Abstract
siRNA treatment has great promise to specifically control gene expression and select cell behaviors but have delivery challenges limiting their use. Particularly for applications in regenerative medicine, uniform and consistent delivery of siRNA to control gene expression and subsequent stem cell functions, such as differentiation, is paramount. Therefore, a diblock copolymer was examined for its ability to effective delivery siRNA to mesenchymal stem cells (MSCs). The diblock copolymers, which are composed of cationic blocks for siRNA complexation, protection, and uptake and pH-responsive blocks for endosomal escape, were shown to facilitate nearly 100% MSC uptake of siRNA, which is vastly superior to a commercially-available control, DharmaFECT, which resulted in only ~60% siRNA positive MSCs. Moreover, the diblock copolymer, at conditions that result in excellent knockdown (down to ~10% of control gene expression), is cytocompatible, causing no negative effects on MSC survivability. In contrast, DharmaFECT:siRNA treatment results in only ~60% survivability of MSCs. Longitudinal knockdown after siRNA treatment was examined and protein knockdown persists for ~6 days regardless of delivery system (diblock copolymer or DharmaFECT). Finally, MSC phenotype and differentiation capacity was examined after treatment with control siRNA. There is no statistically significant differences on cell surface markers of diblock copolymer:siRNA or DharmaFECT:siRNA treated or cells measured 2 weeks after siRNA delivery compared to untreated cells. Upon differentiation with typical media/culture conditions to adipogenic, chondrogenic, and osteogenic lineages and examination of histological staining markers, there is no discernable differences between treated and untreated cells, regardless of delivery mechanism. Thus, diblock copolymers examined herein facilitate uniform siRNA treatment of MSCs, inducing siRNA-specific gene and protein knockdown without adversely affecting MSC survival or differentiation capacity and therefore show great promise for use within regenerative medicine applications.
Introduction
Small, noncoding RNAi molecules known as microRNA (miRNA) posttranscriptionally regulate gene expression to endogenously control of a broad spectrum of biologic processes, including stem cell self-renewal, development, differentiation, growth, and metabolism 1. By perfect or imperfect base pairing with target mRNAs, miRNAs induce either translational repression or cleavage of the target mRNAs via enzyme-mediated mechanisms 1–5. Small interfering RNAs (siRNAs), another family of RNAi molecules, are exogenously delivered double stranded RNAs that can function identically to known miRNAs. Moreover, de novo identification of unique siRNAs is possible just based on mRNA homology without any knowledge of miRNA sequences or targets, providing a virtually unlimited supply of potential therapeutic siRNAs. Thus, effective delivery of siRNA may provide potent cues to direct stem cell behaviors for regenerative medicine applications.
Bone marrow-derived mesenchymal stem cells (MSCs) have demonstrated great potential for a multitude of regenerative medicine strategies 6,7. MSCs are capable of differentiating into cell types responsible for the production of musculoskeletal tissues such as cartilage, bone, and muscle 6,7. And while most stem cell sources require time-consuming enzymatic or mechanical tissue dissociation for isolation, marrow-derived MSCs can be readily isolated from bone marrow aspirates 8. Moreover, MSCs can readily be cultured in vitro to obtain sufficient cell numbers for transplantation 8. Interestingly, it is hypothesized that therapeutically, MSCs might exert beneficial effects by differentiation, directly functioning as a new cell type, or indirectly through the release of trophic factors that fulfill an endocrine/paracrine role 6–10. Regardless of their role, a means to control MSC behaviors could have great utility within regenerative medicine applications and siRNA may provide a means to control MSCs behaviors with great specificity 2–4,11.
Similar to any nucleic acid molecule, siRNA delivery is a great challenge 11–13. First, gaining uptake into cells is a significant hurdle, as the large molecular weight and negative charge of siRNA precludes association with plasma membranes required for nonspecific endosomal uptake. Commonly, cationic polymers or lipids are used to form nanoscale siRNA complexes of negatively-charged siRNA with the positively-charged carrier 12,14. There are numerous examples of cationic carriers that have been developed for siRNA delivery including poly(ethyleneimine) (PEI), chitosan, poly(amidoamine), poly(dimethylaminoethyl methacrylate), and poly(lysine) 12,14. Complexes of carriers and siRNA, particularly ones with overall cationic charges, can associate with negatively charged lipid bilayers and be uptaken endosomally 12,14. Without further intervention, siRNA is trafficked to lysosomes and degraded, as this is the common cellular translocation pathway for endocytosis, subjugating all therapeutic downstream effects of siRNA delivery. In fact, it has been reported that ‘the three biggest problems with RNAi therapeutics remain delivery, delivery, and delivery’ 15. Moreover, successful delivery of siRNA to MSCs has only been established using commercially-available delivery systems, PEI, or electroporation 16 17, resulting in substantial non-specific cytotoxicity and little versatility for further carrier development (e.g., incorporation of specific cell targeting epitopes or controlled release strategies).
To address many of these problems, we have pioneered the development of a family of diblock copolymers specially designed to protect siRNA from nucleases, enhance uptake, and efficiently escape endosomal trafficking 18–20. The copolymers are effective at siRNA delivery to a variety of cancer cell lines and have excellent cytocompatibility; these copolymers are synthesized using controlled, living polymerization, resulting in highly controlled molecular weights, reproducible structures and functions, and flexible end-group chemistries. In this work, we further demonstrate the utility of this delivery system by exploring its ability to mediate siRNA delivery to MSCs. We comprehensively evaluate polymer-mediated siRNA delivery in MSCs, examining siRNA uptake, non-specific cytotoxicity, specificity and longevity of target mRNA and protein knockdown, and any aberrant effects of siRNA delivery on MSC phenotype and multilineage capacity. The diblock copolymer shows great promise for siRNA-mediated control of MSC function and may therefore provide great therapeutic utility in a host of regenerative medicine approaches.
Experimental Section
Materials
All materials were obtained from Sigma Aldrich unless otherwise specified.
Synthesis of RAFT chain transfer agent (ECT)
ECT (4-Cyano-4-(ethylsulfanylthiocarbonyl) sulfanylvpentanoic acid) was synthesized as described previously 18,21.
siRNA delivery polymer synthesis
siRNA complexation block (pDMAEMA) synthesis
The copolymer design consists of two blocks, where the first block is composed of cationic dimethylaminoethyl methacrylate (DMAEMA) which complexes with negatively charged molecules such as siRNA or microRNA. In order to synthesize the DMAEMA polymer block, the radical initiator 2,2′-Azobis(4-methoxy-2.4-dimethyl valeronitrile) (V-70) (Wako Chemicals), and the chain transfer agent ECT was used. The polymerization was conducted in a nitrogen atmosphere in DMF at 30 °C for 12 hours. The initial ECT to initiator ratio was 10 to 1 ([CTA]o/[I]o ), while the initial monomer to ECT ratio ([M]o/[CTA]o), assuming 100% conversion, was such that number average molecular weight was 10,000 g/mol. The product was isolated by precipitation in 50:50 diethyl ether/pentane. To further purify the pDMAEMA macroCTA was dissolved in acetone, precipitated in pentane and dried under house vacuum overnight.
Endosomal escape (tercopolymer of PAA, BMA, and DMAEMA) block synthesis
In order to synthesize the second diblock copolymer block, pDMAEMA macroCTA was added to DMAEMA, propylacrylic acid (PAA), and butyl methacrylate (BMA) in N,N-dimethylformamide (DMF) (25 wt.% monomer and macroCTA to solvent). The ratios used for [M]o/[CTA]o and [CTA]o/[I]o were 250:1 and 10:1 respectively with relative amounts of DMAEMA:PAA:BMA of 25:25:50. This composition of the tercopolymer has previously been shown to be essential for the endosomal escape of siRNA 18. At physiological pH, PAA and DMAEMA and their respective carboxylic acid and tertiary amine groups are equimolar which makes the block amphiphilic. In the acidic endosomal environment, however, the PAA of the tercopolymer becomes protonated, which causes the structure to become hydrophobic and therefore membrane interacting. To the solution of macroCTA, PAA, BMA, and DMAEMA in DMF, V70 initiator was added and it was purged with nitrogen for 30 min. The reaction was allowed to proceed at 30 °C for 18 hours and the product was then precipitated into 50:50 diethyl ether/pentane, redissolved in acetone, precipitated into pentane and dried in vacuum overnight. To characterize the molecular weight and polydispersities of the first pDMAEMA block and diblock copolymer, gel permeation chromatography was performed comparing the products to polymethyl methacrylate standards using a 5 µm AM Gel mixed bed GPC column from American Polymer Standards. The mobile phase used was HPLC-grade DMF containing 0.1 wt.% LiBr at 60 °C flowing at 1 ml/minute. Both the pDMAEMA and diblock copolymers were analyzed via 1H NMR spectroscopy (Bruker Avance400) and UV spectroscopy to verify composition.
