Abstract
The osmoprotectant 3-dimethylsulfoniopropionate (DMSP) occurs in Gramineae and Compositae, but its synthesis has been studied only in the latter. The DMSP synthesis pathway was therefore investigated in the salt marsh grass Spartina alterniflora Loisel. Leaf tissue metabolized supplied [35S]methionine (Met) to S-methyl-l-Met (SMM), 3-dimethylsulfoniopropylamine (DMSP-amine), and DMSP. The 35S-labeling kinetics of SMM and DMSP-amine indicated that they were intermediates and, consistent with this, the dimethylsulfonium moiety of SMM was shown by stable isotope labeling to be incorporated as a unit into DMSP. The identity of DMSP-amine, a novel natural product, was confirmed by both chemical and mass-spectral methods. S. alterniflora readily converted supplied [35S]SMM to DMSP-amine and DMSP, and also readily converted supplied [35S]DMSP-amine to DMSP; grasses that lack DMSP did neither. A small amount of label was detected in 3-dimethylsulfoniopropionaldehyde (DMSP-ald) when [35S]SMM or [35S]DMSP-amine was given. These results are consistent with the operation of the pathway Met → SMM → DMSP-amine → DMSP-ald → DMSP, which differs from that found in Compositae by the presence of a free DMSP-amine intermediate. This dissimilarity suggests that DMSP synthesis evolved independently in Gramineae and Compositae.
DMSP is a sulfonium betaine accumulated by many marine algae (Blunden and Gordon, 1986; Keller et al., 1989) and by certain angiosperms from the Compositae and Gramineae families. The best studied of these are the coastal strand plant Wollastonia biflora (L.) DC. (Compositae) and the salt marsh grass Spartina alterniflora Loisel. (Gramineae) (Storey et al., 1993; Colmer et al., 1996). DMSP is environmentally important as the main biogenic precursor of atmospheric DMS, which has roles in the biogeochemical S cycle, in cloud formation, and in acid precipitation (Malin, 1996). Whereas oceanic DMS fluxes are globally the largest source of atmospheric DMS, those from salt marshes dominated by S. alterniflora are 1 to 2 orders of magnitude higher per unit area and may significantly affect atmospheric S budgets on a regional or local scale (Steudler and Peterson, 1984; Aneja and Cooper, 1989).
DMSP is also physiologically important. Like its analog, Gly betaine, DMSP functions as a compatible solute for enzymes in vitro (Gröne and Kirst, 1991; Nishiguchi and Somero, 1992) and as an osmoprotectant for bacteria (Mason and Blunden, 1989; Paquet et al., 1994). Consistent with its having such functions in plants, DMSP has been shown to accumulate to high levels (≥ 100 mm) in the cytoplasm of algal cells (Dickson et al., 1980; Dickson and Kirst, 1986) and in W. biflora chloroplasts (Trossat et al., 1998). There is also evidence that DMSP is an effective cryoprotectant (Karsten et al., 1996).
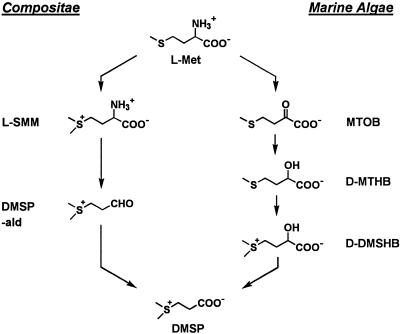
The biosynthesis of DMSP from Met has been partially elucidated in the Compositae W. biflora (Hanson et al., 1994; James et al., 1995) and Ratibida pinnata (Vent.) Barnhart (Paquet et al., 1995), and its subcellular compartmentation has been established (Trossat et al., 1996). The only known intermediates in this pathway are SMM and DMSP-ald (Fig. 1). Although conversion of SMM to DMSP-ald entails the loss of both the amino and carboxyl groups, there is no evidence that this occurs via DMSP-amine or any other stable intermediate, and 15N-labeling data support a mechanism in which SMM is transaminated and decarboxylated by the same enzyme or by a transaminase-decarboxylase complex (Rhodes et al., 1997). Most, if not all, angiosperms synthesize SMM (Giovanelli et al., 1980; Bezzubov and Gessler, 1992), and many have dehydrogenases that can mediate oxidation of DMSP-ald to DMSP (Trossat et al., 1997; Vojtechová et al., 1997). It is therefore the conversion of SMM to DMSP-ald that is special to DMSP synthesis.
Figure 1.
Known steps in DMSP biosynthesis in W. biflora (Compositae) and in the marine alga E. intestinalis. The enantiomers of chiral compounds are indicated. No intermediate between SMM and DMSP-ald has been identified in W. biflora (James et al., 1995).
The DMSP synthesis pathway has also been studied in the marine macroalga Enteromorpha intestinalis (L.) Link and in three microalgae (Gage et al., 1997; Summers et al., 1998). It proceeds via the intermediates 4-methylthio-2-oxobutyrate, 4-methylthio-2-hydroxybutyrate, and DMSHB and therefore has no steps in common with the Compositae pathway (Fig. 1). This dissimilarity shows that DMSP synthesis evolved independently in algae and in the ancestors of Compositae. It also suggests that the pathway in Gramineae might differ from that in Compositae because these families stand far apart phylogenetically, their progenitors having diverged more than 100 million years ago (Crane et al., 1995). This prompted us to examine the DMSP synthesis route in S. alterniflora. We found that it is not the same as either the algal or the Compositae pathway. Although it resembles the latter in having SMM and DMSP-ald intermediates, it differs in the key SMM → DMSP-ald step.
MATERIALS AND METHODS
Spartina alterniflora Loisel. and Spartina patens (Ait.) Muhl. were collected in Florida from coastal marshes in Crescent Beach and Archie Creek (east Tampa Bay), respectively. Blocks of soil (3–5 dm3) containing plants were dug up and transferred to undrained plastic containers. The plants were then maintained for up to 4 months in a naturally lit greenhouse (minimum temperature, 18°C) with the water table close to the soil surface to replace water lost by evapotranspiration; this kept the salinity close to that of the collection site. Because a high N supply decreases DMSP levels (Colmer et al., 1996), no fertilization was given. Other species were fertilized and not salinized: wheat (Triticum aestivum L. cv Florida 310), maize (Zea mays L. cv NK 508), Cortaderia selloana Aschers. & Graebn., and Oplismenus compositus Beauv. were greenhouse grown; Pennisetum purpureum Schumach PI 300086 was grown in a shade house; and Cynodon dactylon Pers. cv Floratex and Bambusa glaucescens (Willd.) Sieb. ex Munro cv Stripestem were field grown. DMSP was assayed in leaf samples (30 mg fresh weight) by a GC method (Paquet et al., 1994). Autumn fern (Dryopteris erythrosora [Eaton] Kuntze) was purchased locally and its fronds used to prepare an acetone powder with Met decarboxylase activity (Stevenson et al., 1990).
