Abstract
Background
Cyclophosphamide-methotrexate-5-fluorouracil (CMF) is often selected as adjuvant chemotherapy for older patients with early-stage breast cancer due to perceived superior tolerability. We sought to measure persistence with CMF, adherence to oral cyclophosphamide, and the association of these with toxic effects.
Patients and methods
CALGB 49907 was a randomized trial comparing standard chemotherapy (CMF or AC, provider/patient choice) with capecitabine in patients aged ≥65 with stage I–IIIB breast cancer. Those randomized to standard therapy and choosing CMF were prescribed oral cyclophosphamide 100 mg/m2 for 14 consecutive days in six 28-day cycles. Persistence was defined as being prescribed six cycles of at least one of the three CMF drugs. Adherence was the number of cyclophosphamide doses that women reported they had taken divided by the number prescribed. Persistence and adherence were based on case report forms and medication calendars.
Results
Of 317 randomized to standard chemotherapy, 133 received CMF. Median age was 73 (range 65–88). Seventy-one percent submitted at least one medication calendar; 65% persisted with CMF. Non-persistence was associated with node negativity (P = 0.019), febrile neutropenia (P = 0.002), and fatigue (P = 0.044). Average adherence was 97% during prescribed cycles.
Conclusions
Self-reported adherence to cyclophosphamide was high, but persistence was lower, which may be attributable to toxic effects.
Keywords: antineoplastic combined chemotherapy protocols, breast neoplasms, geriatrics, medication adherence, patient compliance, toxicity
introduction
Non-persistence with and non-adherence to oral antineoplastic therapy may reduce the efficacy of oncologic treatment. Persistence is defined by the International Society for Pharmacoeconomics and Outcome Research (ISPOR) as the duration of time from initiation to discontinuation of therapy [1]. Optimal persistence occurs when a person keeps taking the medication until the end of the prescribed course. In contrast, ISPOR defines adherence as conforming to provider recommendations with respect to the timing, dosage, and frequency of medication taking [1]. Optimal adherence occurs when no doses are skipped, doubled, or taken at the wrong time or dosage.
Medication calendars are one method that can be used to enhance adherence by reminding patients to take oral drugs at home [2]. However, the efficacy of calendars is limited [3]. The data from these calendars, particularly on clinical trials, can also be utilized to provide information about persistence with and adherence to a therapy. Knowledge of patient persistence with and adherence to an intervention on a clinical trial is critical to the interpretation of the results of the study, including both therapeutic effect and toxic effects. Non-persistence or non-adherence could lead investigators to wrongly conclude that an effective drug was not beneficial, or that a toxic drug was non-toxic. The public health implications of either misassumption could be detrimental. Previous research has suggested that older age, in particular, is a risk factor for non-adherence to adjuvant hormonal therapy for breast cancer [4–6]. In this study, we sought to evaluate persistence with adjuvant cyclophosphamide-methotrexate-5-fluorouracil (CMF) and adherence to the oral component of the regimen in a multicenter study of older women with breast cancer.
methods
The Cancer and Leukemia Group B (CALGB) led a randomized trial in women with early-stage breast cancer aged 65 years and older to compare the efficacy of (i) six cycles of standard chemotherapy with CMF (oral cyclophosphamide 100 mg/m2 for 14 consecutive days, intravenous methotrexate 40 mg/m2 on days 1 and 8, and intravenous 5-fluorouracil 600 mg/m2 on days 1 and 8, in six 28-day cycles) or doxorubicin and cyclophosphamide (AC) with (ii) six cycles of experimental chemotherapy with the oral 5-fluorouracil pro-drug capecitabine [7]. Patients with stage I–IIIB breast cancer were randomized to standard chemotherapy or capecitabine, and the primary trial end point was relapse-free survival. Women who received capecitabine were offered participation in an adherence substudy using microelectronic monitoring system (MEMS) caps [8]. Women who chose to receive CMF were prescribed oral cyclophosphamide, and were asked to use printed medication calendars to record the days that they took doses of cyclophosphamide. Although funding did not allow for the use of MEMS caps or other adherence measures among the women receiving CMF, medication calendars were used to evaluate adherence in this group. Persistence data were collected through standard case report forms (CRFs) submitted by the study-site investigators on each patient. Toxic effects were reported based on the NCI CTCAE version 2. We sought to evaluate persistence, adherence, and their association with toxic effects in patients who received CMF in this study.
