Abstract
Objective
Cholesterol accumulation by macrophages plays a key role in atherogenesis. To begin to develop a global picture of this process, we used proteomics and transcriptomics to analyze foam cells generated with acetyl-LDL, a classic ligand for scavenger receptors.
Methods and Results
Tandem mass spectrometry and stringent statistical analysis revealed that foam cells differentially expressed 15 of the 542 proteins (2.8%) detected in macrophage-conditioned medium. Apolipoprotein E was one of the most up-regulated proteins, confirming that proteins involved in lipid metabolism are important targets for regulation by sterol accumulation. However, levels of proteins linked to complement activation and lysosomal proteolysis also changed markedly. Transcriptional analysis demonstrated that 698 of 19,700 genes (3.5%) were regulated in foam cells, including many genes important in sterol metabolism. We also found that cholesterol accumulation regulated genes implicated in complement activation, but failed to affect genes linked to proteolysis and macrophage polarization. Changes in protein levels in macrophage-conditioned medium were largely independent of changes in mRNA levels.
Conclusions
Loading sterol into macrophages regulates levels of complement proteins and lysosomal proteases—key players in the immune system and plaque rupture. Posttranscriptional mechanisms appear important for controlling levels of most of the proteins detected in macrophage medium.
Keywords: cholesterol, proteolysis, complement, macrophage, atherosclerosis
INTRODUCTION
Scavenger receptors expressed by macrophages play key roles in host defense by clearing bacterial pathogens and apoptotic cells 1. However, scavenger receptors also mediate the uptake of modified lipoproteins during atherosclerosis 2-5. When macrophages take up and degrade more lipoprotein-derived cholesterol than they can excrete, they convert free cholesterol to cholesteryl ester 2. Macrophages loaded with cholesteryl ester-laden lipid droplets—termed foam cells because of their microscopic appearance—are the hallmark of early atherosclerotic lesions 2.
Moreover, genetic, biochemical, and clinical studies provide compelling evidence that macrophages engorged with cholesterol are of central importance in both the initiation and progression of atherosclerotic lesions 2-5. Macrophages do not become foam cells in vitro if incubated with LDL, a major risk factor for atherosclerosis, because cholesterol uptake down-regulates the LDL receptor 2. In contrast, when the lysine residues of apoB, the major LDL protein, are acetylated chemically (acetyl-LDL), the modified lipoprotein binds to macrophage receptors that are not regulated by intracellular sterol content 2. Thus, macrophages incubated with acetyl-LDL in vitro rapidly endocytose the lipoprotein to become foam cells laden with cholesteryl ester.
Studies of mouse macrophages exposed to acetyl-LDL have provided important insights into the role of sterol metabolism in foam cell biology 2, 3. To begin to develop a global view of this model system, we used transcriptomics and proteomics to investigate mRNA and protein expression by mouse macrophages incubated with acetyl-LDL. Our results indicate that proteins that are differentially expressed in the medium of macrophage foam cells are linked to three functional modules 6: lipid metabolism, lysosomal biology, and complement activation. These observations raise the possibility that sterol accumulation by macrophages makes previously unsuspected contributions to the regulation of complement activation and proteolysis in atherosclerotic lesions and other inflammatory tissues.
METHODS
An expanded Methods section is available in online-only Data Supplement.
Lipoproteins
LDL (density 1.063-1.25 g/mL) isolated by sequential ultracentrifugation 7, 8 was acetylated (acetyl-LDL) with acetic anhydride .
Mouse Macrophages
Macrophages harvested from the peritoneum of C57Bl/6J mice 5 days after injection of thioglycolate were plated, washed, and cultured in medium supplemented with 20 μg/mL of LDL or acetyl-LDL 9 .
Proteomics
Proteins were harvested from macrophage conditioned medium, digested with trypsin, and subjected to LC-ESI-MS/MS analysis 9. MS/MS spectra were searched against the mouse International Protein Index (IPI) database 10 (version 2006/04/18). Proteins were quantified by spectral counting, using dual statistical criteria and replicate analyses of each sample 9, 11-13.