Chemicals
SMM iodide (Sigma) was converted to the HCl form by adsorption to a Dowex-50 (H+) column and elution with 2.5 n HCl; it was lyophilized and neutralized before use with one equivalent of KHCO3. DMSP-amine chloride was prepared from 1 mmol of 3-methylthiopropylamine (Chem Service, West Chester, PA) by neutralizing with HCl and treating with 0.7 mL of 6 n HCl containing 2 mmol of MeOH at 110°C for 4 h (Lavine et al., 1954). The product (yield, 75%) was isolated using Dowex-50 as described above and lyophilized; purity was ≥ 98%, as determined by TLC and TLE. DMSP-ald iodide was synthesized as described previously (James et al., 1995). DMSP hydrochloride was obtained from Research Plus, Inc. (Bayonne, NJ).
Labeled Compounds
[35S]Met (44 GBq μmol−1, NEN-DuPont) was mixed with Met to give the desired specific activity; for experiments with leaf tissue it was then treated with Dowex-1 (formate) and Dowex-50 (NH4+) to remove acidic and basic impurities, respectively. [35S]SMM (370 kBq nmol−1) was synthesized by chemical methylation of [35S]Met, as described by Gage et al. (1997). To prepare [35S]DMSP-amine (165 kBq nmol−1), [35S]Met (25 nmol) was first decarboxylated by incubating (1 or 2 h, 37°C) in 0.1 mL of 0.2 m succinate-NaOH buffer, pH 5.0, containing 1 mm pyridoxal 5′-phosphate and 11 mg of D. erythrosora acetone powder. The reaction mixture was then applied to 1-mL Dowex-1 (OH−) and BioRex-70 (H+) columns arranged in series. After washing both columns with water, [35S]methylthiopropylamine was eluted from the BioRex-70 column with 5 mL of 1 n HCl and lyophilized. It was then treated (110°C, 4 h) with 0.3 mL of 6 n HCl containing 50 μmol of MeOH to give [35S]DMSP-amine, which was isolated by TLC on 0.25-mm silica-gel G plates (Machery-Nagel, Düren, Germany) developed with MeOH:acetone:concentrated HCl (90:10:4, v/v). The overall radiochemical yield from [35S]Met was 24%; radiochemical purity was 99%, as determined by TLC and TLE. d- and l-DMSHB (37 kBq nmol−1) were prepared as described by Summers et al. (1998), and [methyl-14C]DMSP-ald (0.41 kBq nmol−1) was prepared as described by James et al. (1995). [35S]DMSP was isolated from S. alterniflora leaf sections that had been given [35S]Met. [C2H3,C2H3]SMM, [13CH3,C2H3]SMM, and [C2H3,C2H3]DMSP were prepared as described by Hanson et al. (1994).
Metabolism of Precursors by Leaf Tissue
Sections (about 10 × 5 mm) cut from leaves at or near full expansion were given shallow incisions spaced 1 to 2 mm apart on the whole abaxial surface. For most experiments, 0.2-g batches of sections were then incubated (cut surface down) in 6-cm Petri dishes on a 4.25-cm circle of Whatman no. 1 filter paper containing 0.5 or 1.0 mL of precursor solution. For C. dactylon, 0.2-g batches of shoot tips with four or five leaves were used. For the [35S]SMM pulse-chase experiment, eight sections were incubated on 4 cm2 of paper in 200 μL of [35S]SMM solution and then transferred to 1 mL of water for the chase. For experiments in which DMSP-ald was analyzed, two leaf sections were incubated in 30 μL of solution. Incubation was at 25 ± 2°C, with rotary agitation at 75 rpm, in fluorescent light (PPFD, 150 μE m−2 s−1); water was added to replace that lost by evapotranspiration. Sections were rinsed before extraction or transfer to chase media as follows: for time-course experiments, for 15 s in water; when DMSP-ald was to be analyzed, for 15 s in 10 mL of a 10−5 m solution of the corresponding unlabeled compound; and for other 35S-labeling experiments, for 5 to 15 min in 10 mL of a 10−4 m solution of the corresponding unlabeled compound. The washings were pooled with the remaining incubation medium, and a sample was counted to determine 35S uptake. For experiments with [13CH3,C2H3]SMM, DMSP for fast-atom-bombardment MS analysis was isolated as described by Hanson et al. (1994). For all other experiments, the extraction procedures, the ion-exchange-fractionation methods, and the TLC and TLE systems used were as described by James et al. (1995).
MS
DMSP was analyzed without derivatization by fast-atom-bombardment MS, using the hexaethylene glycol/K+ nonofluorobutylsulfonate matrix described previously (Hanson et al., 1994). SMM and DMSP-amine were analyzed without derivatization by MALDI-MS; the instrumentation and procedures were as described by Trossat et al. (1998), except that fluorosilicic acid was omitted from the matrix used to analyze DMSP-amine.
Computer Modeling of 35S-Labeling Data
The computer model used was that described by Mayer et al. (1990), except that programs were written in Microsoft Visual Basic 5.0. Programs used a 0.06-min iteration interval to generate simulations of the labeling patterns of metabolites, partitioning pools into metabolically active and inactive (storage) components, and varying the fluxes between pools until the match between observed and simulated data was satisfactory.
RESULTS AND DISCUSSION
Metabolism of [35S]Met, [35S]SMM, and [35S]DMSHB
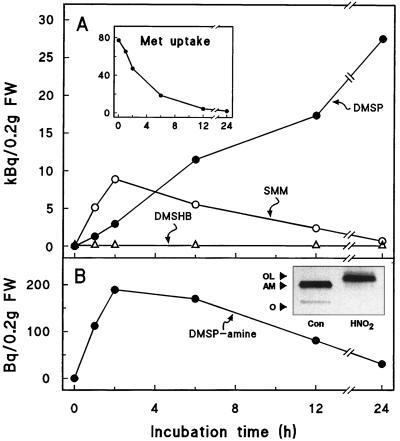
A diagnostic distinction between the algal- and Compositae-type DMSP synthesis pathways is that if radiotracer Met is supplied, the major labeled intermediate is DMSHB in the former and SMM in the latter (Hanson et al., 1994; Gage et al., 1997). We therefore fed [35S]Met to S. alterniflora leaf tissue and monitored the labeling of DMSHB, SMM, and DMSP (Fig. 2A). As in Compositae, no 35S was detected in DMSHB, but SMM acquired label rapidly and lost it as the [35S]Met was metabolized, as expected for an intermediate. However, unlike the pattern in Compositae, DMSP-amine acquired and lost 35S in a way that suggested that it too could be an intermediate (Fig. 2B). DMSP-amine [(CH3)2S+CH2CH2CH2NH3+] is the decarboxylation product of SMM. The [35S]DMSP-amine pool was far smaller than the [35S]SMM pool, with its peak 35S content (between 1 and 6 h) being about 2.5% that of SMM (Fig. 2); two similar experiments gave peak values of 2.9 and 5.0% (not shown).