Characterization of polymer/siRNA complexes
Diblock copolymers were solubilized in highly concentrated stock solutions (~1 g/ml) in ethanol and diluted to 2 mg/ml in PBS. This solution was then utilized to form complexes with siRNA (Dharmacon ON-TARGETplus Non-targeting siRNA #1). Complexes of siRNA with diblock copolymer were formed as follows: siRNA was added to 1.5 ml tubes from 10 µM stock solutions. PBS was added to dilute the siRNA then diblock copolymer (2 mg/ml stock) was added. The resulting solution, which was typically 5–15x more concentrated than the treatment conditions, was incubated at room temperature for 20 min to allow for complete stabilization of complexes. Size and zeta potential measurements were made on a range of siRNA/polymer nanoparticles charge ratios and siRNA treatment concentrations using a Malvern Zetasizer. Charge ratio, which represents the ratio between the protonated DMAEMA residues of the pDMAEMA block, where 50% of the residues are protonated at physiological pH, and negatively charged siRNA, was tested from 1:1 to 8:1. Overall siRNA concentrations tested ranged from 10 to 100 nM. siRNA/diblock copolymer nanoparticles were compared to a commercially-available delivery reagent, DharmaFECT (Dharmacon) at conditions recommended by the manufacturer.
Mesenchymal stem cell (MSC) culture
Human MSCs (MSCs) were isolated from bone marrow aspirates obtained from Lonza 8, maintained in low glucose Dulbecco's Modified Eagle's Medium (LG-DMEM, Hyclone) with 1% penicillin-streptomycin, 1 ng/ml basic fibroblast growth factor (bFGF), and 10% fetal bovine serum (Atlanta Biologicals) and kept at 37 °C with 5% CO2. hMSCs were utilized at passage 5 or less. Mouse MSCs (mMSCs) isolated from GFP transgenic mice (C57BL/6-Tg(UBC-GFP)30Scha/J) were obtained from the mesenchymal stem cell distribution center at Texas A&M (passage 6). GFP-MSCs were cultured as recommended in Iscove’s Modified Dulbecco’s Medium (IMDM) with 1% penicillin-streptomycin, and 10% each of fetal bovine serum and horse serum (Atlanta Biologicals) and utilized prior to passage 10.
Assessing diblock copolymer-mediated siRNA uptake in MSCs
Carrier-mediated MSC uptake of siRNA was analyzed using fluorescently labeled siRNA (6-carboxyfluorescein (FAM) siRNA, Dharmacon #D-001530-01-05). Polymer/siRNA nanoparticles were formulated as described in the ‘Characterization of polymer/siRNA complexes’ section. MSCs were seeded at 12,000 cells/cm2 and treated after 24 hours. Experimental groups used were (1) FAM-siRNA/polymer nanoparticles at 4:1 charge ratio and 37.5 nM siRNA, (2) FAM-siRNA with DharmaFECT carrier (37.5 nM concentration), (3) FAM siRNA without a carrier (37.5 nM), and (4) no treatment. MSCS were treated with siRNA/carrier nanoparticles and after 1, 4 and 24 hours post-treatment, cells were analyzed on an Accuri C6 Flow Cytometer. Briefly, cells were trypsinized and resuspended in PBS with 0.5% bovine serum albumin (BSA) (EMD Biosciences) and 0.01% trypan blue to quench extracellular fluorescence 22 and analyzed in triplicate in three independent experiments. Further analysis was performed using gating of fluorescence to compare treated cells to the no treatment experimental group. In addition, plated cells were fixed with 4% paraformaldehyde 24 hours after treatment and mounted with ProLong Antifade with DAPI (4',6-diamidino-2-phenylindole, Invitrogen). Images were taken on a Nikon E600 Upright fluorescence microscope.
Measurement of polymer-mediated MSC cytotoxicity
MSCs were seeded at 12,000 cells/cm2 in 24 well plates. After 24 hours, MSCs were treated with polymer/siRNA nanoparticles at a variety of charge ratios and siRNA doses ranging from 10 to 37.5 nM. Untreated MSCs and DharmaFECT-mediated siRNA delivery were used as controls, respectively. After a 48 hour incubation period, an alamarBlue (AdB Serotec) metabolic assay for cell viability was performed, where MSCs were incubated with a 10% alamarBlue solution in growth medium and incubated for an additional 2 to 4 hours. Fluorescence of the resulting cell culture media due to metabolism of the active agent in alamarBlue to a fluorescent product was analyzed (excitation wavelength of 570 nm, emission of 600 nm, Tecan M200 Infinite). Cell viability was calculated by normalizing treated cells to untreated cells.
Assessing MSC gene and protein knockdown after diblock-copolymer mediated siRNA delivery
To quantify the efficacy of delivered siRNA to MSCs, mRNA expression was investigated after treatment with siRNA against a housekeeping gene, glyceraldehyde 3 phosphate dehydrogenase (GAPDH). MSCs were seeded as previously described, and treated with GAPDH-siRNA (Thermo Scientific Dharmacon #J-004253-07) polymer complexes at concentrations from 10 to 37.5 nM and at 37.5 nM at charge ratios of 1:1–8:1. Control experiments to analyze non-specific knockdown, MSCs were also treated with complexes at equivalent conditions but with Dharmacon ON-TARGETplus Non-targeting siRNA #1. After 48 hours, RNA was extracted using Homogenizer Mini columns and an e.Z.N.A. Total RNA Kit (Omega Bio-Tek). RNA was reverse transcribed using an iSCRIPT kit (Bio-Rad) and RT-PCR was performed using SsoFast EvaGreen Supermix (Bio-Rad) on a CFX96 Real-time PCR Detection System (Bio-Rad). The housekeeping gene used for RT-PCR was β-actin. Primer sequences for GAPDH and β-actin are listed in Table 1. GAPDH expression was quantified by comparing threshold cycles through the Pfaffl Equations, normalizing to β-actin expression and comparing to untreated cell populations 23.
Table 1.
RT-PCR primer sequences utilized in this work.
| Gene | Forward Sequence (5’-3’) | Reverse Sequence (5’-3’) |
|---|---|---|
| GAPDH | GCAAGAGCACAAGAGGAAGAG | AAGGGGTCTACATGGCAA |
| β-actin | TGTGATGGTGGGAATGGGTCAG | TTTGATGTCACGCACGATTTCC |
Knockdown of protein was longitudinally analyzed using GFP-MSCs. GFP-MSCs were plated at 12,000 cells/cm2 and treated 24 hours later with 37.5 nM GFP siRNA (Dharmacon) alone as a control or with polymer or DharmaFECT delivery systems, respectively. GFP protein expression was analyzed at days 1, 2, 3, 4, 6, 8, and 12 after treatment. Cells were rinsed with PBS twice and lysed with cell lysis buffer for 1 h at 4 °C (200 µL/well, 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate). GFP fluorescence was analyzed on a plate reader (excitation wavelength of 480 nm, emission of 520 nm, Tecan M200 Infinite) and normalized to DNA content of the same lysed solutions, as analyzed via the PicoGreen Assay (Invitrogen).
Effect of siRNA delivery carriers on MSC phenotype and multilineage differentiation capacity
MSCs treated with negative control siRNA (37.5 nM) alone or with polymer or DharmaFECT delivery systems were subsequently analyzed for cell surface markers consistent with MSCs. As an additional control, polymer alone (at identical concentrations to siRNA+polymer treatments), was added to cells. Specifically, markers included CD90 (FITC mouse anti-human, #555595, BD Pharmingen), CD105 (PE mouse anti-human, #560839, BD Pharmingen), CD44 (Pacific Blue anti-human, #103019, Biolegend), and CD45 (PE-Cy7 mouse anti-human, #557748, BD Pharmingen). MSCs are characterized as CD90+/CD105+/CD44+/CD45− 24. Two weeks after treatment, treated and control (untreated) cells were trypsinized, and stained for 20 min in each of the antibodies detailed above in PBS+5% FBS. Antibody labeling was analyzed using flow cytometry (Becton Dickinson LSR Benchtop Analyzer) after optimizing antibody labeling concentrations using titration curves. 10,000 cells were analyzed per sample and fluorescence gating was established using both unstained cells and untreated cells.
In addition, siRNA-treated MSCs were differentiated into osteogenic, chondrogenic, and adipogenic lineages as previously described 25–27. Briefly, for osteogenic and adipogenic differentiation, MSCs were seeded at a density of 12,000 cell/cm2 and treated with siRNA (37.5 nM) alone or delivered via polymer or DharmaFECT delivery systems with polymer alone (at identical concentrations to siRNA+polymer treatments) as an additional control. Two days after treatment, MSCs were differentiated using standard media conditions for three weeks. Osteogenic media consisted of normal growth media with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 50 µM ascorbic acid-2-phosphate 25. Similarly, for adipogenic differentiation, MSCs were cultured with adipogenic supplements, switching between 3 days in adipogenic differentiation medium (normal growth media made with high glucose DMEM with 1 µM dexamethasone, 0.2 mM indomethacin, 10 µg/mL insulin, 0.5 mM methylisobutylxanthine) and 1 day in adipogenic maintenance medium (normal growth media made with high glucose DMEM and 10 µg/mL insulin) 27. Differentiated two-dimensional cell cultures were fixed in 24-well tissue culture plates for 48 hrs in 4% paraformaldehyde and the monolayers were stained for mineralization (von Kossa, osteogenic), or the presence of lipid droplets (oil red o, adipogenic). Undifferentiated cultures were also stained as negative controls. Images were taken on a Motic AE20 inverted light microscope using a Canon EOS Rebel T2i with an eyepiece modification.