Microarrays
Total cellular RNA (RNeasy Mini Kit, Qiagen) was analyzed with Affymetrix Mouse Gene 1.0 ST arrays by the Center for Array Technologies (University of Washington). Raw microarray data were processed with Affymetrix Expression Console Software, using RMA normalization (http://affymetrix.com). P-values were calculated with a modified t-test and an empirical Bayes method 14. P-values were adjusted for multiple comparisons, using the Benjamini-Hochberg method 15. Microarray data are available from the Gene Expression Omnibus Database (accession number GSE19926).
Functional annotation
The Gene Ontology knowledgebase and DAVID (Database for Annotation, Visualization and Integrated Discovery) were used to functionally annotate proteins and genes 16. Enriched functional categories were identified relative to the entire mouse genome.
Protein and gene interaction networks
Protein/gene interaction networks were created by pathway analysis 17, using Ingenuity System software, BIND, DIP, MIPS, IntAct, BioGRID, and MINT. Protein and gene networks were derived by pathway analysis of direct and indirect physical, transcriptional, and enzymatic interactions derived from a global molecular network. All nodes in the protein network were proteins detected by MS/MS analysis of macrophage-conditioned medium.
RESULTS
The shed and secreted macrophage proteome is enriched in specific functional modules
We used tandem mass spectrometry to compare the proteomes of conditioned medium harvested from control and cholesteryl ester-laden macrophages generated by incubating cultured mouse peritoneal macrophages with LDL or acetyl-LDL. Acetyl-LDL treatment doubled the cells’ free cholesterol content and markedly increased their cholesteryl ester mass (for macrophages exposed to LDL or acetyl-LDL, 19.3±2.1 and 38.3±3.8 μg free cholesterol per mg protein and 0.8±0.5 and 42.3±4.3 μg cholesteryl ester per mg protein, respectively). The cells were washed and incubated with serum-free medium for 6 h, and the macrophage-conditioned medium was subjected to LC-ESI-MS/MS to identify proteins.
To ensure high confidence identification of proteins, we processed our MS/MS search results with two Bayesian algorithms 18, 19. We further required that each protein be detected in every analysis of one biological condition, which markedly diminishes the false-positive rate for protein detection 20. This approach generated a list of 542 proteins identified with high confidence in macrophage-conditioned medium.
To organize the shed and secreted macrophage proteome into functional modules 6, we used Gene Ontology annotations, which associate gene products with biological processes, cellular components, and molecular functions (Fig. 1A). This approach identified significant enrichment (relative to the entire mouse genome) of a wide variety of functional modules: cytoskeletal regulation (p<10−4), lysosomal biology (p<10−4), oxidation (p<10−4), cell motility (p <10−4), coagulation (p<10−4), complement regulation (p=10−4), regulation of apoptosis (p=10−4), immunity (p=10−3), and extracellular matrix (p=0.03). Although we detected 14 proteins that mapped to lipid metabolism, that module was not significantly enriched in macrophage-conditioned medium (p=0.1).
Figure 1. Functional and pathway analyses of proteins shed and secreted by macrophages.
Serum-free medium harvested from mouse peritoneal macrophages that had been exposed to LDL (control cells) or acetyl-LDL (foam cells) was analyzed with LC-ESI-MS/MS. Pathway analysis was performed on all 542 proteins detected with high confidence.
Panel A: Gene Ontology (GO) analysis of network proteins. Modules enriched in GO functional categories relative to the entire mouse genome were identified by Fisher’s exact test. The number of proteins in each module is proportional to the size of the box, while the number of proteins shared by 2 modules is proportional to the thickness of the line connecting the boxes.
Panel B: Schematic representation of the protein interaction network determined by pathway analysis. The network is comprised of 238 proteins (nodes) and 505 interactions (edges).
Panel C: The 6 most highly connected proteins (circled in black in Panel B) in the interaction network. Sub-network of the 6 most highly connected proteins (hubs) in the overall network. Note that 5 of the 6 hubs are themselves highly interconnected.
Panel D: Immune response and complement regulation modules (purple and pink spheres in Panel B, respectively).
Analysis of the macrophage proteome reveals a network of potential interactions
We constructed a relational network based on interactions 17 of the 542 proteins detected with high confidence in macrophage-conditioned medium. Protein interactions could be direct or indirect, and were physical, transcriptional, or enzymatic. The single, spanning network was comprised of 238 proteins (nodes) and 505 interactions (edges) (Fig. 1B).