Figure 2.
Labeling patterns of metabolites in S. alterniflora leaf sections supplied with [35S]Met (77 Bq [10 nmol] per 0.2 g fresh weight). A, SMM, DMSHB, and DMSP. Labeling of DMSHB was below the detection limit (0.03 kBq) at all times. Inset shows [35S]Met uptake from the medium. B, DMSP-amine. [35S]DMSP-amine was identified by co-migration with authentic DMSP-amine in TLC (systems 1 and 2 of James et al. [1995]) and TLE (system 1 of James et al. [1995]), and by deamination to 3-dimethylsulfoniopropanol with nitrous acid (Zappia et al., 1969) as shown in the inset. The inset is an autoradiograph of a TLC-system 1 separation of [35S]DMSP-amine from S. alterniflora, before (Con) and after (HNO2) nitrous acid treatment; the positions of the origin (O), DMSP-amine (AM), and 3-dimethylsulfoniopropanol (OL) are marked. FW, Fresh weight.
Another diagnostic difference between the two known DMSP synthesis pathways is the extent of conversion of supplied SMM or DMSHB to DMSP. In this respect, S. alterniflora again resembled Compositae, for leaf segments readily converted [35S]SMM to DMSP but did not metabolize either the d- or l-enantiomer of DMSHB (Table I).
Table I.
Conversion of supplied SMM but not DMSHB to DMSP by S. alterniflora leaf sections
| 35S Precursor | 35S Uptake | [35S]DMSHB | [35S]DMSP |
|---|---|---|---|
| kBq | |||
| l-SMM | 18.9 | <0.03 | 6.9 |
| l-DMSHB | 11.8 | 11.2 | <0.1 |
| d-DMSHB | 6.1 | 5.7 | <0.1 |
Leaf sections (0.2 g fresh weight) were incubated for 24 h with a tracer dose (0.2–1.0 nmol) of [35S]SMM or [35S]DMSHB. The amount of 35S was 19.2 to 19.6 kBq in all cases. Uptake was calculated from the disappearance of 35S from the medium. The experiment was repeated using 100-nmol doses of 35S precursors, with very similar results. The values shown are per 0.2 g fresh weight and have been corrected for recovery.
Evidence from Stable Isotope Labeling that SMM and DMSP-amine Are Intermediates
The above 35S-labeling data are consistent with the reaction sequence Met → SMM → → DMSP. However, because Met and SMM are potentially interconvertible via the SMM cycle (Mudd and Datko, 1990), they are also consistent with the sequence SMM ↔ Met → → DMSP. To distinguish between these alternatives, leaf segments were given SMM labeled with 13C in one methyl group and with 2H in the other, and the DMSP formed was analyzed. Only [13CH3,C2H3]DMSP was detected (Table II). This shows that SMM was converted to DMSP directly, not via Met, because conversion via Met would give 13CH3,13CH3-, 13CH3,C2H3-, and C2H3,C2H3-labeled species of DMSP in a 1:2:1 ratio. These results therefore confirm that SMM is an intermediate of DMSP synthesis in S. alterniflora. They also indicate that flux through the SMM cycle in this plant is small compared with the flux to DMSP.
Table II.
Labeling of DMSP synthesized by S. alterniflora leaf sections given [13CH3,C2H3]SMM
| Precursor | Relative Intensity of DMSP Ions
|
||
|---|---|---|---|
| 13CH3,13CH3 (m/z 137) | 13CH3,C2H3 (m/z 139) | C2H3,C2H3 (m/z 141) | |
| % | |||
| [13CH3,C2H3]SMM | −0.27 | 7.63 | 0.10 |
| Unlabeled SMM | −0.01 | 0.09 | 0.02 |
| lsd0.05 | 0.45 | 1.80 | 0.64 |
Leaf sections (0.2 g fresh weight) were incubated with 5.0 μmol of [13CH3,C2H3]SMM (experimental samples) or unlabeled SMM (controls) for 24 or 48 h. The intensities of the labeled DMSP ions are expressed relative to that of endogenous unlabeled DMSP (m/z 135, 100%); the endogenous DMSP level was 23 ± 1 μmol g−1 fresh weight (mean ± se, n = 4). All signals were corrected for background noise from the matrix, and those at m/z 141 were further corrected for the contribution from the natural-abundance isotope peaks associated with [13CH3,C2H3]DMSP (m/z 139). Because labeled DMSP yields at 24 and 48 h did not differ significantly, results for both times were pooled. Data are means from eight experimental samples and four controls, and were subjected to analysis of variance. That all of the values for control sections given unlabeled SMM are close to 0 confirms the validity of the corrections applied.
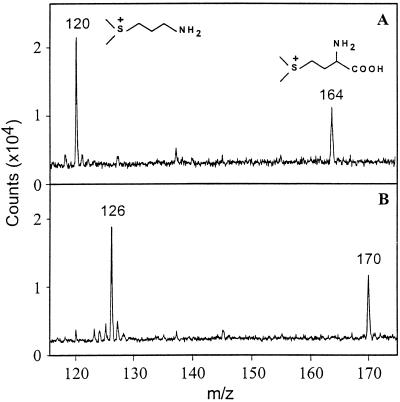
To supplement the radiolabeling evidence that SMM is converted to DMSP via DMSP-amine, leaf segments were fed unlabeled or C2H3,C2H3-labeled SMM, and DMSP-amine was sought by a sensitive MALDI-MS method. Strong signals appeared at m/z 120 or 126, corresponding to unlabeled and C2H3,C2H3-labeled DMSP-amine, respectively (Fig. 3). Confirmation that these peaks represent DMSP-amine was obtained by MALDI postsource decay experiments; these showed the expected fragment at m/z 58 formed from the precursor ion by neutral loss of (CH3)2S or (C2H3)2S. That [C2H3,C2H3]SMM gave rise to little or no [C2H3]DMSP-amine (m/z 123) (Fig. 3B) indicates that the dimethylsulfonium group enters DMSP-amine as a unit, which is consistent with direct conversion of SMM to DMSP-amine by decarboxylation.