For chondrogenic differentiation, treated MSCs were centrifuged (1000 g, 5 min) to form pellet cultures (250,000 cells/pellet) and cultured for three weeks with chondrogenic supplements (normal growth media made with high glucose DMEM without FBS and with 10 ng/mL TGF-β3, 100 nM dexamethasone, 50 µg/mL ascorbic acid-2-phosphate, 100 µg/mL sodium pyruvate, 40 µg/mL proline, and ITS-plus (0.01 ml/ml media; final concentrations: 6.25/µg/ml bovine insulin, 6.25 µg/ml transferrin, 6.25 /µg/ml selenous acid, 5.33 µg/ml linoleic acid, and 1.25 mg/ml bovine serum albumin) 26. Pellet cultures were fixed for 48 hrs in 4% paraformaldehyde, cryosectioned, and stained for glycosaminoglycan production (toluidine blue, chondrogenic). Stained sections were imaged as described above.
Statistical analysis
Data was collected in triplicate in three independent experiments unless otherwise noted. One-way analysis of variance with Tukey’s post-hoc test was utilized to assess differences in mean data values (α=0.05).
Results and Discussion
siRNA delivery polymer synthesis and characterization
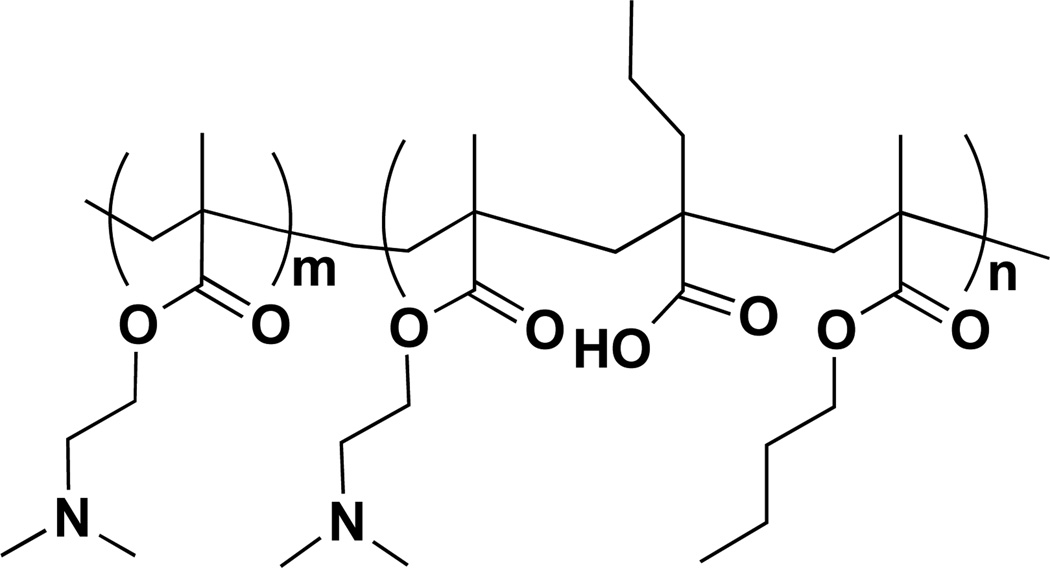
Diblock copolymers for siRNA delivery were synthesized using RAFT polymerizations 18,20. First, macromolecular chain transfer agents (macroCTAs) of dimethylaminoethyl methacrylate (DMAEMA), to condense and protect siRNA, enabling efficient cellular uptake, were polymerized and isolated. The pH-responsive blocks of DMAEMA, butyl methacrylate (BMA), and propylacrylic acid (PAA) was reacted in the presence of the mCTA, pDMAEMA, to form diblock copolymers (Figure 1). The pH-responsive block of the diblock copolymer modulates endosomal escape due to hydrophobic transition and membrane interaction as endosomal pH drops during typical endo-lysosomal trafficking 28–36. These RAFT polymerizations are characteristically well-controlled as evidenced by linear increases in molecular weights both with time and conversion and result in polymers of low polydispersity indices (PDI), as previously detailed for this class of diblock copolymers 20,37. For these studies, GPC was utilized to analyze molecular weight and polydispersity index of the mCTA (pDMAEMA) and the diblock copolymer, which were 9,800 g/mol, PDI = 1.2 and 28,500, PDI = 1.3, respectively.
Figure 1.
Poly(dimethylaminoethyl methacrylate)-b-poly(dimethylaminoethyl methacrylate-co-propylacrylic acid-co-butyl methacrylate) (pDMAEMA-b-p(DMAEMA-co-PAA-co-BMA)) polymer design for siRNA delivery to MSCs. Importantly, the first block, poly(dimethylaminoethyl methacrylate) (pDMAEMA, m) was designed to be partially protonated at physiological pH to allow for siRNA complexation and protection; the second block, n, was designed to be nearly charge neutral at physiologic pH (approximately 50% DMAEMA protonation and 50% PAA deprotonation) but to undergo a transition to more hydrophobic and membrane disruptive in lower pH environments of endo-lysosomal trafficking (m~60, n~70).
Characterization of polymer/siRNA complexes
Polymer diblock alone (at ~40 µg/ml) and polymer:siRNA complexes were characterized for both particle size and zeta potential at the same ranges of charge ratios and concentrations to those tested for siRNA delivery in vitro and the data is reported in Table 2. Irrespective of the various charge ratios or concentrations, the sizes of complexes (or polymer alone) were 50–60 nm. Similarly, zeta potential was similar across all conditions with values of ~ 10 mV. These data are consistent with previous reports of similar diblock copolymers 18,20,37. Importantly, these sizes are well over the minimum size cut-off (8 nm) to avoid renal clearance, yet small enough to minimize uptake by the reticuloendothelial system (particles > 100 nm), and avoid mechanical clearance by the lungs or spleen (>200 nm) 38, indicating that these particles would likely provide for long drug circulation times in vivo. In addition, the complexes were observed to be very stable, maintaining equivalent sizes and zeta potentials over several weeks (data not shown). In contrast to polymer diblocks, the DharmaFECT siRNA delivery system explored herein formed much larger sized particles in the presence of siRNA (136 ± 18.5 nm at 30 nM siRNA and 159 ± 25.6 nm at 37.5 nM siRNA). Interestingly, in the absence of siRNA, the size of DharmaFECT alone is ~ 24 nm, indicating very different particle properties that are dependent upon complexation with siRNA. However, similar to the polymer diblocks, DharmaFECT particles, regardless of siRNA complexation, exhibit surface charges of ~12 mV. The lack of siRNA dependence on surface charge regardless of delivery system is likely due to charge shielding as these measurements were performed in physiologically-relevant buffering conditions (PBS), which is relevant for both in vitro and in vivo siRNA delivery applications.
Table 2.
Sizes and Zeta potentials of diblock polymer:siRNA particles utilized to deliver siRNA to MSCs.