study subjects
Participants in the randomized controlled trial, CALGB 49907, have been described in detail previously [7]. These were women 65 years of age or older with operable stage I–IIIB breast cancers and a performance status of 0 to 2 (according to the National Cancer Institute criteria). Patients had to have an expected survival of more than 5 years and no medical condition that would make treatment with chemotherapy unreasonably hazardous. Women with any other active cancer or a previous cancer with a risk of relapse that >30% were excluded. Each participant signed an IRB-approved, protocol-specific informed consent for CALGB 49907 in accordance with federal and institutional guidelines. For the present analysis, only patients assigned to standard therapy and choosing to receive CMF instead of AC were included.
definitions
Persistence with CMF was defined as being prescribed all six cycles of at least one of the three CMF drugs. Adherence to oral cyclophosphamide was calculated as the number of cyclophosphamide doses that women reported they had taken according to patient medication calendars divided by the number of doses they were prescribed. Adherence was calculated for each cycle (28 days) for which a participant submitted a medication calendar. If no calendar was submitted for a cycle, this was considered missing data and not included in adherence analyses. Overall adherence was the averaged proportion of pills taken for all participants for the cycles for which medication calendars were submitted for the days up until the day each patient was told to stop taking the drugs (based on CRFs) either because of completion of therapy or because of a clinical decision to stop therapy before completion.
Consistent with prior literature, we defined a participant as non-adherent if <80% of the cyclophosphamide doses expected (between the time she began and the time she stopped treatment) were recorded in the cycles for which she submitted calendars [2]. Because each cycle should have included 14 consecutive daily doses of cyclophosphamide, non-adherence in a particular cycle was defined as report of 11 or fewer doses taken. When analyzing adherence over the whole study, the number of doses that qualified as non-adherent varied by individual, depending on the number of cycles over which a patient persisted with treatment and the number of calendars she submitted.
statistical methods
Patient registration, randomization, and data collection were managed by the CALGB Statistical Center. Data quality was insured by review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were carried out CALGB statisticians using the SAS® software, version 9.2.
Patient characteristics of interest include age (continuous), tumor size (continuous), race (white versus other), ethnicity (Hispanic versus Non-Hispanic), ECOG performance status (normal, ambulatory, >50% time in bed), type of primary surgery (mastectomy versus lumpectomy), estrogen receptor status (negative versus positive), progesterone receptor status (negative versus positive), and lymph node status (negative versus positive). Adverse event (AE) categories of interest were selected as any AE of grade 3 or 4 severity possibly, probably, or definitely related to CMF for which five or more patients experienced the AE. AE categories include diarrhea, fatigue, febrile neutropenia, leukopenia, nausea, thrombocytopenia, thromboembolism, and vomiting. Each AE category was analyzed as a dichotomous variable.
Group comparisons between treatment regimens within the standard arm (CMF versus AC) were carried out using a Wilcoxon rank-sum test for continuous variables and Fisher's exact test for categorical variables. Similarly, comparisons were carried out between persistent and non-persistent groups, using a Wilcoxon rank-sum test for continuous variables and Fisher's exact test for categorical variables. Univariate logistic regression modeling was used to identify marginally significant (P ≤ 0.2) predictors for persistence. The significant univariate predictors were then used as candidate variables in building a multivariable logistic regression model with a forward stepwise selection. Odds ratios with corresponding 95% confidence intervals were taken from respective logistic models. This procedure was completed twice; first time with independent variables limited to patient characteristics and a second time with independent variables limited to AE categories. Fisher's exact tests were used to compare patient characteristics based on the occurrence of AEs. Descriptive statistics were used to summarize patient characteristics and AEs among the adherent and non-adherent patients.
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements. Such on-site review of medical records was carried out for a subgroup of 153 patients (24%) of the 633 patients in CALGB 49907.