The number of functional interactions exhibited by individual proteins in the network varied widely, indicating that the network’s overall architecture was heterogeneous 21. Thus, the 6 most highly connected proteins (Fig. 1B, C) accounted for 30% of the interactions, while each of 125 proteins had only 1 or 2 interactions (25% of the total). Topological analysis of this network demonstrated that, consistent with many biological networks, it is scale-free 21. Five of the 6 most highly connected proteins (coagulation factor II, fibronectin, insulin-like growth factor 1, growth factor receptor bound protein 2, and Ras homolog gene family member A) were themselves densely interconnected (Fig. 1C), suggesting that they were important hubs in the network’s overall topology.
Many of the proteins in the different functional classes mapped to specific regions of the protein interaction network 22. Two examples of such subnetworks were proteins involved in oxidation (Fig. 1B, D; cyan nodes; 100% mapped to subnetwork) and those involved in complement regulation (Fig. 1B, D; magenta nodes; 100% mapped to subnetwork). These observations raise the possibility that proteins that are shed and secreted from macrophages interact to form functionally important modules in the extracellular milieu.
Dual statistical criteria identify proteins differentially expressed by macrophage foam cells
To identify proteins that differed in relative abundance in media of cholesteryl ester-loaded and control macrophages, we used a log likelihood test (G-test) together with a 2-tailed t-test to find significant differences in spectral counts (the sum of all peptides detected by MS/MS) for each protein 11-13. Permutation analysis 23, 24 revealed that requiring G >1.5 (G-test) and α<0.05 (t-test) yielded the maximum number of true-positive protein identifications with an acceptable FDR of 7%. Using these stringent analytical criteria, we identified 15 proteins that were differentially expressed in medium from macrophages loaded with acetyl-LDL (Table 1).
Table 1. Proteins differentially expressed by cultured macrophage foam cells.
| Protein | Control Cells (spectral counts) |
Foam Cells (spectral counts) |
G- statistic* (G-test) |
P value (t-test) |
|---|---|---|---|---|
| Prosaposin (PSAP) | 23.6 | 44.3 | 6.4 | 0.018 |
| CD5 antigen-like (CD5L) | 0 | 4.4 | 6.1 | 0.0004 |
| Apolipoprotein E (APOE) | 36.9 | 58.1 | 4.9 | 0.038 |
| Cathepsin D (CTSD) | 75.1 | 97.4 | 2.9 | 0.030 |
| Complement C3 (CO3) | 22.9 | 13.4 | 2.5 | 0.030 |
| Procollagen, type I, α2 (COL1A2) | 11.6 | 5.3 | −2.5 | 0.0017 |
| Histone cluster 1 (H2BF) | 9.4 | 17.4 | −2.4 | 0.037 |
| Cathepsin L (CTSL) | 28 | 40.9 | 2.4 | 0.029 |
| Puromycin-sensitive aminopeptidase (NPEPPS) |
3.5 | 0.6 | −2.2 | 0.017 |
| Ferritin light chain 1 (FTL1) | 3.3 | 7.9 | 2.0 | 0.0060 |
| Protein phosphatase 1, catalytic subunit, gamma isoform (PPP1CC) |
0.8 | 3.4 | 1.8 | 0.014 |
| GPI-anchored a2 macroglobulin- related protein (CD109) |
9 | 4.4 | −1.6 | 0.011 |
| Complement factor properdin (PFC) | 0.5 | 2.6 | 1.6 | 0.0025 |
| Cystatin C (CYSC) | 23.8 | 33.1 | 1.6 | 0.030 |
| Colony stimulating factor 1 receptor (CSF1R) |
10 | 16.3 | 1.5 | 0.023 |
A positive or negative value for the G-test indicates an increased or decreased level of protein expression, respectively, relative to control macrophages.
As assessed by these dual criteria, the fraction of differentially expressed proteins (2.8%, Table 1) and genes (3.5%, microarray analysis; Supplemental Table 1) in macrophages were similar following sterol loading by exposure to acetyl-LDL.
Biochemical validation of differential protein expression by macrophage foam cells
We investigated the effectiveness of our analytical strategy by biochemically quantifying changes in relative abundance for 3 of the 15 macrophage proteins that met both of our statistical criteria (Table 1). Immunoblot analysis of medium isolated from macrophages incubated with acetyl-LDL demonstrated significant changes in levels of all 3 proteins (Fig. 2A, B): apolipoprotein E (APOE; p=0.007), cathepsin L (CTSL; p=0.005), cathepsin D (CTSD; p=0.016).
