies, attack rates ranged between 58% and 100%. However, differences in case definitions, incomplete documentation of vaccination history and antibiotic therapy, and retrospective data collection make the results from these studies difficult to interpret. In studies in which household contacts of confirmed index cases were prospectively followed with active surveillance, the average attack rate for unvaccinated children was 76% (range, 64%–86%) [4–9]. Attack rates outside of the household setting are difficult to quantify, but in classroom contact studies, attack rates were low, ranging from 0% to 36% [10–12]. Taken together, these observations indicate that transmission of pertussis requires repeated or prolonged exposure and/or close contact.
Although it is often stated that pertussis is transmitted via aerosolized respiratory droplets, no controlled study has ever documented airborne transmission of pertussis. These studies have been hampered by the fact that all of the current animal models of pertussis, including mice, rats, and pigs, do not exhibit transmission from infected to naive animals, even in close contact [13]. We hypothesized that our recently developed baboon model of pertussis would allow study of airborne transmission of pertussis due to the close genetic relationship of this species to humans, and because we observed intense paroxysmal coughing in pertussis-infected baboons. Naive baboons were cohoused with directly inoculated baboons, either in the same cage or in separate cages located 7 feet away. One hundred percent of naive baboons became infected, demonstrating airborne transmission of pertussis.
MATERIALS AND METHODS
Ethics Statement
All animal procedures were performed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with protocols approved by the Center for Biologics Evaluation and Research Animal Care and Use Committee and the principles outlined in the Guide for the Care and Use of Laboratory Animals by the Institute for Laboratory Animal Resources, National Research Council.
Bacterial Strains and Media
B. pertussis strain D420 was provided by the Centers for Disease Control. Bordet-Gengou (BG) agar plates were prepared with Bordet-Gengou agar (Becton-Dickinson, Sparks, MD) containing 1% proteose peptone (Becton-Dickinson) and 15% defibrinated sheep blood. Regan-Lowe plates were prepared from Regan-Lowe Charcoal Agar Base (Becton-Dickinson) with 10% defibrinated sheep blood and 40 µg/mL cephalexin.
Infection of Baboons
Baboons were obtained from the Oklahoma Baboon Research Resource at the University of Oklahoma Health Sciences Center and housed in a biocontainment unit designed to study airborne transmission (Figure 1). Inoculums were prepared to a concentration between 109 and 1010 bacteria/mL as previously described [14]. Baboons were anesthetized with 10–15 mg/kg ketamine administered intramuscularly. For directly inoculated (index) animals, the pharynx was swabbed with 2% lidocaine solution and animals were intubated using a 2–3 mm endotracheal tube to deliver 1 mL inoculum to the top of the trachea. A 24-gauge, 3.2 cm intravenous (IV) catheter (Abbocath) was used to deliver 0.5 mL inoculum to the back of each naris. Animals were placed in a sitting position for 3 minutes, returned to their cages, and observed until they recovered from anesthesia.
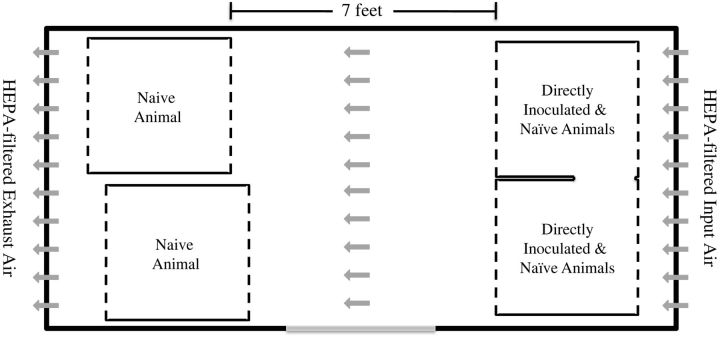
Figure 1.
Biocontainment unit for airborne transmission studies. The biocontainment unit for demonstrating airborne transmission of B. pertussis is diagrammed. Gray arrows indicate direction of airflow. Abbreviation: HEPA, high-efficiency particulate air.