results
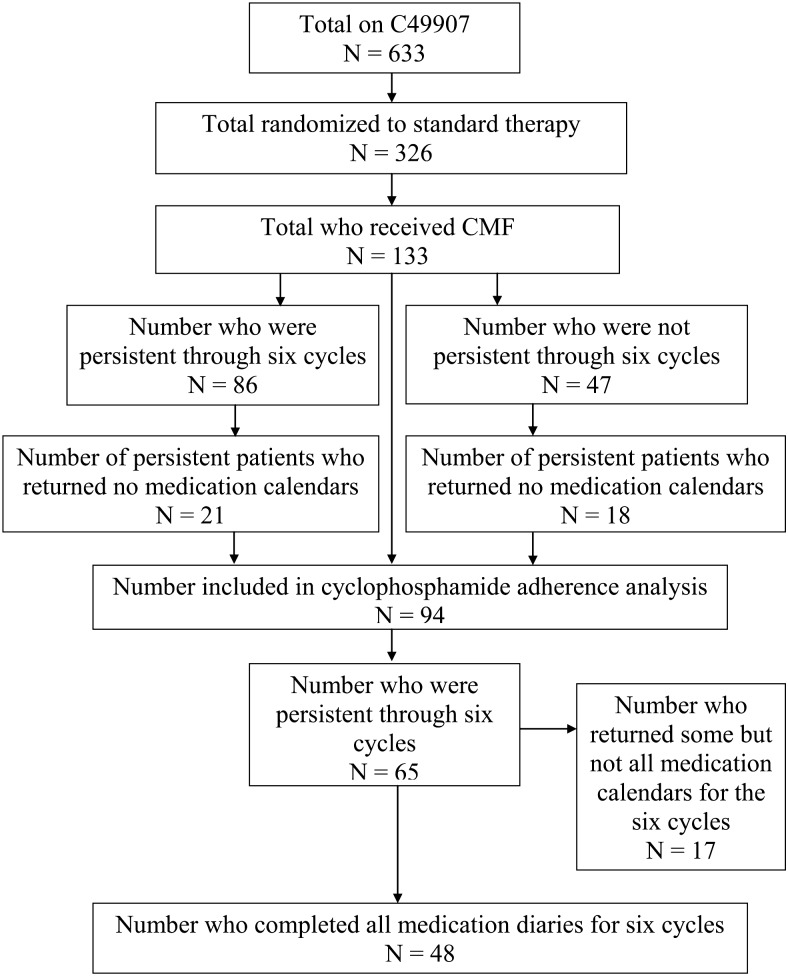
CALGB 49907 opened in 2001 and closed in November 2006, when a pre-planned boundary for futility was crossed. This occurred after the first planned analysis of 600 patients showed that patients randomized to capecitabine were two times more likely to relapse (P = 0.0006) and almost two times more likely to die (P = 0.019) than patients assigned to standard (CMF or AC) therapy [7]. When the study closed, 633 patients had been accrued to the clinical trial, 326 of whom had been randomized to standard therapy. Nine patients withdrew before they chose a regimen on the standard care arm. One hundred and thirty-three of these 326 women elected to receive CMF rather than AC on the standard arm. The flow diagram of the patient selection process is presented in Figure 1. Patient characteristics of those who chose to receive CMF or AC are presented in Table 1. The median age of CMF patients was 73 (range 65–88). CMF patients were older (P = 0.008) and less likely to be Hispanic (P < 0.001) than AC patients.
Figure 1.
Flow diagram of participants.
Table 1.
Baseline participant demographic and clinical characteristics for patients who received CMF compared with AC
| Characteristic | CMF (n = 133) | AC (n = 184) | P-valuea |
|---|---|---|---|
| Median age, years | 73.4 | 71.7 | 0.008* |
| Median tumor size, cm | 2.00 | 2.20 | 0.724 |
| Race | |||
| White | 116 (87%) | 155 (84%) | |
| Other | 17 (13%) | 29 (16%) | 0.520 |
| Ethnicity | |||
| Hispanic or Latino | 0 | 14 (8%) | |
| Non-Hispanic | 133 (100%) | 170 (92%) | <0.001* |
| ECOG performance status | |||
| 0 | 100 (75%) | 134 (73%) | |
| 1 | 29 (22%) | 48 (26%) | |
| 2 | 4 (3%) | 2 (1%) | 0.344 |
| Most extensive primary surgeryb | |||
| Lumpectomy | 62 (47%) | 88 (48%) | |
| Mastectomy | 71 (53%) | 94 (52%) | 0.820 |
| Hormone receptor status | |||
| Negative | 43 (32%) | 60 (33%) | |
| Positive | 90 (68%) | 123 (67%) | 1.000 |
| Lymph node status | |||
| Negative | 31 (23%) | 59 (32%) | |
| Positive | 102 (77%) | 124 (67%) | 0.101 |
aFisher's exact test for categorical variables, Wilcoxon's rank-sum test for continuous variables.
bPrimary surgery type was not reported for two patients.