Figure 3.
MALDI-MS analysis of the base fractions from S. alterniflora leaf segments (0.2 g fresh weight) incubated for 6 h with 5.0 μmol of unlabeled SMM (A) or [C2H3,C2H3]SMM (B). The peaks at m/z 164 and 170 correspond to the unlabeled SMM and [C2H3,C2H3]SMM supplied, respectively, and those at m/z 120 and 126 correspond to unlabeled and C2H3,C2H3-labeled DMSP-amine formed during the experiment, respectively. In these spectra, matrix background peaks are largely suppressed, but residual signals can be seen at m/z 123, 137, and 146. The relative response of DMSP-amine and SMM in MALDI-MS analyses under these conditions was approximately 30:1, determined by spiking the samples with known amounts of DMSP-amine and SMM (data not shown). In leaf segments not given SMM, the intensities of the peaks corresponding to DMSP-amine and SMM were about 5 and 15%, respectively, of those in A. No peaks attributable to DMSP-amine were detected in the SMM substrates supplied to the leaf segments.
MALDI-MS was also used to quantify the endogenous SMM pool in S. alterniflora using an internal standard of [C2H3,C2H3]SMM. Duplicate analyses gave values of 143 and 160 nmol g−1 fresh weight. A signal attributable to endogenous DMSP-amine was detectable by MALDI-MS; it was too small to quantify accurately, but indicated that the DMSP-amine level was not more than 20 nmol g−1 fresh weight, i.e. far lower than the SMM level.
Labeling Kinetics of DMSP-amine during and after a Pulse of [35S]SMM
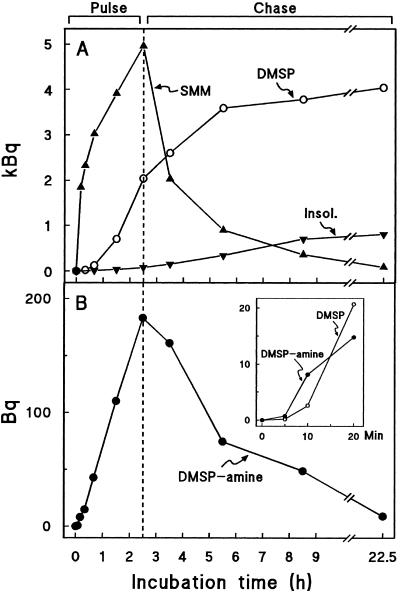
A pulse-chase experiment with [35S]SMM was used to further test the possibility that DMSP-amine is a DMSP synthesis intermediate (Fig. 4). In the first minutes of the pulse, 35S accumulated in DMSP-amine more rapidly than in DMSP (Fig. 4B, inset), which fits qualitatively with DMSP-amine being an intermediate. During the chase, DMSP-amine lost label as the [35S]SMM was depleted and [35S]DMSP synthesis slowed, again consistent with its being an intermediate. The data also confirm that there is only a minor SMM → Met flux via the SMM cycle, because little 35S accumulated in free Met at any time (<0.1 kBq; not shown) and labeling of the insoluble fraction, a maximum estimate of protein-bound [35S]Met, was small relative to that of DMSP (Fig. 4A).
Figure 4.
Labeling kinetics of SMM, DMSP, and insoluble material (Insol., A) and DMSP-amine (B) in a pulse-chase experiment with [35S]SMM. Batches of eight S. alterniflora leaf sections (approximately 0.1 g fresh weight) were supplied with 18.5 kBq (0.16 nmol) of [35S]SMM; after 2.5 h they were rinsed for 15 s and transferred to water. That more 35S was lost from SMM during the chase than appeared in other products may be ascribed to efflux of [35S]SMM present in the apoplast. The inset in B is an expanded-scale plot of the labeling of DMSP-amine and DMSP at early times; note that DMSP-amine is initially more heavily labeled than DMSP.
[35S]SMM Metabolism in Grasses That Do Not Accumulate DMSP
If DMSP-amine is an intermediate specific to the DMSP synthesis pathway, then species that lack DMSP should not synthesize it from supplied SMM. This prediction was tested by comparing the metabolism of [35S]SMM in S. alterniflora with that in eight grasses that were shown to lack detectable amounts of DMSP (Table III). These DMSP-free grasses were chosen to include another species of Spartina, S. patens, and otherwise for maximum diversity; together, they represented all five subfamilies of Gramineae. The DMSP-free grasses took up 56 to 97% of the [35S]SMM supplied, but none produced a detectable quantity of [35S]DMSP-amine or more than a trace of [35S]DMSP (Table III). This incapacity to form DMSP-amine among a wide range of species of Gramineae that do not accumulate DMSP indicates that DMSP-amine is an intermediate in the DMSP pathway, and not simply a common minor metabolite of grasses.
Table III.
[35S]DMSP-amine is not a metabolite of [35S]SMM in grasses that lack DMSP
| Species | Endogenous DMSP | 35S Uptake | [35S]DMSP-amine | [35S]DMSP |
|---|---|---|---|---|
| μmol g−1 fresh weight | kBq | Bq | ||
| S. alterniflora | 29.0 | 18.3 | 389 | 8180 |
| S. patens | <0.03 | 28.6 | <30 | <30 |
| T. aestivum | <0.03 | 37.0 | <30 | <30 |
| Z. mays | <0.03 | 36.6 | <30 | 90 |
| B. glaucescens | <0.03 | 36.3 | <30 | <30 |
| O. compositus | <0.03 | 37.6 | <30 | <30 |
| C. dactylon | <0.03 | 35.1 | <30 | <30 |
| P. purpureum | <0.03 | 36.8 | <30 | 70 |
| C. selloana | <0.03 | 21.6 | <30 | <30 |
Leaf tissue (0.2 g fresh weight) of eight grasses shown not to accumulate DMSP was incubated for 6 h with 38.9 kBq (0.12 nmol) of [35S]SMM; S. alterniflora was included as a benchmark. Uptake was calculated from 35S disappearance from the medium. The radioactivity values shown are per 0.2 g fresh weight and have been corrected for recovery. Endogenous DMSP contents were determined on duplicate 30-mg fresh weight leaf samples. The comparison between S. alterniflora and S. patens was repeated, with similar results.