| Sample | Charge Ratio | siRNA Concentration | Size (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| Polymer:siRNA | 1:1 | 37.5 nM | 56.9 ± 13.1 | 12.2 ± 6.7 |
| Polymer:siRNA | 2:1 | 37.5 nM | 59.8 ± 16.1 | 13.9 ± 9.4 |
| Polymer:siRNA | 4:1 | 37.5 nM | 55.6 ± 13.4 | 10.8 ± 9.8 |
| Polymer:siRNA | 8:1 | 37.5 nM | 56.2 ± 15.7 | 14.8 ± 7.4 |
| Polymer:siRNA | 4:1 | 10 nM | 54 ± 12.7 | 9.6 ± 5.4 |
| Polymer:siRNA | 4:1 | 20 nM | 57.2 ± 13.2 | 11 ± 3.5 |
| Polymer:siRNA | 4:1 | 25 nM | 52.7 ± 11.7 | 14.6 ± 11.1 |
| Polymer:siRNA | 4:1 | 30 nM | 56.4 ± 16.7 | 15.3 ± 9.5 |
| Polymer:siRNA | 4:1 | 37.5 nM | 55.6 ± 13.4 | 13.7 ± 5.4 |
| Polymer only | NA | NA | 54.1 ± 11.3 | 14.2 ± 7.2 |
| DharmaFECT:siRNA | NA | 30 nM | 159 ± 25.6 | 11.8 ± 5.2 |
| DharmaFECT:siRNA | NA | 37.5 nM | 136 ± 18.5 | 13.8 ± 9.5 |
| DharmaFECT only | NA | NA | 24 ± 4.3 | 13.5 ± 8.3 |
Assessing diblock copolymer-mediated siRNA uptake in MSCs
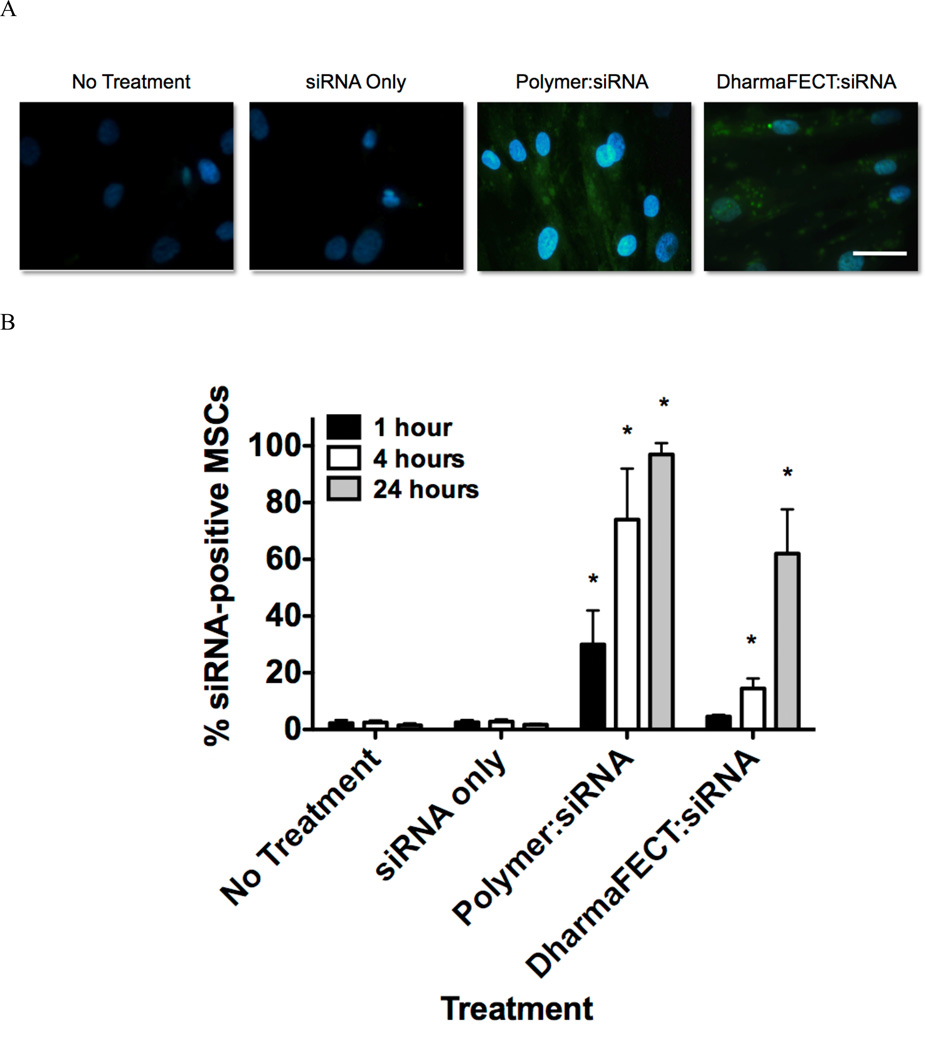
To explore polymer-mediated siRNA delivery into MSCs, fluorescently-labeled siRNA (FAM-siRNA) was utilized. Successful NP-mediated uptake in MSCs is shown in Figure 2A, where siRNA fluoresces green and cellular nuclei fluoresce blue (DAPI staining). Within cells that are untreated or treated only with siRNA there is no detectable green fluorescence within MSCs, indicating no siRNA internalization, as expected. siRNA is large and negatively charged and is not readily endocytosed by cells in the absence of a carrier 12. In contrast, there is clear polymer-mediated, intracellular accumulation of siRNA in all imaged cells and the accumulation is diffuse rather than punctate, indicating that cytosolic, rather than endosomal, localization of siRNA has been achieved. In DharmaFECT controls, however, there is a significant amount of siRNA with punctate appearance within the cytosol, indicative of endosomal or lysosomal compartmentalization. To quantitatively analyze uptake over time, we utilized flow cytometry to evaluate % of FAM-siRNA positive cells 1 h, 4 h, and 24 h after treatment. As shown in Figure 2B, untreated cells and siRNA treated cells were found to have no uptake of siRNA (<3% siRNA positive cells) longitudinally over the study period. However, polymer-mediated delivery of siRNA resulted in 30% positive cells after just 1 hour of treatment. At 4 hours, 75% of MSCs are positive for siRNA and at 24 hours, this level further increases to over 96%. This is in contrast to DharmaFECT-mediated delivery of siRNA, where at 1 hour, uptake is only positive for 4% of cells, a level not statistically increased over controls. However, the percentage of siRNA positive cells increased to ~15% at 4 hours and further increased to 64% at 24 hours. Interestingly, percent siRNA positive cells at both 4 and 24 hours using DharmaFECT as the delivery reagent was still significantly lower than the uptake observed for polymer-mediated delivery. In all applications involving therapeutic siRNA, it is incredibly important to guarantee complete and consistent treatment of all target cells. Particularly for applications in regenerative medicine this is true, where uniform treatment of MSCs with siRNA will result in consistent and reproducible effects on cell fate and function.
Figure 2.
(A) Representative images demonstrating limited siRNA (green) uptake in untreated and siRNA only treated MSCs and punctate siRNA localization in DharmaFECT:siRNA treated MSCs while polymer:siRNA treated MSCs show robust, diffuse staining within the cytosol. Samples were treated for 24 hours with 37.5 nM siRNA with 4:1 charge ratio (polymer:siRNA) or standard treatment conditions (DharmaFECT:siRNA). Nuclei are stained with DAPI (blue fluorescence), bar = 100 µm. (B) Flow cytometry was used to quantify %-siRNA positive MSCs at 1, 4, and 24 hours after treatment (37.5 nM siRNA treatment with 4:1 charge ratio (polymer:siRNA) or standard treatment conditions (DharmaFECT:siRNA). Data are from three independent experiments conducted in triplicate with error bars representing standard deviation. Statistical significance was evaluated at a level of p<0.05 using 1-way analysis of variance to compare between groups at the same timepoints. * indicates significance compared to all other treatments.
Measurement of carrier-mediated MSC cytotoxicity
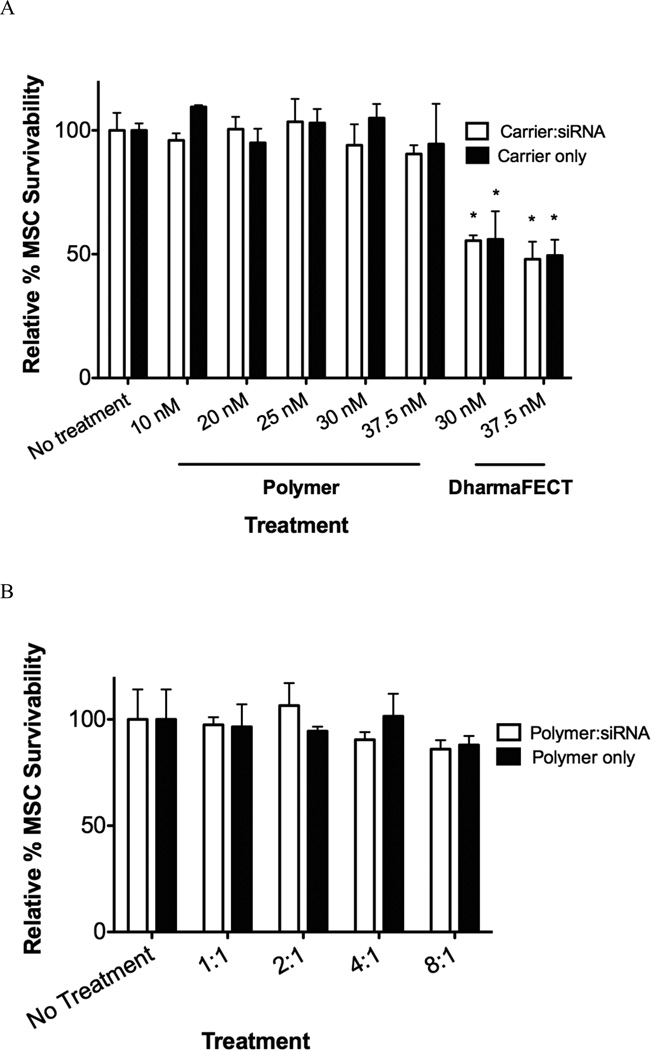
Non-specific cytotoxicity is a common drawback to cationic nucleic acid delivery systems 12. For example, poly(ethyleneimine) (PEI), the most well-studied, oft-used cationic polymer for nucleic acid delivery suffers from high levels of cytotoxicity, which is primarily attributed to necrosis and mitochondrial apoptosis activation 39. To characterize non-specific MSC cytotoxicity associated with diblock copolymer-mediated siRNA delivery, MSCs were treated with scrambled siRNA-copolymer nanoparticles at a variety of concentrations and charge ratios. MSCs treated with DharmaFECT/scrambled siRNA and polymer only were also analyzed. Forty-eight hours after treatment, an alamarBlue (AdB Serotec) metabolic assay for cell viability was performed and the data is summarized in Figure 3. Over a range of siRNA concentrations (10–37.5 nM with charge ratio of 4:1), diblock copolymer nanoparticle-mediated delivery exhibits no significant impact on cell viability compared with no treatment controls (Figure 3A). Similar results are shown for the nanoparticles over a range of charge ratios (1:1–4:1), where there was no significant reduction in MSC survivability compared with no treatment controls. There was also no differences between survivability observed with polymer:siRNA nanoparticles and polymer alone (Figure 3A and 3B). These data indicates that there is no MSC cytotoxicity due nanoparticle treatment at the conditions tested, similar to data previously observed with Hela cells and NIH3T3 fibroblasts 18,20,40. However, DharmaFECT-siRNA treatment was associated with a significant amount of MSC cytotoxicity of about 50% at both 30 and 37.5 nM siRNA concentrations.