Figure 2. Biochemical and sub-network analysis of protein expression by macrophages.
Panel A: Biochemical validation of differential protein expression by macrophage foam cells. Equal amounts of protein from serum-free medium conditioned by control or foam cells were subjected to SDS-PAGE, using 4%–12% gradient gels. Proteins were transferred to PVDF membranes, and probed with antibodies raised against murine APOE, CTSD, CTSL or MAC2.
Panel B: Immunoblots were quantified by densitometry. Statistical significance was assessed using the two-tailed Student’s t-test.
Panel C: Pathway analysis was used to construct a protein interaction subnetwork centered on proteins that were differentially expressed by macrophage foam cells. Upregulated (red) and down-regulated (green) proteins were linked by incorporating all first neighbors of differentially expressed proteins. Note that 7 of the first neighbors added were differentially expressed if only the t-test or G-test was used to assess significance (light red, up-regulated; light green, down-regulated). The resulting subnetwork consists of 40 proteins (nodes) and 69 interactions (edges). GO analysis of the subnetwork revealed modules enriched in proteins implicated in complement regulation, lipid metabolism, and lysosomal biology.
Differentially expressed macrophage proteins map to modules implicated in lysosomal biology, lipid metabolism, and complement regulation
We used proteins that were differentially expressed by macrophage foam cells to identify a subnetwork of the full protein interaction network (Fig. 1B) that is regulated when cholesterol accumulates in macrophages. Inclusion of first neighbors yielded a spanning subnetwork centered on the differentially expressed proteins (Fig. 2C). Importantly, many of the first neighbors included in the subnetwork (alpha-2-macroglobulin [A2M], complement component C1Q beta chain [C1QB], C1Q gamma chain [C1QC], heat shock protein-5 [HSPA5], integrin beta-2 [ITGB2], galectin-3 [LGALS], and Y box protein-1 [YBX1]) were differentially expressed by macrophage foam cells when only the t-test or G-test was used to assess significance, suggesting that they might also be regulated by sterol loading.
Gene Ontology analysis of the network of sterol-regulated proteins identified significant enrichment in three functional modules: lysosomal biology (p<10−4), complement activation (p <10−4), and lipid metabolism (p=0.03) (Fig. 2C). Taken together, our observations indicate that proteins linked to lipid metabolism, lysosomes and complement activation are differentially expressed by macrophage foam cells.
Statistical and fold-change criteria identify genes differentially expressed by macrophage foam cells
To investigate the role of altered mRNA levels in regulating protein expression by foam cells, we analyzed total RNA extracted from control macrophages and macrophages exposed to acetyl-LDL. For a change to be considered significant, we required genes to show an expression change of at least 1.5-fold with p<0.05 as assessed by Student’s t-test (adjusted for multiple hypothesis testing). Based on these criteria, we identified 449 genes that were up-regulated and 249 genes that were down-regulated (Supplemental Table 1). Consistent with previous studies 25, genes involved in cholesterol synthesis were a major target for down-regulation in macrophages exposed to acetyl-LDL.
Cd5l, a secreted protein that is a member of the SCSR (scavenger receptor cysteine rich) superfamily, showed the greatest in increase in both mRNA levels (8-fold) and protein levels (Table 1) when macrophages were exposed to acetyl-LDL. CD5L is also highly prevalent on B-1 cells, but we failed to detect mRNA characteristic of these cells (such as CD20) in our microarray data; we also failed to detect the protein in the conditioned medium of elicited macrophages 25.
Multiple genes that regulate complement activation were also up-regulated in macrophages incubated with acetyl-LDL. Four striking examples were C1qa, C1qb, and C1q, which are key early components in the classic pathway for complement activation, and Cfp (properdin), a component of the alternative pathway for complement activation.