[35S]DMSP-amine Metabolism in S. alterniflora and Grasses Lacking DMSP
As an intermediate of DMSP synthesis, supplied DMSP-amine should be readily converted to DMSP by S. alterniflora. Figure 5 confirms that this was true for both tracer and substrate doses of [35S]DMSP-amine. It also shows that the eight grasses lacking DMSP produced relatively little [35S]DMSP, although their [35S]DMSP-amine uptake was generally greater than that of S. alterniflora. This supports the view that DMSP-amine is a characteristic intermediate of the DMSP pathway in S. alterniflora, and underscores the distinction between this species and Compositae, in which DMSP-amine is not as readily metabolized to DMSP (James et al., 1995). That the DMSP-free grasses made a little [35S]DMSP agrees with previous data for T. aestivum and for dicot species that lack DMSP (James et al., 1995). This seemingly nonspecific DMSP production may be caused by the tandem action of diamine oxidase and ω-aminoaldehyde dehydrogenase, both of which occur in grasses and dicots and can attack substrates with a dimethylsulfonium group (Bardsley et al., 1971; Awal et al., 1995; Suzuki, 1996; Trossat et al., 1997).
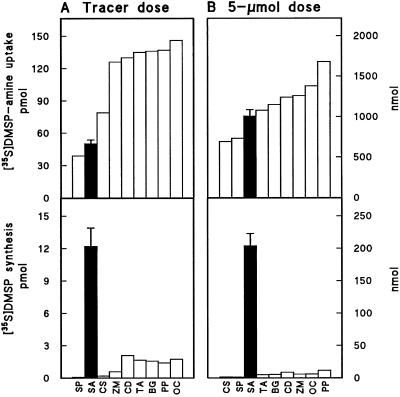
Figure 5.
Uptake and conversion to [35S]DMSP of tracer (A) or substrate-level (B) doses of [35S]DMSP-amine. Leaf tissue (0.2 g fresh weight) of nine grass species was incubated with 140 ± 15 pmol (tracer) or 5.0 μmol (substrate level) of [35S]DMSP-amine for 6 h. Uptake was calculated from disappearance of 35S from the medium. Species are arranged in order of ascending label uptake. Species are as follows: SP, S. patens; SA, S. alterniflora; CS, C. selloana; ZM, Z. mays; CD, C. dactylon; TA, T. aestivum; BG, B. glaucescens; PP, P. purpureum; and OC, O. compositus. All species except S. alterniflora lacked detectable DMSP (Table III). Data for S. alterniflora are means + se for four similar experiments; those for other species are single determinations.
A remarkable feature of the metabolism of [35S]DMSP-amine in S. alterniflora was that whether the amount taken up was 0.05 or 1000 nmol, about 20% of it was metabolized to [35S]DMSP (Fig. 5). Such a high capacity for metabolizing DMSP-amine to DMSP suggests that unlabeled DMSP-amine might not act as an effective trap for label coming from [35S]SMM. This proved to be the case; combining a 3 × 104-fold excess of unlabeled DMSP-amine with tracer [35S]SMM resulted in a high internal DMSP-amine level, but did not cause 35S to accumulate in DMSP-amine (Table IV). The unlabeled DMSP-amine reduced [35S]SMM uptake (and [35S]DMSP synthesis), but this is unlikely to have masked a trapping effect because the size of the internal [35S]SMM pool hardly changed (Table IV).
Table IV.
DMSP-amine does not act as a trapping pool for label from [35S]SMM
S. alterniflora leaf sections (0.2 g fresh weight) were incubated for 4 h with 18.5 kBq (0.15 nmol) of [35S]SMM, with or without a 5-μmol trapping pool of unlabeled DMSP-amine. The size of the DMSP-amine pool in the tissue was estimated in a parallel experiment in which leaf sections were incubated with 5 μmol (18.5 kBq) of [35S]DMSP-amine for 4 h. The values shown are per 0.2 g fresh weight and have been corrected for recovery. The experiment was repeated using an 8-h incubation time, with similar results.
| DMSP-amine
Trap
|
[35S]SMM Uptake | Label
Distribution
|
|||
|---|---|---|---|---|---|
| Supplied | In tissue | SMM | DMSP-amine | DMSP | |
| nmol | kBq | kBq | Bq | kBq | |
| 0 | 0 | 5.22 | 1.96 | 111 | 2.52 |
| 5000 | 568 | 2.44 | 1.48 | 126 | 0.78 |
Evidence for DMSP-ald as an Intermediate
On comparative biochemical grounds, the most plausible route between DMSP-amine and DMSP would be via DMSP-ald. We therefore tested for [35S]DMSP-ald as a metabolite of [35S]SMM or [35S]DMSP-amine. During a pulse of either precursor, DMSP-ald acquired detectable 35S, and lost much of it during a subsequent chase period (Table V). These data are consistent with DMSP-ald being an intermediate in DMSP synthesis. The data for the [35S]SMM pulse indicate that the DMSP-ald pool is very small (about 2.5% of the size of the DMSP-amine pool) and turns over very fast (at least once every 100 s).
Table V.
Labeling of DMSP-ald from [35S]SMM or [35S]DMSP-amine in S. alterniflora
| 35S Precursor | Treatment | Label
Distribution
|
|||
|---|---|---|---|---|---|
| SMM | DMSP-amine | DMSP-ald | DMSP | ||
| kBq | Bq | Bq | kBq | ||
| SMM | Pulse | 69.3 | 936 | 24.1 | 2.44 |
| Pulse-chase | 23.8 | 466 | 8.1 | 13.0 | |
| DMSP-amine | Pulse | –a | 23,400 | 11.8 | 1.41 |
| Pulse-chase | – | 3,990 | 1.1 | 2.74 | |
Pairs of leaf segments were supplied with a pulse of [35S]SMM (77 kBq, 0.99 nmol) or [35S]DMSP-amine (35 kBq, 1.33 nmol) for 3 h, rinsed for 15 s, and then transferred to water for a 9-h chase period. DMSP-ald was analyzed as the corresponding alcohol after reduction with NaBH4 (see Methods); a correction was made for traces of alcohol detectable in controls that were not treated with NaBH4. That more 35S was lost from SMM or DMSP-amine during the chase than appeared in DMSP may be ascribed primarily to efflux of unabsorbed label from the apoplast. The values shown are per pair of segments and have been corrected for recovery. The experiment was repeated, with similar results.
–, Below detection limit (0.05 kBq).