Figure 3.
Nonspecific cytotoxicity in MSCs at a variety of siRNA concentrations and charge ratios delivered via polymer diblock or DharmaFECT. (A) MSCs were treated with siRNA at different concentrations with charge ratio of 4:1 (diblock copolymer carrier) or by manufacturer’s recommendations (DharmaFECT) and after 24 hours analyzed for cell survivability using the alamarBlue metabolic assay. (B) MSCs were treated with siRNA at 37.5 nM with charge ratios of 1:1–8:1. Data are from three independent experiments conducted in triplicate relative to survivability of untreated cells (No Treatment) with error bars representing standard deviation. Statistical significance was evaluated at a level of p<0.05 use 1-way analysis of variance with * indicating significance from no treatment controls.
Assessing MSC gene and protein knockdown after diblock-copolymer mediated siRNA delivery
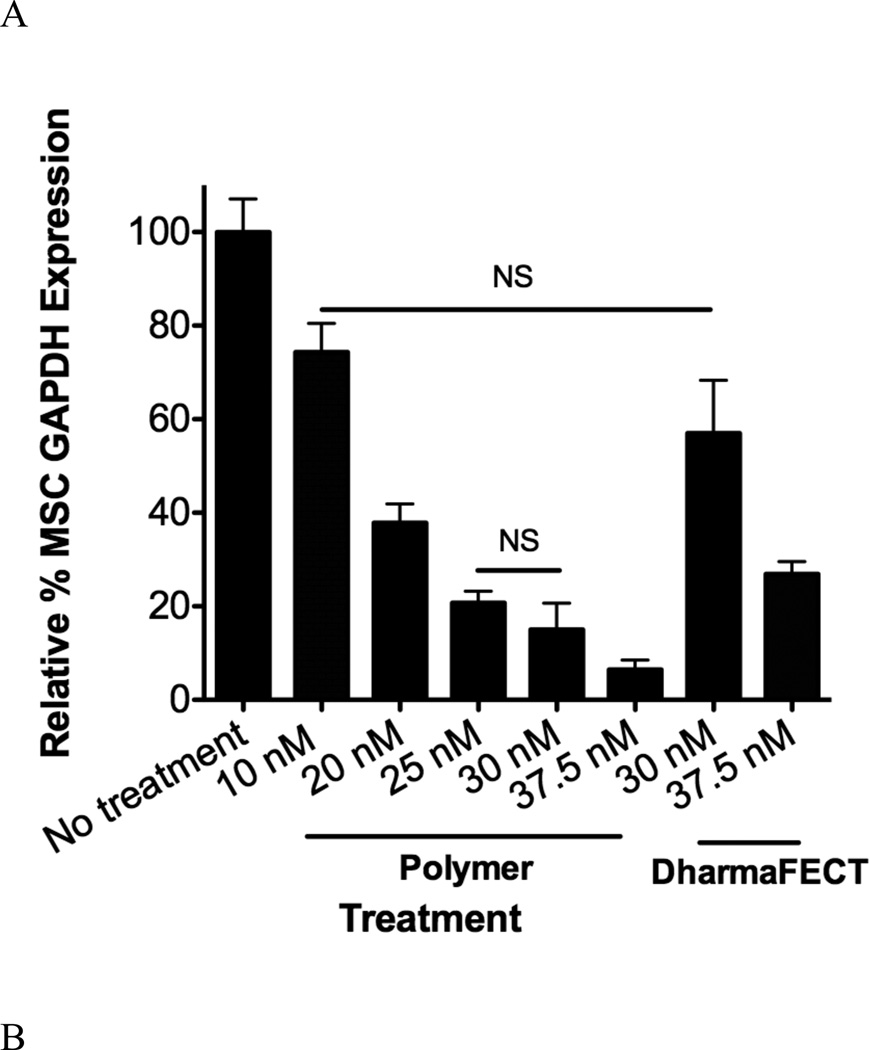
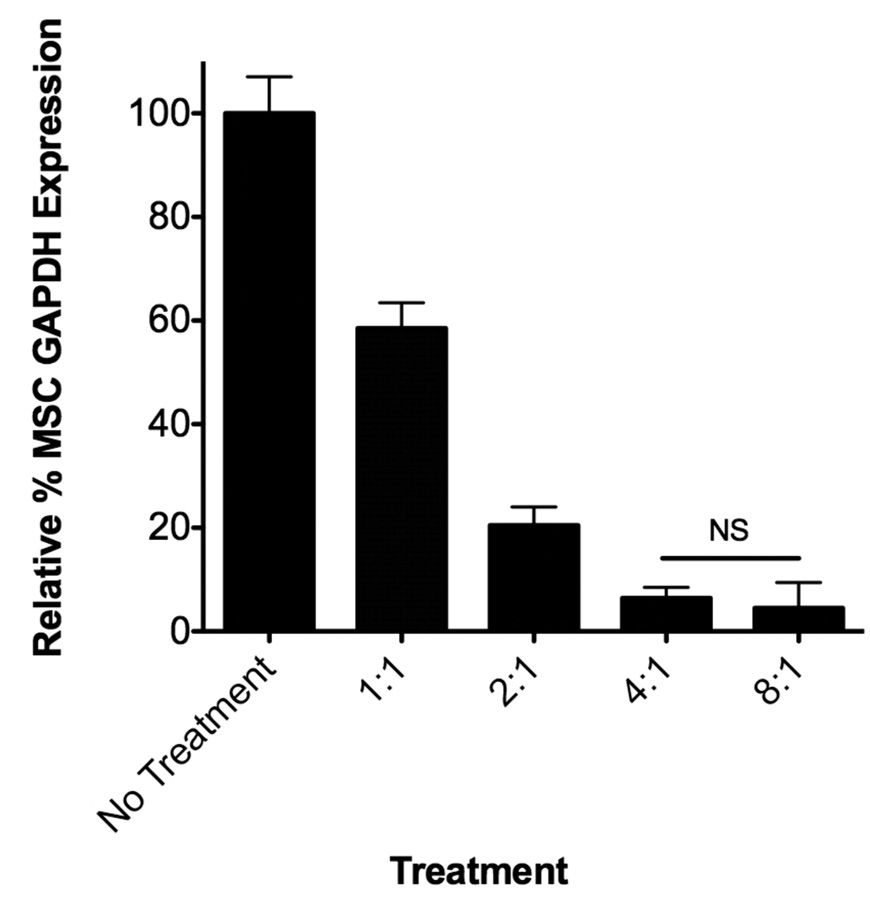
MSCs were seeded, subsequently treated with siRNA targeting GAPDH and allowed to incubate for 48 hours after which RT-PCR was performed to analyze alterations in GAPDH mRNA levels. A variety of siRNA doses (10–37.5 nM) and charge ratios (polymer alone, 1:1–8:1) were analyzed. As detailed in Figure 4A, GAPDH gene expression, which is shown relative to untreated controls, varied between 80% and 8% for doses ranging from 10 nM-37.5 nM siRNA delivered via the polymer nanoparticles at a fixed charge ratio of 4:1. DharmaFECT, a commercially available control used in this work, resulted in less GAPDH knockdown compared with diblock copolymer mediated siRNA delivery, realizing 60% and 25% of control GAPDH expression at 30 nM and 37.5 nM siRNA delivery. At these same siRNA doses, the polymer delivery system resulted in 15% and 8% of control gene expression, significantly more knockdown versus DharmaFECT-mediated siRNA delivery at equivalent concentrations. Importantly, no changes in GAPDH gene expression were observed when a non-specific, control siRNA was utilized under the same delivery conditions (see Supporting Information, Figure S1). Charge ratio of the polymer delivery system also significantly impacts GAPDH gene expression. Altering the polymer:siRNA charge ratio from 1:1 to 2:1 to 4:1 and to 8:1 resulted in 59%, 21%, 8%, and 5% of control GAPDH levels, respectively. This trend of increasing charge ratio and enhanced siRNA activity has been described previously for a variety of cationic nucleic acid delivery polymers and lipids, typically by decreasing overall particle size to more favorable sizes for uptake (~50–200 nm) or through increased surface charge and membrane association 18,19,41,42. In this particular work, charge ratio does not affect the size nor surface properties of polymer:siRNA particles at least at the physiologically-relevant buffer conditions we utilized to analyze the particles (see size and Zeta Potential measurements, Table 2). However, by increasing the overall charge ratio at equivalent siRNA doses, it may be possible that greater overall concentrations of pH-responsive blocks are endocytosed. Therefore, the overall efficiency of uptake and/or extent to which endosomal membrane disruption occurs may be intrinsically increased by default of the carrier design (e.g., by increasing the charge ratio and cationic characteristics, the diblock copolymer concentration must increase as does the pH-responsive characteristics), thereby resulting in the observed increases in cytosolic delivery of siRNA and subsequent siRNA-mediated gene knockdown effects.