Differentially expressed macrophage genes map to modules implicated in lipid and protein metabolism, complement activation, and oxidative phosphorylation
We used Gene Ontology annotation to organize acetyl-LDL regulated genes into functional modules. This approach identified significant enrichment in a wide variety of modules: ribosomes (p<10−4), lipid metabolism (p<10−4), proton transporting ATP synthase complex (p<10−4), complement regulation (p<10−4), intracellular organelles (p<10−4), oxidoreductase activity (p=0.01), immune response (p=0.02), chemotaxis (p=0.02), and phagocytosis (p=0.04). Mapping differentially expressed genes onto the KEGG integrated database 26 indicated that 3 major metabolic pathways were regulated in macrophage foam cells: steroid biosynthesis, the large and small ribosomal subunits, and oxidative phosphorylation (Supplemental Fig. 1-3). These observations suggest that the major impact of loading sterol into macrophages is on protein and lipid metabolism, mitochondrial function, and complement activation. It is noteworthy that we also detected enrichment of complement proteins in conditioned medium of macrophage foam cells, and that recent studies implicate oxidative phosphorylation in the function of alternatively activated macrophages 27.
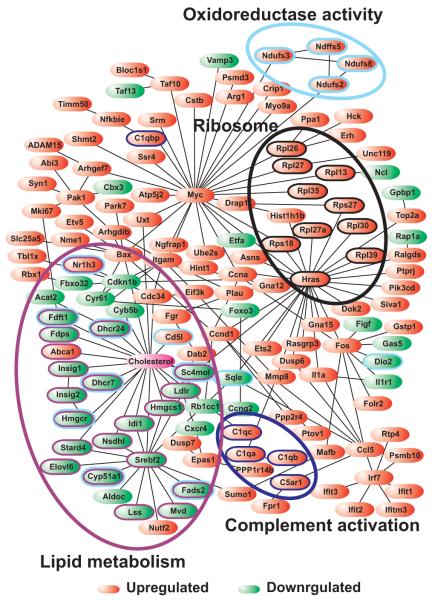
We constructed an interaction network based on pathway analysis of the 698 genes differentially expressed in macrophage foam cells. The single, spanning network contained 179 interactions between 144 nodes (cholesterol and 143 genes) (Fig. 3). Many of the genes clustered into specific regions of the gene interaction network. For example, Ndufs2, Ndufs2, Ndufs8, and Ndffs5 are all mitochondrial oxidoreductases. This approach indicates that multiple genes linked to lipid metabolism, oxidoreductase activity, ribosomes, and complement activation could potentially interact with one another to form a functional network.
Figure 3. Network of genes differentially expressed by macrophages incubated with acetyl-LDL.
Total RNA extracted from LDL (control) and acetyl-LDL exposed macrophages were subjected to microarray analysis. Pathway analysis was used to construct an interaction network centered on genes that were differentially expressed by macrophage foam cells (Supplemental Table 1). “Cholesterol” was also included as a node in the network to highlight its central role in this model. Upregulated (red) and down-regulated (green) genes were linked by direct (binding, enzymatic) or indirect (transcriptional) interactions. The resulting network consists of 144 nodes (143 genes and “cholesterol”), and 179 interactions (edges). GO analysis of the sub-network revealed modules enriched in genes implicated in lipid metabolism, oxidoreductase activity, ribosomes, and complement activation.
Cholesterol loading fails to affect macrophage polarization but suppresses lipopolysaccharide (LPS) induction of inflammatory genes
The release of pro-inflammatory cytokines is markedly increased when peritoneal macrophages deficient in ABCA1 and ABCG1 are stimulated with LPS, leading to the proposal that cholesterol loading renders the cells pro-inflammatory 28, 29. However, we observed a different response in peritoneal macrophages incubated with acetyl-LDL: sterol-loading blunted the response of the macrophages to LPS without altering the overall pattern of LPS-induced gene expression (Fig. 4D). Moreover, we observed no consistently altered expression of pro-inflammatory (M1) or anti-inflammatory (M2) genes in macrophages exposed to acetyl-LDL (Fig. 4B, C). The pattern of gene expression we observed in LPS-stimulated peritoneal macrophages was remarkably similar to that reported for macrophages derived from bone marrow cells 30. These data indicate that loading macrophages with cholesterol derived from acetyl-LDL does not consistently affect polarization or enhance the inflammatory response when macrophages are subsequently challenged with LPS.
Figure 4. Analysis of genes differentially expressed by macrophages incubated with acetyl-LDL.