Modeling of DMSP Synthesis in S. alterniflora
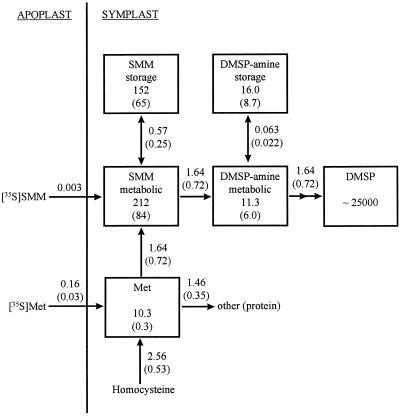
The kinetic data of Figures 2 and 4, and results from an additional pulse-chase experiment with [35S]Met (not shown), were used to develop the model summarized in Figure 6. For each experiment, flux rates and pool sizes were progressively adjusted until a close match between observed and simulated values was obtained (overall r2 = 0.991, n = 80). Means and se values of these best-fit estimates for the three independent experiments are shown in Figure 6. For simplicity, the model did not include a DMSP-ald intermediate because the pool size of DMSP-ald was so small as to have no effect on estimates of the DMSP-amine → DMSP flux.
Figure 6.
A model of DMSP synthesis from Met in S. alterniflora, based on computer-assisted analyses of the data shown in Figures 2 and 4 and an additional pulse-chase experiment with [35S]Met (not shown). Values in boxes are mean pool sizes (nmol g−1 fresh weight) and those next to arrows are mean flux rates (nmol min−1 g−1 fresh weight). Values in parentheses are se. The DMSP pool size shown is based on the experimental values given in Tables II and III because modeling did not permit estimation of this parameter (modeling permitted estimation only of the amount of label that accumulated in DMSP, which is independent of the pool size assumed). The rates of [35S]SMM and [35S]Met uptake from the apoplast (assumed to equilibrate rapidly with the medium) apply only to the pulse phase of the pulse-chase experiments. During the chase the uptake rates were reduced by 20- to 40-fold, depending on the dilution of the medium plus apoplastic precursor pools achieved during the chase. For the experiment shown in Figure 2, which did not involve a chase, the [35S]Met uptake rate was assumed to be 0.192 nmol min−1 g−1 fresh weight initially, and thereafter to decline in direct proportion to the size of the exogenous [35S]Met pool (see Fig. 2A, inset). For all experiments it was necessary to postulate that there was a large endogenous flux of (unlabeled) homocysteine to Met, SMM, DMSP-amine, and DMSP, and that portions of the SMM and DMSP-amine pools were sequestered in “storage” compartments. These storage pools were postulated to be in slow equilibrium with the “metabolically active” pools that participated as intermediates in DMSP synthesis. Labeling of DMSP-ald (an intermediate between DMSP-amine and DMSP) was not considered.
These modeling results show that the observed labeling patterns are quantitatively consistent with a pathway of DMSP synthesis in which DMSP-amine is an intermediate. A noteworthy point is that the model postulates “storage” pools of both SMM and DMSP-amine in addition to “metabolically active” pools, the flux rates between these pools being low compared with the rate of DMSP synthesis. One possible physical basis for this is that SMM conversion to DMSP occurs in the chloroplast, as in W. biflora (Trossat et al., 1996); in this case, the metabolic pools of SMM and DMSP-amine would be chloroplastic and the storage pools extrachloroplastic (cytosolic and/or vacuolar). For DMSP-amine, which is doubly positively charged at cellular pH, another possible basis for the storage component is reversible adsorption to negative charges on macromolecules, as occurs with di- and polyamines (Kumar et al., 1997, and refs. cited therein).
The mean total pool size of SMM estimated from the model is more than twice that measured by MALDI-MS. However, the modeling results indicated appreciable variation in pool sizes and fluxes between different batches of S. alterniflora leaves (see the se values in Fig. 6), and the range of model-derived total SMM pool sizes (90–511 nmol g−1 fresh weight) encompasses the experimental value of approximately 150 nmol g−1 fresh weight. The model-estimated total DMSP-amine pool has a mean value of about 7% of the SMM pool, which is broadly consistent with the MALDI-MS estimates (see “Evidence from Stable Isotope Labeling that SMM and DMSP-amine are Intermediates”). When expressed per day, the model-estimated flux rate to DMSP is 2.36 μmol d−1 g−1 fresh weight, which is equivalent to about 10% of the total DMSP pool.
The model was used to estimate the SMM and DMSP-amine pool sizes and the SMM → DMSP-amine and DMSP-amine → DMSP fluxes in two additional labeling experiments. The first experiment was that described in Table IV, with and without a trapping pool of DMSP-amine. The results with no trapping pool were satisfactorily accommodated by using flux and pool size values that fell within the ranges given in Figure 6. When DMSP-amine was supplied, the model gave a good fit to experimental data if (a) the exogenous DMSP-amine was postulated to enter the DMSP synthesis pathway by passing via the storage pool, (b) the DMSP-amine storage pool expanded massively from approximately 5 to 2700 nmol g−1 fresh weight, (c) the DMSP-amine metabolic pool expanded from 4 to 75 nmol g−1 fresh weight, and (d) the DMSP-amine → DMSP flux in-creased from 1 to 3.78 nmol min−1 g−1 fresh weight. These assumptions seem biologically reasonable; note that assumption (a) is consistent with exogenously supplied DMSP-amine passing through a cytosolic (storage) pool en route to a chloroplastic (metabolic) pool. The model thus provides an acceptable quantitative explanation for the failure of added DMSP-amine to trap label from SMM.
The second additional experiment to which the model was applied (not shown) involved supplying a large dose of [35S]SMM (5 μmol/200 mg fresh weight) for 4 h. The data were best accommodated by postulating an expansion of the metabolic pool of SMM from 75 to 2700 nmol g−1 fresh weight coupled with an increase in the SMM → DMSP-amine flux from 1 to 4.8 nmol min−1 g−1 fresh weight. This resulted in an expansion of the metabolic DMSP-amine pool from 4 to 100 nmol g−1 fresh weight and an increase in the DMSP-amine → DMSP flux from 1 to 4.4 nmol min−1 g−1 fresh weight.
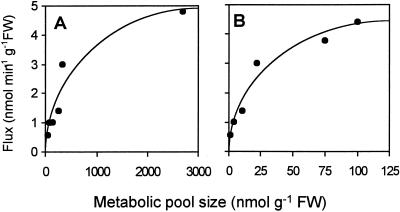
Taken together with the data summarized in Figure 6, these modeling results revealed hyberbolic relationships between the SMM metabolic pool size and the SMM → DMSP-amine flux, and between the DMSP-amine metabolic pool size and the DMSP-amine → DMSP flux (Fig. 7). Such saturable kinetics are to be expected given that the fluxes are enzyme mediated, and the fact that they are observed supports the validity of the model. Double-reciprocal plots gave apparent Km values for SMM and DMSP-amine of 310 and 5.8 nmol g−1 fresh weight, respectively. These values cannot be converted to concentrations because the size of the compartment(s) containing the metabolic pools of SMM and DMSP-amine are unknown.