*P-value <0.05.
persistence with cyclophosphamide
Eighty-six patients (65%) persisted with CMF to the completion of planned protocol therapy. Characteristics of persistent versus non-persistent patients are presented in supplementary Table S1, available at Annals of Oncology online. Two of these 86 had stopped cyclophosphamide but continued on methotrexate and 5-fluorouracil for six cycles, and the rest received all three drugs to completion. In a multivariable logistic regression model (presented in Table 2), non-persistence was associated with lymph node negativity (P = 0.016) and hormone receptor-positive tumors (P = 0.014). In a multivariable logistic regression model focusing on grade 3 and 4 toxic effects as independent variables, non-persistence was significantly associated with the report of fatigue (P = 0.025) and febrile neutropenia (P = 0.003), but not with any other grade 3 or 4 AE (Table 3). By univariate testing, using CTCAE v.2, no patient or tumor characteristic was found to be associated with a grade 3 or 4 AE.
Table 2.
Logistic regression modeling predicting persistence with patient characteristic covariates
| Univariate logistic regression models | ||||
|---|---|---|---|---|
| Effect | Description | Odds ratio | 95% confidence interval | P-value |
| Age | Five-year increments | 1.28 | 0.87–1.88 | 0.212 |
| Performance status | 0 versus 2 | 0.16 | 0.02–1.64 | 0.117 |
| 1 versus 2 | 0.20 | 0.02–2.21 | 0.327 | |
| Node status | Negative versus positive | 2.05 | 0.90–4.65 | 0.086 |
| Surgery | Mastectomy versus lumpectomy | 0.99 | 0.49–2.02 | 0.974 |
| Hormone receptor status | Negative versus positive | 0.43 | 0.19–0.99 | 0.047* |
| Race | Other versus white | 1.33 | 0.47–3.76 | 0.591 |
| Multivariable logistic regression model (entry P-value = 0.2, keep P-value = 0.2) | ||||
| Effect | Description | Odds ratio | 95% confidence interval | Adjusted P-value |
| Node status | Negative versus positive | 3.12 | 1.24–7.84 | 0.016* |
| Hormone receptor status | Negative versus positive | 3.17 | 1.27–7.94 | 0.014* |
*P-value <0.05.
Table 3.
Logistic regression modeling predicting persistence with grade 3–4 adverse event covariates
| Univariate logistic regression models | ||||
|---|---|---|---|---|
| Effect | Description | Odds ratio | 95% confidence interval | P-value |
| Leukopenia | No leukopenia versus leukopenia | 1.24 | 0.60–2.56 | 0.569 |
| Thrombocytopenia | No thrombocytopenia versus thrombocytopenia | 1.26 | 0.20–7.81 | 0.806 |
| Fatigue | No fatigue versus fatigue | 4.50 | 1.44–14.11 | 0.010* |
| Nausea | No nausea versus nausea | 4.15 | 0.99–17.45 | 0.052 |
| Vomiting | No vomiting versus vomiting | 3.37 | 0.77–14.81 | 0.107 |
| Diarrhea | No diarrhea versus Diarrhea | 3.08 | 0.82–11.52 | 0.095 |
| Febrile neutropenia | No febrile neutropenia versus febrile neutropenia | 23.61 | 2.91–191.32 | 0.003* |
| Thromboembolism | No thromboembolism versus Thromboembolism | 0.36 | 0.04–3.19 | 0.358 |
| Multivariable logistic regression model (entry P-value = 0.2, keep P-value = 0.2) | ||||
| Effect | Description | Odds ratio | 95% confidence interval | Adjusted P-value |
| Fatigue | No fatigue versus Fatigue | 4.08 | 1.20–13.91 | 0.025* |
| Vomiting | No vomiting versus Vomiting | 4.53 | 0.99–20.64 | 0.051 |
| Febrile neutropenia | No febrile neutropenia versus febrile neutropenia | 24.34 | 2.93–201.94 | 0.003* |
*P-value <0.05.