Panel A: qRT-PCR confirmation of genes expressed differentially by macrophage foam cells. Control macrophages (open bars); macrophage foam cells (filled bars); N=3. ** p<0.01 by Student’s t-test.
Panel B,C: Levels of mRNA for M1 (inflammatory) and M2 (anti-inflammatory) polarization in control versus acetyl-LDL exposed macrophages. Values represent the log2 ratio of mRNA for control and macrophage foam cells. A positive or negative value for log2 indicates an increased or decreased level of protein expression, respectively, relative to control macrophages.
Panel D: Levels of LPS-inductions. Control and acetyl-LDL exposed macrophages were washed, exposed for 4 h to serum-free medium (No-stim) or serum-free medium supplemented with 100 ng/mL of LPS (LPS-treated). Data represent fold change of each gene in LPS-treated from average (n=4) of each gene expression in No-Stim. Data represent the 15 genes that were most upregulated by LPS in control cells. N=4. ** p<0.01, *p<0.05 by Student’s t-test.
Differential protein expression in macrophage conditioned medium is largely independent of differences in mRNA expression
We used two complementary approaches to explore the relationship between mRNA and protein levels in macrophage-conditioned medium. First, to provide a general overview, we performed this analysis using all proteins in macrophage conditioned medium that exhibited an average of >2 spectral counts by MS/MS analysis in both control and macrophage foam cells (Fig. 5A). We used this criterion because it is difficult to use spectral counting to quantify low-abundance proteins 11, 13. For the 313 proteins that met this criterion, no correlation was observed between the expression of proteins in conditioned medium of control and acetyl-LDL exposed cells and their cognate mRNAs (p=0.84, r2<0.01).
Figure 5. Correlation of differences in mRNA and protein expression levels in control macrophages and macrophages incubated with acetyl-LDL.
Values represent the log2 ratio of mRNA or spectral counts for control and macrophage foam cells.
Panel A: Relationship for all genes for proteins detected by MS/MS analysis of macrophage conditioned medium that exhibited an average of >2 spectral counts in both control and macrophage foam cells. CD5L, which had an infinite ratio of change in protein expression level, was not plotted.
Panel B: Relationships for genes and/or proteins that exhibited significant changes in relative abundance. Values are plotted for: i) mRNAs that exhibited a ≥2-fold change that was significant as assessed by an adjusted p-value and ii) differentially expressed proteins, as assessed by both the G-test and t-test. CD5L, which had an infinite ratio of change in protein expression level, was not plotted.
Second, we examined the relationship between the proteins and mRNAs that exhibited the largest differences in relative values between control and cholesterol-loaded cells (Fig. 5B). For this analysis, we included: i) all genes that exhibited a significant, >2-fold change at the mRNA level (as assessed by the t-test) and that were detected by MS/MS analysis, and ii) all 15 proteins that were differentially expressed as assessed by both the G-test and t-test. For this analysis we excluded CD5L, because the protein was not detected in the medium of control macrophages, yielding a ratio of differential protein expression that was infinite. The correlation between protein level and mRNA level was borderline significant (p=0.069, r2=0.16). Collectively, these observations indicate that mRNA can be an important regulator of protein expression if its relative level increases significantly, but that there is little overall relationship between changes in mRNA levels and protein levels in macrophage-conditioned medium. However, we did observe that similar functional categories—lipid metabolism and the complement pathway—were regulated by sterol loading at both the protein and mRNA levels.
DISCUSSION
Macrophages incubated with acetyl-LDL are a widely used model system for studying foam cell biology 2. To begin to develop a global view of the foam cell, we used the 15 proteins that were differentially expressed by cultured macrophage foam cells and their first neighbors to construct a subnetwork of shed and secreted proteins. This approach linked functional modules involved in lysosomal proteolysis, lipid metabolism, and complement regulation to cholesterol deposition in macrophages 6.
In macrophages, lysosomal digestion of endocytosed lipoproteins promotes cholesterol accumulation 2, 31, and proteolysis of prosaposin by cathepsins regulates the expression of ABCA1 31. Thus, lysosomal proteases are likely to be important in macrophage cholesterol homeostasis. Studies of hypercholesterolemic mice have implicated lysosomal proteases and cystatin C in atherogenesis and degradation of structural proteins in vascular lesions 32. Proteolysis and regulation of cholesterol accumulation in macrophages may play a critical role in triggering plaque rupture, the major cause of myocardial infarction and sudden death in humans with coronary artery disease 33.