Evaluation of Animals
Baboons were anesthetized with ketamine. Whole blood was evaluated for the number of circulating white blood cells by complete blood count (CBC). The back of each naris was flushed with 1 mL phosphate-buffered saline using a 24-gauge/3.2 cm IV catheter. The recovered nasopharyngeal washes from both nares were combined and 100 µL of the recovered sample was divided and plated onto 2 Regan-Lowe plates. The number of colony-forming units per plate was recorded after incubation at 37°C for 4–5 days. Infection with B. pertussis was confirmed by examining colony morphology and hemolysis on Bordet-Gengou blood agar plates and polymerase chain reaction amplification of IS481, a genomic insertion site that is specific for B. pertussis [1].
Statistics
For the leukocytosis data, statistical analysis was performed by comparing preinfection to peak infection with the 1-tail paired t test.
RESULTS
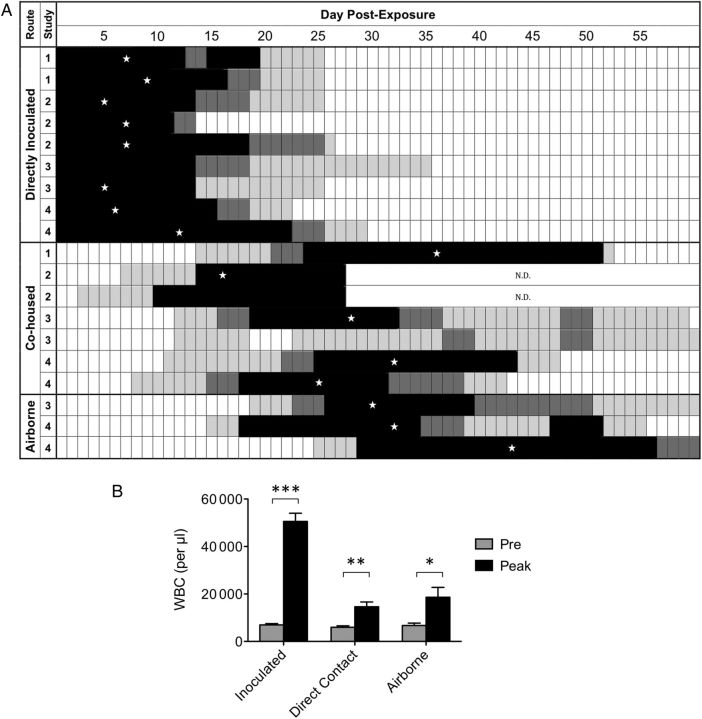
Although it is widely believed that transmission of B. pertussis occurs via aerosolized respiratory droplets, no controlled study has ever documented airborne transmission of B. pertussis. We utilized the baboon model of pertussis, which was developed in our laboratory [14], to determine if airborne transmission occurs between directly inoculated (index) baboons and naive baboons. We first studied whether transmission occurs between index and naive baboons under conditions where the animals are in close contact with one another. In our first transmission study, 2 baboons were directly inoculated as described in the “Materials and Methods” section, and cohoused in the same unit with 1 naive baboon. The naive baboon was introduced to the unit 24 hours after introduction of the index animals in the unit. Subsequently, all animals were evaluated for colonization of the upper airway by plating of nasopharyngeal washes and quantifying the number of colony-forming units, and were evaluated for circulating white blood cells in peripheral blood. A second study was performed in which 3 index baboons were placed in the same cages with 2 naive baboons. In these 2 studies, all 3 naive cohoused animals became infected as demonstrated by recovery of B. pertussis from their upper airways (Figure 2A) and by significant increases in circulating white blood cells (Figure 2B).
Figure 2.
Colonization and leukocytosis in directly inoculated and exposed baboons. Weanling baboons were either inoculated directly with B. pertussis, as described in “Materials and Methods,” or cohoused with inoculated baboons in the same caging unit, or housed in a caging unit 7 feet away from the caging unit housing the inoculated baboons. Nasopharyngeal washes were performed as described in “Materials and Methods” and the recovered wash was plated on 2 Regan-Lowe plates (50 μL per plate). A, The number of colony-forming units per plate is indicated for each day by the shading of the boxes in the row corresponding to each individual animal: white = 0, light gray = 1–200 colonies, dark gray = 201–2000 colonies, and black = >2000 colonies. A star indicates the day of peak leukocytosis for each animal except for 2 animals that did not achieve a peak at least 2-fold higher than baseline. B, Once prior to challenge and on the indicated days post challenge, blood was drawn from each baboon and the number of circulating WBCs per µl of peripheral blood was determined as described in “Materials and Methods.” The average preinoculation WBC count and the average peak WBC count following inoculation is shown for the inoculated group (n = 9). The average preexposure WBC count and the average peak WBC count following exposure is shown for the cohoused group (n = 7) and for the airborne transmission group (n = 3). Within each group, significance was determined for pre- vs peak values: *P < .05, **P < .005, ***P < .0005. Abbreviations: N.D., not determined; WBC, white blood cell.