Out of the 19 non-persistent patients whose non-persistence was attributed to the toxicity of the treatment, 8 patients experienced three or more different types of grade 3 or 4 AE that were thought to be possibly, probably, or definitely related to CMF over the course of treatment. The grade 3 and 4 AEs that were documented as possibly, probably, or definitely related to CMF in these 19 patients included the following (with the number experiencing each toxicity during the course of treatment in parentheses): pain (1), diarrhea (3), nausea (3), fever (1), vomiting (3), neutropenia/leukopenia (14), thromboembolism (1), coagulopathy (1), febrile neutropenia/neutropenic infection (5), infection with normal neutrophil count (1), thrombocytopenia (1), renal insufficiency (2), syncope (2), ataxia (2), speech impairment (1), fatigue (3), dehydration (1), hypokalemia (1), hypotension (2), hyperglycemia (1) and anemia (1). Statistical testing was not carried out on this subset of 19 patients due to small sample size.
adherence to cyclophosphamide
Ninety-four of 133 (71%) CMF-treated patients submitted at least one medication calendar documenting their use of cyclophosphamide. Fewer calendars were submitted in later treatment cycles (supplementary Table S2, available at Annals of Oncology online). There were no obvious differences in reasons for treatment discontinuation between those who did and did not submit calendars (supplementary Table S3, available at Annals of Oncology online). Average adherence across all cycles with available data was 97%. Five percent (five women) reported taking <80% of total expected doses (number of observed doses/number of expected doses <0.80) and were therefore considered non-adherent. Since a small number of patients were observed in the non-adherent group, there was limited statistical power to identify associations with patient and tumor characteristics (Table 4).
Table 4.
Adherent versus non-adherent patients
| Adherent | Non-adherent | |
|---|---|---|
| Characteristic | n = 89 (95%) | n = 5 (5%) |
| Median age (years) | 73.5 | 75.8 |
| Median tumor (cm) | 2.00 | 1.70 |
| Race | ||
| White | 78 (88%) | 4 (80%) |
| Other | 11 (12%) | 1 (20%) |
| Ethnicity | ||
| Hispanic | 0 | 0 |
| Non-Hispanic | 89 (100%) | 5 (100%) |
| ECOG performance status | ||
| 0 | 68 (76%) | 3 (60%) |
| 1 | 20 (22%) | 2 (40%) |
| 2 | 1 (1%) | 0 |
| Most extensive primary surgery | ||
| Lumpectomy | 43 (48%) | 3 (60%) |
| Mastectomy | 46 (52%) | 2 (40%) |
| Hormone receptor status | ||
| Positive | 53 (60%) | 5 (100%) |
| Negative | 36 (40%) | 0 |
| Lymph node status | ||
| Positive | 68 (76%) | 5 (100%) |
| Negative | 21 (24%) | 0 |
| Grade 3 or 4 adverse eventsb | ||
| Neutropenia | 25 (28%) | 0 |
| Leukopenia | 33 (37%) | 3 (60%) |
| Fatigue | 11 (12%) | 1 (20%) |
| Nausea | 4 (4%) | 1 (20%) |
| Diarrhea | 6 (7%) | 1 (20%) |
| Febrile neutropenia | 7 (8%) | 1 (20%) |
| Thromboembolism | 5 (6%) | 0 |
aA progesterone receptor status was not provided for one patient in the non-adherent group.
bAdditional grade 3 or 4 adverse events reported in less than five patients: anemia, thrombocytopenia, mucositis and vomiting.
discussion
Non-persistence and non-adherence may impair the efficacy of oral oncologic therapies. In recent years, attention has focused on improving the way that oral therapies are ordered, dispensed, administered, and monitored in an effort to improve the quality of cancer care [9]. In clinical trials, it has been recognized that differences in adherence rates between two regimens may impact results, so medication calendars have conventionally been used as a convenient method to measure and promote adherence. In women with breast cancer, persistence with and adherence to oral adjuvant hormonal therapy have been shown to affect a variety of outcomes, including survival [4, 5, 10–15]. However, less is known about persistence with and adherence to oral chemotherapy regimens for this disease. In one study, Mayer et al. showed that more than a third of patients were over-adherent (i.e. took more than their prescribed doses) to capecitabine and gefitinib in a phase I metastatic breast cancer trial, but there were too few participants to evaluate for an impact on outcomes [16]. Furthermore, patients with metastatic disease may be more motivated to take their oral antineoplastics agents than early-stage patients, so it is important to also study patients without advanced disease.
In the current study, there were too few women on CMF to have adequate power to detect a difference in disease outcomes based on adherence or persistence, but one prior study did suggest that there may be a dose effect for CMF (i.e. patients who take fewer cycles obtain less benefit from the treatment) [17]. Also, the results of C49907 showed that standard chemotherapy reduced risk even in this older population, suggesting that non-persistent and non-adherent patients may be more likely to experience recurrence.