Quantification of transcribed mRNA has been widely used to identify pathways induced by targeted perturbations of cells 34. We therefore complemented our proteomic studies by using microarrays to analyze mRNA from control and cholesteryl ester-laden macrophages. These studies confirmed previous observations that multiple genes involved in cholesterol biosynthesis are down-regulated in cholesteryl ester-laden macrophages and that certain genes, such as Abca1, that promote cholesterol excretion are up-regulated 35.
Unexpectedly, we also found that loading cholesterol into macrophages regulated the expression of multiple complement regulatory proteins at both the mRNA and protein levels. It is noteworthy that several of those proteins, including C1q and C3, are of central importance in triggering complement activation, and that activated components of the complement pathway have been detected in advanced human atherosclerotic lesions 36. Altered release of lysosomal proteases and complement regulatory proteins by macrophage foam cells may therefore promote plaque rupture, ischemic injury, and activation of the coagulant cascade—key pathogenic events in human atherosclerosis.
It is generally believed that the conversion of macrophages to foam cells is pro-inflammatory, but several lines of evidence indicate that this is not the case for peritoneal macrophages exposed to acetyl-LDL. First, we found no evidence for increased expression of inflammatory proteins induced by cholesterol-loading of macrophages at both the mRNA and protein level. Second, we observed no consistent differences in the expression of a wide range of genes used to monitor the pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes. Third, we demonstrated that sterol-loading blunted the response of the macrophages to LPS without altering the overall pattern of LPS-induced gene expression. Collectively, these observations strongly suggest that macrophages are not polarized towards a pro-inflammatory state when they cholesterol-loaded by incubation with acetyl-LDL.
A strength of our proteomics approach is the unbiased nature of the analysis and the emphasis on protein (as opposed to mRNA) expression. A limitation of untargeted MS/MS is the inability to detect low-abundance proteins, such as cytokines 13. However, we also observed no consistent increases in mRNA levels for a wide range of inflammatory cytokines or chemokines. In particular, we observed no increased expression of classic inflammatory genes such as ICAM1, TNF, or MCP-1.
Different results have been reported for ABCA1- and ABCA1/ABCG1-deficient macrophages, which have elevated levels of cholesterol and clearly exhibit a pro-inflammatory phenotype 37, 38. It is important to note that deficiency of those proteins impairs the efflux of free cholesterol from the macrophage plasma membrane. The resulting increase in free cholesterol has been proposed to alter cell signaling by pro-inflammatory membrane-associated receptors such as TLR4 37, 38.
Modified lipoproteins increase cellular cholesterol levels by a completely different mechanism. In that system, endocytosis delivers lipoproteins to lysosomes, which release free cholesterol that is then delivered to the endoplasmic reticulum 25. The increased level of free cholesterol in the endoplasmic reticulum activates ACAT. This enzyme converts free cholesterol to cholesteryl ester, which is subsequently stored in lipid droplets in the cells. Under these conditions, one would expect little or no change in the free cholesterol content of the plasma membrane and no activation of cell surface receptors such as TLR4. These observations suggest that ABCA1/ABCG1 deficiency and endocytosis of modified LDL increase the concentration of free cholesterol in different cellular pools, and that this in turn is likely to have different effects on the phenotype of macrophages.
Changes in mRNA levels do not necessarily predict changes in protein levels 39. We therefore examined the relationship between changes in mRNA and protein levels induced by loading cholesterol into macrophages. Overall there was no correlation between the two even if we limited the analysis to genes that exhibited a significant change. However, we observed a large increase in both mRNA and CD5L protein in the medium of macrophages incubated with acetyl-LDL. We also found that two functional modules, lipid metabolism and complement activation, were regulated at both the mRNA and protein level, suggesting co-regulation by pre- and post-transcriptional mechanisms. However, post-transcriptional mechanisms appear to be important for the regulation of most of the proteins we detected in the medium of macrophages. Collectively, these observations suggest that mRNA can play a major role in regulating macrophage protein expression if its relative level is markedly altered or if multiple genes in a pathway are coordinately regulated 25, 34.