Having determined that transmission can be reliably observed in the weanling baboon model when animals are allowed to directly interact with each other over a prolonged period of time, we proceeded with studies to determine if airborne transmission of B. pertussis can occur. In order to study airborne transmission, we designed a biocontainment unit (BCU) that ensured containment of infectious organisms and provided a unidirectional flow of air across the space housing the infected and naive baboons (Figure 1). The unit was designed so that following high-efficiency particulate air (HEPA) filtration, air enters the BCU through a perforated wall, travels the length of the BCU, exits the BCU through a perforated wall, and is HEPA-filtered again prior to being exhausted from the BCU. The individual cages housing the baboons within the BCU were modified so that both the front and back of the cages were made of wire mesh, allowing free passage of air through the cages. In our first airborne transmission study, 2 index baboons were housed in the same cage with 2 naive baboons. A third naive baboon was housed in a separate cage placed approximately 7 feet away from the cage holding the other baboons, making it impossible for this animal to have contact with the other animals (Figure 1). In the second airborne transmission study, 2 index baboons were housed in the same cages with 2 naive baboons, and 2 additional naive baboons were housed in separate cages placed approximately 7 feet away from the cage holding the directly inoculated baboons. The 2 cages each holding 1 naive baboon had a solid stainless steel barrier between them and were placed so that line-of-sight and physical contact between the animals housed in the 2 cages was not possible (Figure 1). In both airborne transmission studies, care was taken to ensure that feeding and handling of the naive animals housed in the “clean” cages was performed prior to caring for animals housed in the cage holding the index animals in order to eliminate any possibility of physical transfer of bacteria from the index to the naive animals in clean cages by researchers. As in the previous studies, naive baboons were introduced into the BCU 24 hours after introduction of the index animals. All 6 animals were followed and evaluated for colonization of the upper airway by plating of nasopharyngeal washes and for circulating white blood cell counts in peripheral blood. In these 2 studies, 100% of naive animals became colonized following exposure to infected animals (Figure 2A) and exhibited leukocytosis (Figure 2B).
DISCUSSION
It is widely believed that pertussis is spread by the transfer of aerosolized droplets directly from infected to naive individuals; however, no controlled study has ever documented airborne transmission of pertussis. Although numerous retrospective hospital and school outbreak studies and prospective household contact studies have documented examples of transmission, these studies could not practically control for contact between individuals [4–12]. Therefore, the possibility of contact-mediated or indirect transmission could not be ruled out. The lack of animal models of pertussis transmission prevented the execution of carefully controlled laboratory studies to demonstrate airborne transmission [13]. To our knowledge, the only documented case of pertussis transmission in an animal model was demonstrated using Macaca cyclopsis, a primate species that is no longer available for experimental use. In this study, transmission was observed to a cohoused naive animal, again making it impossible to rule out transmission by contact [15]. We recently described a baboon model of clinical pertussis [14]. Using this model, we demonstrated that 100% of baboons inoculated with a clinical isolate of B. pertussis exhibit all the clinical manifestations of human pertussis, including paroxysmal coughing, mucus production, and leukocytosis [14]. The baboon model provided the opportunity to study transmission of pertussis in a tightly controlled environment. Our results demonstrated that transmission does occur between infected baboons and uninfected baboons, indicating that the baboon model likely will be useful for studying transmission of pertussis. Most significantly, our results are the first to document airborne transmission of pertussis, demonstrating that pertussis can be transmitted by aerosolized respiratory droplets.
