Abstract
EMBO J (2012) 31 22, 4276–4288 doi:; DOI: 10.1038/emboj.2012.250; published online September 18 2012
AgRP/NPY neurons are critical regulators of body weight and food intake. Concordant with their orexigenic effects, it is expected that AgRP ablation leads to the appearance of a lean phenotype. In the current issue of The EMBO Journal, Joly-Amado et al (2012) describe an obese phenotype in a model of AgRP-ablated mice, and link it to a shift in metabolic profile in efferent tissues such as the liver, muscle and pancreas.
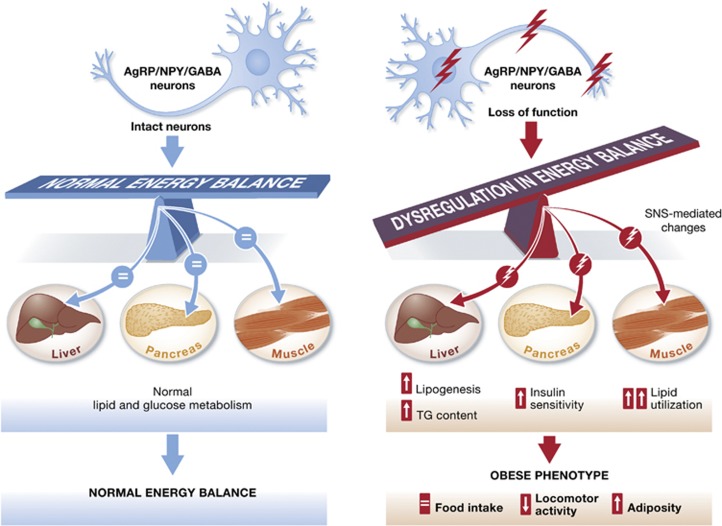
Hypothalamic AgRP/NPY neurons are known to play a key role in the regulation of body weight and food intake. With the advent of ‘toxin-receptor mediated cell knockout’ technology, several reports have tried to address the physiological relevance of this set of neurons. The ablation of Agrp-expressing neurons has different consequences depending on the age of the mice. Thus, in the neonatal stage, temporal deletion of AgRP neurons by diphtheria toxin (DT) injection has no major effects on energy balance (Luquet et al, 2005, 2007). However, in adult mice, as expected due to the potent orexigenic role of AgRP neurons, DT administration to these AgRPDTR mice promotes a huge reduction in body weight and food intake in a short time (Bewick et al, 2005; Gropp et al, 2005; Luquet et al, 2005), which can even lead to starvation (Luquet et al, 2005). It has been hypothesized that the absence of effect in the neonates could be due to ‘compensatory mechanisms’ developed during the neonatal stage, when the neurocircuitry is not fully formed (Luquet et al, 2005). It has been shown that DT injection to adult mice, which have been previously treated with DT during the neonatal stage, does not have the same drastic effect as seen in starvation-induced DT treatment (Luquet et al, 2005). In this issue of The EMBO Journal, Joly-Amado et al (2012) report an increase in feeding efficiency in 3-month-old AgRPDTR mice after DT injection during the neonatal stage. This change in feeding efficiency leads to an obese phenotype related to a decrease in locomotor activity. These results, contrary to those expected due to the orexigenic function of AgRP neurons, provide clues about the existence of a ‘compensatory mechanism’. Furthermore, in this study, the development of obesity is concomitant with an increase in fat depot weight.
It is known that AgRP released from the AgRP/NPY neurons in the hypothalamus acts like an endogenous inhibitor of melanocortin receptors (MCRs) in the melanocortin system (Cone 2005). AgRP antagonizes the effects of POMC cleavage subproducts (e.g., α-msh) on these receptors to affect energy balance. It has been reported by Nogueiras et al (2007) that central manipulation of MCRs (using inhibitors or agonists) is able to control adiposity by modifying lipogenesis in WAT. Moreover, they found that hypothalamic MCRs act like a switch between carbohydrate and fat utilization: the blockade of MCRs decreases the percentage of fat utilized (Nogueiras et al, 2007). In the current issue, Joly-Amado et al (2012) describe changes in the same direction as found in this previous study; they report evidence of AgRP/NPY neuron involvement in the control of nutrient partitioning and lipid metabolism in peripheral tissues, in agreement with another report that demonstrated the importance of Sirt1 in AgRP neurons to modulate substrate utilization during fasting (Dietrich et al, 2010). The AgRPDTR mice used in this study show a shift in substrate utilization. AgRP deficiency stimulates lipid utilization, that is, these mice obtain energy from stored fat. Despite this shift in metabolic profile, adult AgRPDTR mice present more adiposity than controls. This can be explained by the fact that these mice show a potent increase in lipogenesis and triglyceride (TG) content in the liver. Post-pandrial plasma TG levels are raised, but they are normalized by fasting, providing evidence that the peripheral tissues of these AgRP-ablated mice obtain the energy required to maintain their functions from lipids. Furthermore, the authors describe a ‘paradoxical benefit’ in HFD-exposed mice. These animals are protected against the effects of HFD—their body weight and fat content are indistinguishable from wild-type mice, probably due to the fact that the mice utilize the excess fat found in the diet for energy.