Figure 4.
Specific siRNA-mediated knockdown of GAPDH in MSCs at a variety of siRNA concentrations and charge ratios delivered via polymer diblock or DharmaFECT carriers. (A) MSCs were treated with siRNA at different concentrations with charge ratio of 4:1 (diblock copolymer carrier) or by manufacturer’s recommendations (DharmaFECT) and after 48 hours analyzed for GAPDH gene expression using RT-PCR with β-actin as the housekeeping gene (see Table 1 for primer sequences used). (B) MSCs were treated with GAPDH siRNA at 37.5 nM with charge ratios of 1:1–8:1 using the diblock copolymer delivery system. Data are from three independent experiments conducted in triplicate relative to gene expression of untreated cells (No Treatment) with error bars representing standard deviation. Statistical significance was evaluated at a level of p<0.05 using one-way analysis of variance. All pairwise comparisons were found significant unless noted by NS (not significant).
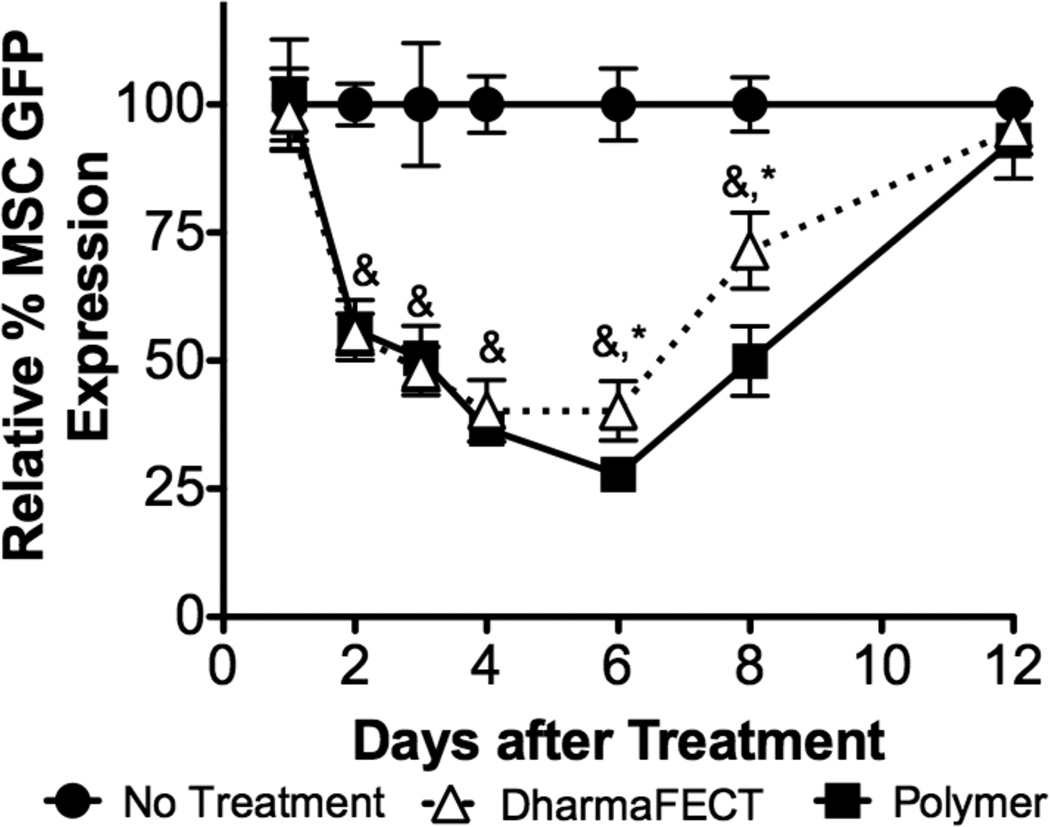
As siRNA delivery is transient due to cell division intracellular siRNA half-life, we sought to examine longitudinal protein knockdown after treatment with siRNA. To simplify the quantification of protein knockdown, we utilized GFP transgenic MSCs, employing siRNA specific to GFP and following GFP protein levels using fluorescence. Figure 5 reports GFP signal, which is normalized to DNA for each treatment to account for seeding fluctuations and nonspecific cytotoxicity of the control, DharmaFECT, and shown relative to untreated control cells at day 1, 2, 3, 4, 6, 8, 12 after siRNA treatment. GFP signal is unaffected 1 day after treatment. However, at day 2, expression is reduced to ~55% of control and further reduces to ~50% and 38% of control at days 3 and 4 for both diblock-copolymer and DharmaFECT-mediated siRNA delivery. At day 6, polymer:siRNA treated MSCs exhibit a further reduction in GFP signal, to ~28% of control while DharmaFECT-treated cells stay at ~40%. At day 8, knockdown is reversed to ~50% for polymer:siRNA treated MSCs and ~70% for DharmaFECT treated MSCs. By day 12, GFP expression recovered to control cell levels. ~8 days of siRNA efficacy is consistent with other studies examining longevity of knockdown after delivery. As an example, gene knockdown was examined using Oligofectamine in cell lines with various cell doubling times. Significant knockdown was observed that was consistent with doubling times; cells with fast doubling times (0.8 days) had maximum knockdown (~40% gene expression of untreated cells) 2 days after treatment, which returned to normal levels after 3 days. For cells with doubling times of ~1.5 days, knockdown was greatest 1 day after treatment (~20% gene expression of untreated cells) and restored to normal after 5 days. Finally, cells considered to be ‘non-dividing’ (ccd-1074sk human skin fibroblast cells, which have reported doubling times of approximately 2–3 days 43) exhibited maximum knockdown ~5 days after treatment (to ~20% gene expression of untreated cells), which slowly recovered to normal after 20 days 44. As MSCs have characteristic doubling times of ~2.5 days, our longitudinal analysis is consistent with this data considering also that our data indicates that GFP expression is restored to untreated levels at ~ 12 days. Interestingly, there is little difference between polymer- and DharmaFECT-mediated kinetics of knockdown suggesting similar processing and division amongst daughter cells once siRNA is successfully delivered cytosolically. Although a short, substantial knockdown of certain targets may be sufficient to trigger a cascade of downstream effects, other situations may require considerably longer knockdown to achieve the desired therapeutic effect. Particularly in regenerative medicine applications, where control over cell fate and function is a central goal, gene knockdown of ~8 days may not be sufficient. Some have attempted to solve this problem by using lentiviral delivery of expressed short-hairpin siRNAs (shRNAs) to achieve sustained gene silencing in vitro and in vivo 45,46. Other more recent and innovative approaches include incorporation of siRNA:delivery system nanoparticles into biomaterials for sustained release 40,47–49. Most recently, microsponges consisting only of shRNA have been synthesized. The microsponges, where the assembled shRNAs are processed intracellularly to siRNAs, have been demonstrated to efficiently deliver over half a million copies of siRNA per microsponge to a cell while maintaining RNA stability in vivo, albeit with slightly lower efficacy than via delivery with PEI 50. However, some data indicates the necessity of long-term delivery, particularly for control of MSC fate, may not be necessary. Through one treatment of MSCs with siRNAs that mimic miRNAs upregulated during MSC osteogenic differentiation, Mariner et al. demonstrated greater sensitivity of MSCs to typical soluble osteogenic factors (e.g., dexamethasone, ascorbic acid, and β-glycerophosphate). siRNA treatment resulted in more rapid and robust increases in the osteogenic markers alkaline phosphatase and calcium deposition compared with untreated MSCs 17.