Figure 7.
Model-derived relationships between metabolic pool sizes and fluxes for SMM and the SMM → DMSP-amine flux (A) and for DMSP-amine and the DMSP-amine → DMSP flux (B). Each data point corresponds to metabolic pool size and flux values obtained using the model shown in Figure 6. The experimental data input for the model came from Figures 2 and 4, from Table IV, and from the two additional experiments described in the text. FW, Fresh weight.
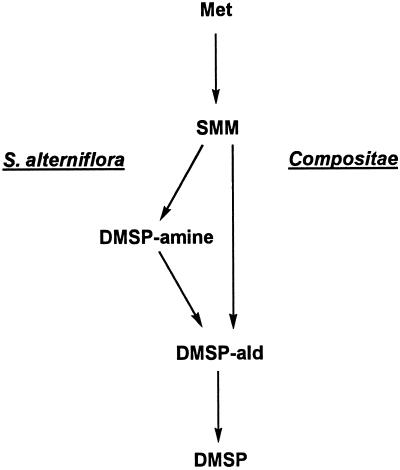
CONCLUSION
We have shown that the DMSP synthesis pathway in S. alterniflora is unlike that in marine algae and resembles that in Compositae by having SMM and DMSP-ald as intermediates. However, it differs crucially in that SMM is converted to DMSP-ald via a pool of free DMSP-amine (Fig. 8). The presence of a free DMSP-amine intermediate is supported by the labeling kinetics of DMSP-amine when [35S]Met or [35S]SMM is fed, by the formation of [C2H3,C2H3]DMSP-amine from [C2H3,C2H3]SMM, and by the ready conversion of supplied DMSP-amine to DMSP. That DMSP-amine is taken up but does not trap label coming from SMM can be explained by its relatively poor access to the metabolic pool and by high capacity for the conversion of DMSP-amine to DMSP.
Figure 8.
DMSP synthesis from Met in S. alterniflora and species of Compositae. Many flowering plants can carry out the S-methylation of Met to SMM and the oxidation of DMSP-ald to DMSP. Only the conversion of SMM to DMSP-ald is unique to DMSP synthesis.
Given the prevalence among angiosperms of both SMM and enzymes able to oxidize DMSP-ald (Giovanelli et al., 1980; Trossat et al., 1997), it is the ability to convert SMM to DMSP-ald that distinguishes plants that produce DMSP from those that do not (Fig. 8). Because S. alterniflora and members of Compositae evidently differ in how they carry out this key conversion, it seems likely that their DMSP pathways had independent evolutionary origins. DMSP synthesis may thus have evolved at least three times, once each in algae, dicots, and monocots. With respect to evolution of the pathway in S. alterniflora, two novel enzymes would presumably be required: an SMM decarboxylase and a DMSP-amine oxidase, dehydrogenase, or aminotransferase. In this regard, it is worth noting that SMM is an analog of S-adenosyl-l-Met, and that S-adenosyl-l-Met decarboxylases are ubiquitous (Kumar et al., 1997). Likewise, DMSP-amine is an analog of a diamine, and diamine oxidases occur widely in plants (Suzuki, 1996). It is therefore easy to imagine how novel enzymes able to decarboxylate SMM and to oxidize DMSP-amine might have originated.
ACKNOWLEDGMENT
We thank Dr. Peter Stiling for identifying and collecting S. patens.
Abbreviations:
- DMS
dimethylsulfide
- DMSHB
4-dimethylsulfonio-2-hydroxybutyrate
- DMSP
3-dimethylsulfoniopropionate
- DMSP-ald
3-dimethylsulfoniopropionaldehyde
- DMSP-amine
3-dimethylsulfoniopropylamine
- MALDI-MS
matrix-assisted laser-desorption ionization MS
- MeOH
methanol
- SMM
S-methyl-l-Met
- TLE
thin-layer electrophoresis
Footnotes
This work was supported in part by the National Science Foundation (grant nos. IBN-9514336 to A.D.H. and IBN-9628750 to D.A.G.) and by an endowment from the C.V. Griffin, Sr., Foundation. Mass spectral data were acquired at the Michigan State University-National Institutes of Health (NIH) Mass Spectrometry Facility, which is supported in part by the NIH, National Center for Research Resources (grant no. RR 00484). This is University of Florida Agricultural Experiment Station journal series no. R-06248.
LITERATURE CITED
- Aneja VP, Cooper WJL. Biogenic sulfur emissions. In: Saltzman ES, Cooper WJ, editors. Biogenic Sulfur in the Environment. Washington, DC: American Chemical Society; 1989. pp. 2–13. [Google Scholar]
- Awal HMA, Yoshida I, Doe M, Hirasawa E. 3-Aminopropionaldehyde dehydrogenase of millet shoots. Phytochemistry. 1995;40:393–395. [Google Scholar]
- Bardsley WG, Ashford JS, Hill CM. Synthesis and oxidation of aminoalkyl-onium compounds by pig kidney diamine oxidase. Biochem J. 1971;122:557–567. doi: 10.1042/bj1220557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzubov AA, Gessler NN. Plant sources of S-methylmethionine. Prikl Biokhim Mikrobiol. 1992;28:423–429. [PubMed] [Google Scholar]
- Blunden G, Gordon SM. Betaines and their sulphonio analogues in marine algae. Prog Phycol Res. 1986;4:39–80. [Google Scholar]
- Colmer TD, Fan TW-M, Läuchli A, Higashi RM. Interactive effects of salinity, nitrogen and sulphur on the organic solutes in Spartina alterniflora leaf blades. J Exp Bot. 1996;47:369–375. [Google Scholar]
- Crane PR, Friis EM, Pedersen KR. The origin and early diversification of angiosperms. Nature. 1995;374:27–33. [Google Scholar]
- Dickson DMJ, Kirst GO. The role of β-dimethylsulphoniopropionate, glycine betaine and homarine in the osmoacclimation of Platymonas subcordiformis. Planta. 1986;167:536–543. doi: 10.1007/BF00391230. [DOI] [PubMed] [Google Scholar]
- Dickson DMJ, Wyn Jones RG, Davenport J. Steady state osmotic adaptation in Ulva lactuca. Planta. 1980;150:158–165. doi: 10.1007/BF00582360. [DOI] [PubMed] [Google Scholar]
- Gage DA, Rhodes D, Nolte KD, Hicks WA, Leustek T, Cooper AJL, Hanson AD. A new route for synthesis of dimethylsulphoniopropionate in marine algae. Nature. 1997;387:891–894. doi: 10.1038/43160. [DOI] [PubMed] [Google Scholar]
- Giovanelli J, Mudd SH, Datko A (1980) Sulfur amino acids in plants. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 5: Amino Acids and Derivatives. Academic Press, New York, pp 453–505