To investigate the cause of this increase in fat utilization, the authors looked into the possibility that the muscles use TG as fuel. They uncovered different results between oxidative (soleus) and fast glycolytic (white gastrocnemius) muscles. The former showed an increase in lipid utilization correlated with a decrease in the maximal OXPHOS complex I respiration rate. No changes were found in the latter. Taken together, these results indicate that the ability to oxidize lipids to obtain energy is ameliorated in the oxidative muscles of adult AgRP-ablated mice.
Joly-Amado et al (2012) address the question about how the hypothalamic AgRP/NPY neurons can affect peripheral tissues. It is well known that the autonomic nervous system connects hypothalamic areas with different tissues (Nogueiras et al, 2007). Coinciding with previous studies that examined the sympathetic nervous system (SNS), the main mediator between the hypothalamus and WAT (Nogueiras et al, 2007), the current authors found that the SNS also mediates the response of the efferent tissues. They show that all of the effects described in liver, muscle and pancreas are dependent upon the SNS outflow from the hypothalamus.
AgRP/NPY neurons also release γ-aminobutyric acid (GABA; Horvath et al, 1997). It has been demonstrated that GABA is necessary for the normal regulation of body weight (Tong et al, 2008). Constitutive inactivation of Vgat in AgRP neurons provokes body weight loss associated with an increase in locomotor activity (Tong et al, 2008). It has been reported that bretazenil (GABA agonist) replacement in adult AgRPDTR mice after DT injection rescues the anorexic phenotype and minimizes changes in body weight and food intake (Wu et al, 2009). Furthermore, that study showed that bretazenil administration solely into the parabrachial nucleus is enough to prevent the anorexia. Joly-Amado et al (2012), following the same strategy as in previous reports, investigated the role of GABA in the phenotype reported. They found that subcutaneous bretazenil treatment rescues the obese phenotype in adult AgRPDTR mice, increasing the RQ, which means an increase in carbohydrate utilization, and significantly decreasing the body fat content, thus emphasizing the importance of GABA release by the AgRP/NPY neurons to modulate energy balance.
In summary (see Figure 1), Joly-Amado et al (2012) in a series of elegant experiments describe a novel phenotype observed in a mouse model of neonatal depletion of AgRP neurons. They link the obese phenotype observed in these mice with an increase in lipogenesis in the liver and an increase in lipid utilization by oxidative muscles. Furthermore, the authors show that these changes in the lipid profile of the peripheral tissues are due to AgRP ablation and are mediated by the SNS, and are not a consequence of adiposity in these mice. This study provides more clues about the existence of a ‘compensatory mechanism’ developed during the postnatal period. In spite of this, the mice are still sensitive to AgRP and GABA treatment. It is apparent that more effort is required to completely and comprehensively elucidate and understand the exact mechanism by which this model of AgRP-ablated mice become obese in adulthood.
Figure 1.
AgRP neurons are essential for normal energy homeostasis. AgRP neurons play a critical role in the regulation of energy balance. Manipulation of these hypothalamic neurons causes changes in the normal phenotype. Joly-Amado et al (2012) describe that AgRP deletion in the neonatal stage brings about the appearance of an obese phenotype in adulthood. AgRP ablation promotes changes in efferent tissues that lead to an increase in adiposity.
Acknowledgments
LV was supported by a Fulbright Fellowship from the Spanish Ministry of Education.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR (2005) Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 19: 1680–1682 [DOI] [PubMed] [Google Scholar]
- Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578 [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, Xu AW, Souza DO, Gao Q, Diano S, Gao XB, Horvath TL (2010) Agrp neurons mediate Sirt1's action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci 30: 11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC (2005) Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8: 1289–1291 [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C (1997) Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res 756: 283–286 [DOI] [PubMed] [Google Scholar]
- Joly-Amado A, Denis RGP, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, Prola A, Flamment M, Foufelle F, Magnan C, Luquet S (2012) Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J 31: 4276–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD (2005) NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685 [DOI] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, Palmiter RD (2007) NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 28: 214–225 [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC et al. (2007) The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB (2008) Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11: 998–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD (2009) Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137: 1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]