Figure 5.
Longitudinal knockdown of GFP signal in GFP transgenic MSCs was assessed for the diblock copolymer and DharmaFECT delivery systems. GFP-MSCs were treated with siRNA at 37.5 nM with charge ratio of 4:1 (diblock copolymer carrier) or by manufacturer’s recommendations (DharmaFECT). At a variety of timepoints, GFP signal, which is normalized to DNA for each treatment to account for seeding fluctuations and nonspecific cytotoxicity of the control, DharmaFECT, was analyzed and is shown relative to untreated control cells at day 1, 2, 3, 4, 6, 8, 12 after siRNA treatment. Data are from three independent experiments conducted in triplicate relative to GFP expression of untreated cells (No Treatment) with error bars representing standard deviation. Statistical significance was evaluated at a level of p<0.05 using 1-way analysis of variance with & and * indicating significance from no treatment controls and polymer delivery, respectively.
Effect of siRNA delivery carriers on MSC phenotype and multilineage differentiation capabilities
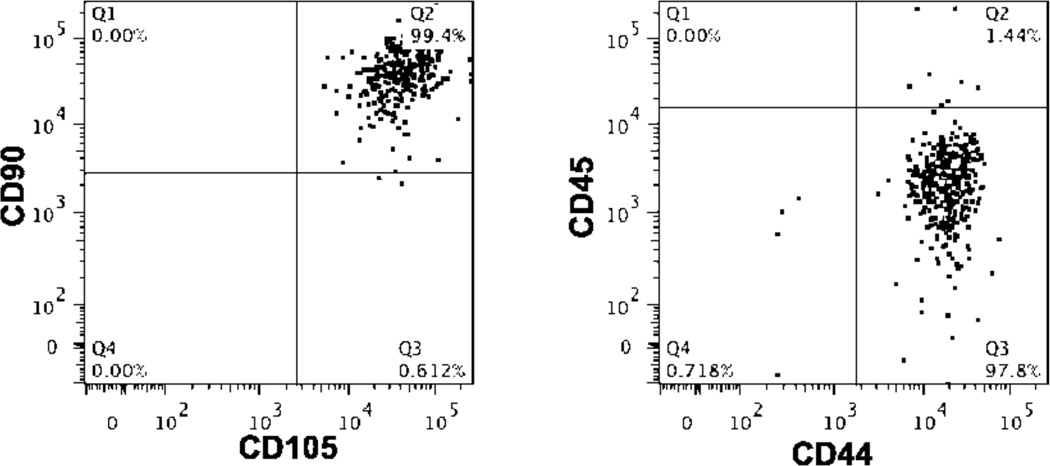
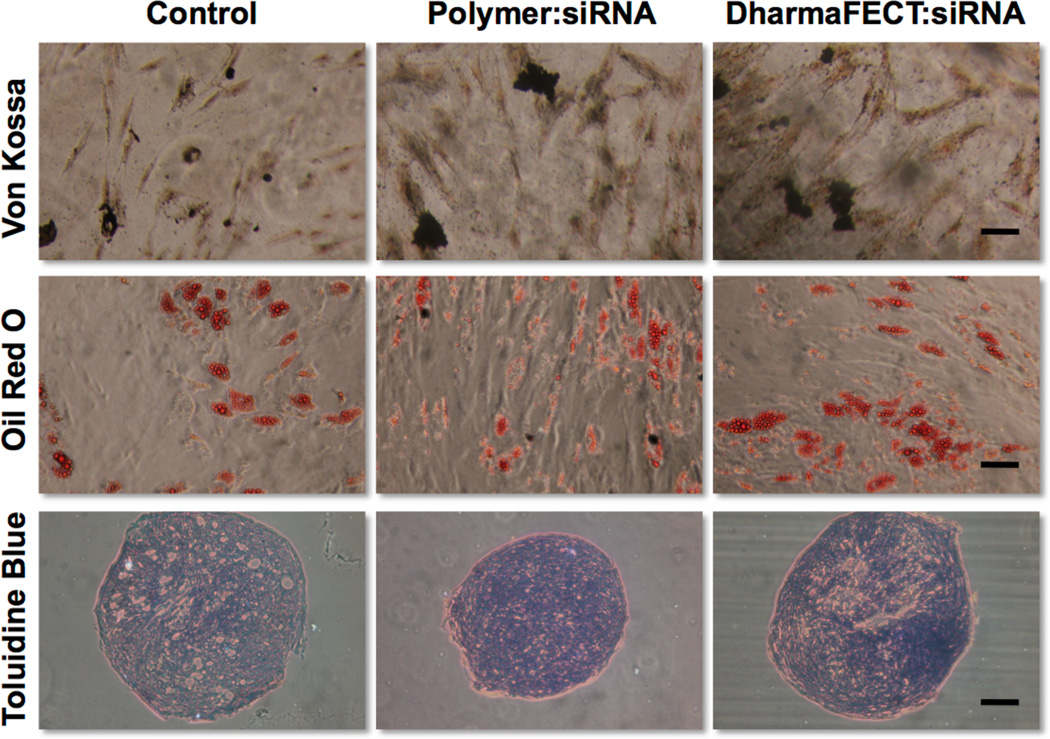
MSCs treated with the newly developed and efficacious siRNA delivery system were also examined for alterations in their phenotype using flow cytometry. MSCs, treated with the dibock copolymer nanoparticles or DharmaFECT, were examined using markers typically used to characterize MSCs. MSCs that were treated with control (scrambled) siRNA/polymer NPs showed no altered MSC phenotype when treated over 15 days. Specifically, cell surface markers of MSCs (CD44+/CD45−/CD90+/CD105+) (see representative flow cytometry dot plots, Figure 6) do not statistically change regardless of treatment (no treatment, siRNA/polymer NPs, or DharmaFECT). In all treatments including polymer:siRNA (4:1 charge ratio, 37.5 nM siRNA), polymer alone (equivalent concentration to 4:1, 37.5 nM siRNA), and DharmaFECT (37.5 nM siRNA), the percentage of cells gated as CD44+/CD45−/CD90+/CD105+ was 94% or greater. To further verify that polymer/siRNA-NP treated MSCs do not suffer aberrant phenotypic changes, treated MSCs were subsequently differentiated using typical soluble cues into osteogenic, adipogenic, and chondrogenic phenotypes. Histology was utilized to examine differentiation and is shown in Figure 7 compared to controls of untreated MSCs. As demonstrated by the Oil Red O, Toluidine Blue, and Von Kossa staining, specifically for adipogenesis, chondrogenesis, and osteogenesis, respectively, treatment of MSCs with siRNA alone or complexed with the diblock copolymer delivery system or DharmaFECT did not alter their multilineage capacity, as staining is qualitatively identical amongst all experimental groups. According to Lonza, the source of the MSCs used in this work (except for longitudinal knockdown studies), MSCs are validated for the following markers: CD105+, CD44+, CD90+, CD45− by flow cytometry. They are also tested using the same in vitro assays as utilized herein and are found to be positive for adipogenic, chondrogenic, osteogenic differentiation using similar staining methods 24. Thus, based on our analysis, treatment of MSCs with control siRNA alone or delivered via polymer:siRNA or DharmaFECT:siRNA nanoparticles does not affect their stemness or ability to differentiate into mesenchymal lineages. To our knowledge, this is the first study examining explicitly the effect of siRNA delivery (with or without delivery systems) on MSC stemness. However, recent studies have identified gene expression changes that are induced with a variety of nucleic acid delivery systems using microarray analysis 51–53. For example, cationic lipids oligofectamine and lipofectin altered the expression of various genes – even some involved in differentiation 53. However, these studies only examined the effect of delivery system alone and did not examine long-term effects on stem cell differentiation capacity. Gene signatures can change when delivery system is formulated with siRNA and can vary greatly for different delivery materials, cells, and treatment times 54. It is extremely important to examine these effects to minimize unintended, off-target effects of siRNA treatment, particularly for applications in regenerative medicine, where consistent, controlled differentiation of all cells is desirable.
Figure 6.
Representative flow cytometry dot plots demonstrating no significant alterations in surface antigen expression by MSCs treated with siRNA. MSCs were treated with control siRNA at 37.5 nM with charge ratio of 4:1 (diblock copolymer carrier) or by manufacturer’s recommendations (DharmaFECT). Two weeks following treatment, MSCs were labeled with anti-CD90, anti-CD105, anti-CD45, and anti-CD44 antibodies and analyzed via flow cytometry. Fluorescence gating was established using both unstained cells and untreated cells. Data were analyzed from three independent experiments conducted in triplicate relative to untreated cells and statistical significance was evaluated at a level of p<0.05. The percentage of MSCs with the phenotype CD90+/CD105+/CD44+/CD45− for no treatment, polymer:siRNA, polymer alone, and DharmaFECT:siRNA treatments were 98.1 ± 0.4, 97.9 ± 0.2, 97.7 ± 0.8, and 93.6 ± 3.7, respectively, with no significant differences obtained upon pairwise comparison.