In summary, our studies indicate that the complement pathway and lysosomal proteins— key components of the immune system and potential players in plaque rupture and—are regulated when mouse macrophages are loaded with cholesterol by exposure to acetyl-LDL. We also observed the upregulation of multiple genes linked to oxidative phosphorylation and ribosomal protein synthesis, but not in genes linked to macrophage polarization. Our findings have identified previously unsuspected links among macrophage sterol accumulation, complement regulation, and proteolysis, all of which are implicated in atherogenesis.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by grants from the National Institutes of Health (HL108897, HL092969, P30DK017047). Mass spectrometry experiments were performed by the Quantitative and Functional Proteomics Core (Diabetes Research Center, University of Washington). Microarray analyses were performed by the Center for Array Technologies (University of Washington).
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009;50(Suppl):S282–286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ory DS. Nuclear receptor signaling in the control of cholesterol homeostasis: have the orphans found a home? Circ Res. 2004;95:660–670. doi: 10.1161/01.RES.0000143422.83209.be. [DOI] [PubMed] [Google Scholar]
- 5.Webb NR, Moore KJ. Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr Drug Targets. 2007;8:1249–1263. doi: 10.2174/138945007783220597. [DOI] [PubMed] [Google Scholar]
- 6.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 7.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendez AJ, Oram JF, Bierman EL. Protein kinase C as a mediator of high density lipoprotein receptor-dependent efflux of intracellular cholesterol. J Biol Chem. 1991;266:10104–10111. [PubMed] [Google Scholar]
- 9.Becker L, Gharib SA, Irwin AD, Wijsman E, Vaisar T, Oram JF, Heinecke JW. A macrophage sterol-responsive network linked to atherogenesis. Cell Metab. 2010;11:125–135. doi: 10.1016/j.cmet.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R. The International Protein Index: an integrated database for proteomics experiments. Proteomics. 2004;4:1985–1988. doi: 10.1002/pmic.200300721. [DOI] [PubMed] [Google Scholar]
- 11.Fu X, Gharib SA, Green PS, Aitken ML, Frazer DA, Park DR, Vaisar T, Heinecke JW. Spectral index for assessment of differential protein expression in shotgun proteomics. J Proteome Res. 2008;7:845–854. doi: 10.1021/pr070271+. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 13.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series. 1995;B:289–300. [Google Scholar]
- 16.Dennis G, Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 17.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 18.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 19.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 20.Rappsilber J, Mann M. What does it mean to identify a protein in proteomics? Trends Biochem Sci. 2002;27:74–78. doi: 10.1016/s0968-0004(01)02021-7. [DOI] [PubMed] [Google Scholar]
- 21.Barabasi AL, Oltvai ZN. Network biology: understanding the cell’s functional organization. Nat Rev Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 22.Schwikowski B, Uetz P, Fields S. A network of protein-protein interactions in yeast. Nat Biotechnol. 2000;18:1257–1261. doi: 10.1038/82360. [DOI] [PubMed] [Google Scholar]
- 23.Heinecke NL, Pratt BS, Vaisar T, Becker L. PepC: Proteomics software for identifying differentially expressed proteins based on spectral counting. Bioinformatics. 2010 doi: 10.1093/bioinformatics/btq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 26.Kanehisa M, Goto S, Kawashima S, Nakaya A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002;30:42–46. doi: 10.1093/nar/30.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjorkbacka H, Fitzgerald KA, Huet F, Li X, Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT, Freeman MW. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics. 2004;19:319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- 31.Haidar B, Kiss RS, Sarov-Blat L, Brunet R, Harder C, McPherson R, Marcel YL. Cathepsin D, a lysosomal protease, regulates ABCA1-mediated lipid efflux. J Biol Chem. 2006;281:39971–39981. doi: 10.1074/jbc.M605095200. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 33.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 34.Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- 35.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 36.Oksjoki R, Kovanen PT, Pentikainen MO. Role of complement activation in atherosclerosis. Curr Opin Lipidol. 2003;14:477–482. doi: 10.1097/00041433-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 38.Becker L, Liu NC, Averill MM, Yuan W, Pamir N, Peng Y, Irwin AD, Fu X, Bornfeldt KE, Heinecke JW. Unique proteomic signatures distinguish macrophages and dendritic cells. PLoS One. 2012;7:e33297. doi: 10.1371/journal.pone.0033297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.