The dilution of aerosolized droplets in the air as a function of distance from the infected subject is predicted to significantly affect the efficiency of transmission. As a result of this dilution, the exposure rate of the animals housed 7 feet away from the index animals is expected to be significantly lower than the exposure rate for the naive animals that are directly cohoused with the index animals. In our studies, the time to infection of the distally housed animals was significantly longer than the time to infection of the cohoused animals (19 vs 10 days, respectively; P = .0027). This result is consistent with household contact studies in which transmission required prolonged and/or close contact. Even in the context of household exposure, 15%–35% of susceptible individuals escape infection [4–9]. In school settings where close contact is less likely to occur, the transmission rates are much lower (0% to 36%) [10–12].
Using the baboon model, our results demonstrate that pertussis transmission can occur via aerosolized respiratory droplets. As expected for an airborne exposure, the rate of transmission is dependent upon distance between the infected and naive individual. Although pertussis is typically described as being highly infectious, efficient transmission requires close contact or prolonged exposure.
Notes
Acknowledgments. We thank Lewis Shankle, Perry Altland, and Ernest Madison for technical assistance, Dr Gary White, Dr Roman Wolf, and Dr James Papin for many helpful discussions, and Dr Gopa Raychaudhuri for careful reading of the manuscript.
Financial support. This work was supported by the Food and Drug Administration and National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) through interagency agreement #Y1-AI-1727-01. Baboons were obtained from the Oklahoma Baboon Research Resource. The Oklahoma Baboon Research Resource was supported by grant numbers P40RR012317 and 5R24RR016556-10 from the NIH National Center For Research Resources.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–82. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pertussis. Epidemiology and prevention of vaccine-preventable diseases. 12 ed. Atlanta: Centers for Disease Control and Prevention; 2011. pp. 215–32. [Google Scholar]
- 3.Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:385–400. [PubMed] [Google Scholar]
- 4.Heininger U, Cherry JD, Stehr K, et al. Comparative efficacy of the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine and Lederle whole-cell component DTP vaccine in German children after household exposure. Pertussis Vaccine Study Group. Pediatrics. 1998;102:546–53. doi: 10.1542/peds.102.3.546. [DOI] [PubMed] [Google Scholar]
- 5.Isomura S. Clinical studies on efficacy and safety of an acellular pertussis vaccine in Aichi Prefecture, Japan. Dev Biolog Standard. 1991;73:37–42. [PubMed] [Google Scholar]
- 6.Mertsola J, Ruuskanen O, Eerola E, Viljanen MK. Intrafamilial spread of pertussis. J Ped. 1983;103:359–63. doi: 10.1016/s0022-3476(83)80403-x. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt HJ. Efficacy of a three-component acellular pertussis vaccine (DTaP) in early childhood after household exposure to “typical” (WHO-defined) pertussis. Dev Biolog Standard. 1997;89:67–9. [PubMed] [Google Scholar]
- 8.Simondon F, Preziosi MP, Yam A, et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine. 1997;15:1606–12. doi: 10.1016/s0264-410x(97)00100-x. [DOI] [PubMed] [Google Scholar]
- 9.Storsaeter J, Gustafsson L. Absolute efficacy of acellular pertussis vaccines in household settings. Dev Biolog Standard. 1997;89:153–9. [PubMed] [Google Scholar]
- 10.Rodman AC, Bradford WL, Berry GP. An epidemiological study of an outbreak of pertussis in a public school. Am J Public Health Nations Health. 1946;36:1156–62. doi: 10.2105/ajph.36.10.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culotta CE, Dominick D, Harrison ER. Epidemiologic studies in whooping cough. Yale J Biol Med. 1938;10:473–83. [PMC free article] [PubMed] [Google Scholar]
- 12.Etkind P, Lett SM, Macdonald PD, Silva E, Peppe J. Pertussis outbreaks in groups claiming religious exemptions to vaccinations. Am J Dis Child. 1992;146:173–6. doi: 10.1001/archpedi.1992.02160140039017. [DOI] [PubMed] [Google Scholar]
- 13.Elahi S, Holmstrom J, Gerdts V. The benefits of using diverse animal models for studying pertussis. Trends Microbiol. 2007;15:462–8. doi: 10.1016/j.tim.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. A Non-human primate model of pertussis. Infect Immun. 2012;80:1530–36. doi: 10.1128/IAI.06310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin TM. Experimental whooping cough in monkey. J Formosan Med Assoc. 1958;57:53–62. [Google Scholar]