Figure 7.
siRNA treated MSCs Retain Multilineage Potential. MSCs were treated with control siRNA at 37.5 nM with charge ratio of 4:1 (diblock copolymer carrier) or by manufacturer’s recommendations (DharmaFECT). 24 hrs after treatment, MSCs were differentiated using standard protocols for 3 weeks to induce osteogenesis (Von Kossa, mineralization), adipogenesis (oil red o, lipid droplets), or chondrogenesis (toluidine blue, glycosaminoglycans), bar = 100 µM.
Conclusions
Here we utilize diblock copolymers to demonstrate successful, efficacious, and safe delivery of siRNA to MSCs. siRNA was uniformly delivered to all MSCs treated using the diblock copolymer formulation, resulting in gene and protein-specific knockdown, with no observed cytotoxicity at efficacious treatment conditions. Moreover, protein knockdown was sustained for ~6 days, similar in longevity to the commercially-available carrier employed as a control. siRNA delivered via this polymer did not alter MSC stem cell phenotype nor MSC differentiation capacity. Thus, we expect this polymer delivery system to have great utility to specifically and potently alter MSC intracellular protein production. Delivery of siRNA, therefore, may prove useful for controlling stem cell behaviors for a variety of applications in regenerative medicine.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the National Science Foundation (BMAT-1206219) and the University of Rochester for funding. We would also like to thank Dr. James McGrath for use of equipment and Andrew Shubin for reading and editing of the manuscript. The GFP-expressing mouse mesenchymal stem cells employed in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH, Grant # P40RR017447.
Footnotes
Supporting Information
Supporting information, particularly knockdown data resulting from control siRNA delivery experiments (Figure S1) is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Zhang B, Pan X, Anderson TA. Journal of cellular physiology. 2006;209:266. doi: 10.1002/jcp.20713. [DOI] [PubMed] [Google Scholar]
- 2.Stadler BM, Ruohola-Baker H. Cell. 2008;132:563. doi: 10.1016/j.cell.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo L, Zhao RC, Wu Y. Exp Hematol. 2011;39:608. doi: 10.1016/j.exphem.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Yau WW, Rujitanaroj PO, Lam L, Chew SY. Biomaterials. 2012;33:2608. doi: 10.1016/j.biomaterials.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Zoldan J, Lytton-Jean AK, Karagiannis ED, Deiorio-Haggar K, Bellan LM, Langer R, Anderson DG. Biomaterials. 2011;32:7793. doi: 10.1016/j.biomaterials.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamnig A, Lepperdinger G. Can J Physiol Pharmacol. 2012;90:295. doi: 10.1139/y11-109. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI, Bruder SP. Trends Mol Med. 2001;7:259. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF. Methods Mol Biol. 2008;449:27. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. J Pathol. 2009;217:318. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan AI, Correa D. Cell Stem Cell. 2011;9:11. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y, Wang C, Varshney RR, Wang DA. Pharm Res. 2009;26:263. doi: 10.1007/s11095-008-9772-3. [DOI] [PubMed] [Google Scholar]
- 12.Pack DW, Hoffman AS, Pun S, Stayton PS. Nat Rev Drug Discovery. 2005;4:581. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 13.Cheema SK, Chen E, Shea LD, Mathur AB. Wound Repair Regen. 2007;15:286. doi: 10.1111/j.1524-475X.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- 14.Kang HC, Huh KM, Bae YH. J Controlled Release. 2012 doi: 10.1016/j.jconrel.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkel JM. Science (New York, N.Y.) 2009;326:454. [Google Scholar]
- 16.Jeon SY, Park JS, Yang HN, Woo DG, Park KH. Biomaterials. 2012;33:4413. doi: 10.1016/j.biomaterials.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Mariner PD, Johannesen E, Anseth KS. J Tissue Eng Regen Med. 2012;6:314. doi: 10.1002/term.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benoit DS, Convertine AJ, Duvall CL, Hoffman AS, Stayton PS. J Controlled Release. 2009;133:221. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoit DS, Henry SM, Shubin AD, Hoffman AS, Stayton PS. Mol Pharmaceutics. 2010;7:442. doi: 10.1021/mp9002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benoit DS, Srinivasan S, Shubin AD, Stayton PS. Biomacromolecules. 2011;12:2708. doi: 10.1021/bm200485b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moad G, Chong YK, Postma A, Rizzardo E, Thang SH. Polymer. 2005;46:8458. [Google Scholar]
- 22.Sahlin S, Hed J, Rundquist I. J Immunol Methods. 1983;60:115. doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonza; Lonza, Ed. 2012. [Google Scholar]
- 25.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. J Cell Biochem. 1997;64:295. [PubMed] [Google Scholar]
- 26.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Tissue Eng. 1998;4:415. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 27.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Dev Cell. 2004;6:483. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 28.Benoit DS, Convertine AJ, Duvall CL, Hoffman AS, Stayton PS. J Control Release. 2009;133:221. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benoit DS, Henry SM, Shubin AD, Hoffman AS, Stayton PS. Mol Pharm. 2010;7:442. doi: 10.1021/mp9002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benoit DS, Srinivasan S, Shubin AD, Stayton PS. Biomacromolecules. 2011 doi: 10.1021/bm200485b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albarran B, To R, Stayton PS. Protein Engineering, Design & Selection: PEDS. 2005;18:147. doi: 10.1093/protein/gzi014. [DOI] [PubMed] [Google Scholar]
- 32.El-Sayed MEH, Hoffman AS, Stayton PS. J Controlled Release. 2005;104:417. doi: 10.1016/j.jconrel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Henry SM, El-Sayed MEH, Pirie CM, Hoffman AS, Stayton PS. Biomacromolecules. 2006;7:2407. doi: 10.1021/bm060143z. [DOI] [PubMed] [Google Scholar]
- 34.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. Bioconjugate Chem. 2002;14:412. doi: 10.1021/bc020056d. [DOI] [PubMed] [Google Scholar]
- 35.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. J Controlled Release. 2003;89:365. doi: 10.1016/s0168-3659(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 36.Yin X, Hoffman AS, Stayton PS. Biomacromolecules. 2006;7:1381. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- 37.Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. Biomacromolecules. 2010;11:2904. doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda H, Bharate GY, Daruwalla J. Eur J Pharm Biopharm. 2009;71:409. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. Mol Ther. 2005;11:990. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Nelson CE, Gupta MK, Adolph EJ, Shannon JM, Guelcher SA, Duvall CL. Biomaterials. 2012;33:1154. doi: 10.1016/j.biomaterials.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erbacher P, Roche AC, Monsigny M, Midoux P. Bioconjugate Chem. 1995;6:401. doi: 10.1021/bc00034a010. [DOI] [PubMed] [Google Scholar]
- 42.Goula D, Remy JS, Erbacher P, Wasowicz M, Levi G, Abdallah B, Demeneix BA. Gene Ther. 1998;5:712. doi: 10.1038/sj.gt.3300635. [DOI] [PubMed] [Google Scholar]
- 43.ATCC. 2012 www.atcc.org.
- 44.Bartlett DW, Davis ME. Nucleic Acids Research. 2006;34:322. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiscornia G, Singer O, Ikawa M, Verma IM. Proc Natl Acad Sci U S A. 2003;100:1844. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. Nat Genet. 2003;33:401. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 47.Cao H, Jiang X, Chai C, Chew SY. J Controlled Release. 2010;144:203. doi: 10.1016/j.jconrel.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Chen M, Gao S, Dong M, Song J, Yang C, Howard KA, Kjems J, Besenbacher F. ACS Nano. 2012;6:4835. doi: 10.1021/nn300106t. [DOI] [PubMed] [Google Scholar]
- 49.Rujitanaroj PO, Wang YC, Wang J, Chew SY. Biomaterials. 2011;32:5915. doi: 10.1016/j.biomaterials.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 50.Lee JB, Hong J, Bonner DK, Poon Z, Hammond PT. Nat Mater. 2012;11:316. doi: 10.1038/nmat3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beyerle A, Irmler M, Beckers J, Kissel T, Stoeger T. Mol Pharmaceutics. 2010;7:727. doi: 10.1021/mp900278x. [DOI] [PubMed] [Google Scholar]
- 52.Merkel OM, Beyerle A, Beckmann BM, Zheng M, Hartmann RK, Stoger T, Kissel TH. Biomaterials. 2011;32:2388. doi: 10.1016/j.biomaterials.2010.11.081. [DOI] [PubMed] [Google Scholar]
- 53.Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. J Drug Targeting. 2003;11:311. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- 54.Akhtar S, Benter I. Adv Drug Delivery Rev. 2007;59:164. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.