- Gröne T, Kirst GO. Aspects of dimethylsulfoniopropionate effects on enzymes isolated from the marine phytoplankton Tetraselmis subcordiformis (Stein) J Plant Physiol. 1991;138:85–91. [Google Scholar]
- Hanson AD, Rivoal J, Paquet L, Gage DA. Biosynthesis of 3-dimethylsulfoniopropionate in Wollastonia biflora (L.) DC. Evidence that S-methylmethionine is an intermediate. Plant Physiol. 1994;105:103–110. doi: 10.1104/pp.105.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James F, Paquet L, Sparace SA, Gage DA, Hanson AD. Evidence implicating dimethylsulfoniopropionaldehyde as an intermediate in dimethylsulfoniopropionate biosynthesis. Plant Physiol. 1995;108:1439–1448. doi: 10.1104/pp.108.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten U, Kück K, Vogt C, Kirst GO (1996) Dimethylsulfoniopropionate production in phototropic organisms and its physiological function as a cryoprotectant. In RP Kiene, PT Visscher, MD Keller, GO Kirst, eds, Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, pp 143–153
- Keller MD, Bellows WK, Guillard RRL. Dimethyl sulfide production in marine phytoplankton. In: Saltzman ES, Cooper WJ, editors. Biogenic Sulfur in the Environment. Washington, DC: American Chemical Society; 1989. pp. 167–182. [Google Scholar]
- Kumar A, Altabella T, Taylor MA, Tiburcio AF. Recent advances in polyamine research. Trends Plant Sci. 1997;2:124–130. [Google Scholar]
- Lavine TF, Floyd NF, Cammaroti MS. The formation of sulfonium salts from alcohols and methionine in sulfuric acid. J Biol Chem. 1954;207:107–117. [PubMed] [Google Scholar]
- Malin G (1996) The role of DMSP and DMS in the global sulfur cycle and climate regulation. In RP Kiene, PT Visscher, MD Keller, GO Kirst, eds, Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, pp 177–189
- Mason TG, Blunden G. Quaternary ammonium and tertiary sulfonium compounds of algal origin as alleviators of osmotic stress. Bot Mar. 1989;32:313–316. [Google Scholar]
- Mayer RR, Cherry JH, Rhodes D. Effects of heat shock on amino acid metabolism of cowpea cells. Plant Physiol. 1990;94:796–810. doi: 10.1104/pp.94.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd SH, Datko AH. The S-methylmethionine cycle in Lemna paucicostata. Plant Physiol. 1990;93:623–630. doi: 10.1104/pp.93.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi MK, Somero GN. Temperature- and concentration-dependence of compatibility of the organic osmolyte β-dimethylsulfoniopropionate. Cryobiology. 1992;29:118–124. doi: 10.1016/0011-2240(92)90011-p. [DOI] [PubMed] [Google Scholar]
- Paquet L, Lafontaine PJ, Saini HS, James F, Hanson AD. Évidence en faveur de la présence du 3-diméthylsulfoniopropionate chez une large gamme d'Angiospermes. Can J Bot. 1995;73:1889–1896. [Google Scholar]
- Paquet L, Rathinasabapathi B, Saini H, Zamir L, Gage DA, Huang Z-H, Hanson AD. Accumulation of the compatible solute 3-dimethylsulfoniopropionate in sugarcane and its relatives but not other gramineous crops. Aust J Plant Physiol. 1994;21:37–48. [Google Scholar]
- Rhodes D, Gage DA, Cooper AJL, Hanson AD. S-Methylmethionine conversion to dimethylsulfoniopropionate. Evidence for an unusual transamination reaction. Plant Physiol. 1997;115:1541–1548. doi: 10.1104/pp.115.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudler PA, Peterson BJ. Contribution of gaseous sulphur from salt marshes to the global sulphur cycle. Nature. 1984;311:455–457. [Google Scholar]
- Stevenson DE, Akhtar M, Gani D. L-Methionine decarboxylase from Dryopteris filix-mas: purification, characterization, substrate specificity, abortive transamination of the coenzyme, and stereochemical courses of substrate decarboxylation and coenzyme transamination. Biochemistry. 1990;29:7631–7647. doi: 10.1021/bi00485a013. [DOI] [PubMed] [Google Scholar]
- Storey R, Gorham J, Pitman MG, Hanson AD, Gage D. Response of Melanthera biflora to salinity and water stress. J Exp Bot. 1993;44:1551–1560. [Google Scholar]
- Summers PS, Nolte KD, Cooper AJL, Borgeas H, Leustek T, Rhodes D, Hanson AD. Identification and stereospecificity of the first three enzymes of 3-dimethylsulfoniopropionate biosynthesis in a chlorophyte alga. Plant Physiol. 1998;116:369–378. [Google Scholar]
- Suzuki Y. Purification and characterization of diamine oxidase from Triticum aestivum shoots. Phytochemistry. 1996;42:291–293. [Google Scholar]
- Trossat C, Nolte KD, Hanson AD. Evidence that the pathway of dimethylsulfoniopropionate biosynthesis begins in the cytosol and ends in the chloroplast. Plant Physiol. 1996;111:965–973. doi: 10.1104/pp.111.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trossat C, Rathinasabapathi B, Hanson AD. Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and ω-aminoaldehydes. Plant Physiol. 1997;113:1457–1461. doi: 10.1104/pp.113.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trossat C, Rathinasabapathi B, Weretilnyk EA, Shen T-L, Huang Z-H, Gage DA, Hanson AD. Salinity promotes accumulation of dimethylsulfoniopropionate and its precursor S-methylmethionine in chloroplasts. Plant Physiol. 1998;116:165–171. doi: 10.1104/pp.116.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtechová M, Hanson AD, Muñoz-Clares RA. Betaine aldehyde dehydrogenase from amaranth leaves efficiently catalyzes the NAD-dependent oxidation of dimethylsulfoniopropionaldehyde to dimethylsulfoniopropionate. Arch Biochem Biophys. 1997;337:81–88. doi: 10.1006/abbi.1996.9731. [DOI] [PubMed] [Google Scholar]
- Zappia V, Zydek-Cwick CR, Schlenk F. The specificity of S-adenosylmethionine derivatives in methyl transfer reactions. J Biol Chem. 1969;244:4499–4509. [PubMed] [Google Scholar]