It is informative to compare our findings with those of our analysis of adherence to capecitabine using MEMS caps in this trial, in which adherence to capecitabine averaged 78%, and 25% of patients took <80% of prescribed capecitabine doses.6 Self-reported measures of adherence generally over-estimate adherence compared with more objective measures (e.g. MEMS caps) [18, 19]. Thus, it is unclear whether adherence to capecitabine in this study was actually less than adherence to the oral component of CMF. Because patients who did not turn in medication calendars may have been less adherent to the oral component of CMF, this present analysis likely over-estimates adherence in the CMF arm.
In this analysis, there were few identifiable demographic and clinical predictors of persistence and adherence. The association between non-persistence and both node-negativity and hormone receptor-positive tumors suggests that those predicted to have less benefit from chemotherapy (because of lower risk and less chemo-sensitive disease, respectively) may have been less likely to be persistent. This is consistent with prior data suggesting that beliefs about the efficacy of a medication may impact medication-taking behaviors [2]. The association between certain toxic effects and non-persistence is likewise an expected but still important finding from this study. Elderly patients may be particularly susceptible to side-effects from chemotherapy that lead to treatment cessation. Bone marrow reserve may be less robust in older patients, and energy levels may be more tenuous. In patients of any age, experiences such as hospital admission for febrile neutropenia may be unpleasant and/or frightening enough to dissuade them from proceeding with the planned oncologic treatment.
Consistent with capecitabine adherence in this study, adherence to cyclophosphamide was not associated with age in women aged 65 or older at diagnosis [8]. However, because non-adherence was rare, the power was limited for analyses focused on adherence. Specific concomitant medication data were not available for our analyses, but poly-pharmacy may have contributed to non-adherence and non-persistence. Future work is warranted to evaluate the effects of age on persistence with and adherence to oral chemotherapy in a population of women including both older and younger women, as well as to explore other factors that may be associated with oral chemotherapy persistence or adherence (including the psychosocial state of the patient, intentions to adhere, and treatment-related factors) [2, 20].
The results from this study must be interpreted in light of its limitations. Self-reported adherence measures can be inaccurate and may over-estimate adherence as noted above. Further, data collected on this study did not include the timing of doses of cyclophosphamide, just the total number per cycle, so we were not able to assess for the proportion of doses that were taken too close together or too far apart. Finally, adherence in the setting of this trial may not reflect persistence with and adherence to CMF in the community both because the knowledge among participants that adherence was being monitored using the medication calendars may have improved adherence with cyclophosphamide among some women and because participants in a clinical trial may be different from those who either opt not to or never have the option to participate [21, 22]. In this study, as usual in clinical trials, comorbidities were minimal at the time of enrollment (partly due to exclusion criteria). Therefore, persistence and adherence may be higher than in the real world, where elderly patients with multiple comorbidities are often prescribed CMF when chemotherapy for early-stage breast cancer is warranted. Further research to evaluate the adherence to and persistence with oral chemotherapies using potentially more sensitive and less subjective methods in non-trial populations is clearly warranted.
Still, our findings have important implications for the treatment of older women with early-stage breast cancer with adjuvant oral CMF and future research using oral medications in oncology. The decision to treat an older woman with CMF should take into consideration the substantial rate of non-persistence seen in this study. Monitoring for and interventions to prevent febrile neutropenia and fatigue, in particular, may help to improve persistence. CMF using intravenous cyclophosphamide is an alternative regimen for which concerns about adherence would be minimized and tolerability may be better, allowing for improved persistence. However, there are limited data regarding the efficacy of this regimen compared with the well-studied oral cyclophosphamide CMF regimen [23]. Future cancer clinical trials evaluating oral agents should closely measure, monitor, and promote adherence to optimize the understanding of the benefits and risks of the treatment. Additional evaluation of adherence to and persistence with oral chemotherapy outside of the clinical trial setting is clearly warranted.
funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers CA32291, CA33601, CA32102, CA77202, CA25224, CA21115, CA105409, CA77651, and CA77406; grant number CA31946 to the Cancer and Leukemia Group B and Richard L. Schilsky; and grant number CA33601 to the CALGB Statistical Center and Stephen George, PhD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The following institutions participated in this study:
University of Oklahoma, Oklahoma, OK—Howard Ozer, MD, supported by CA37447;
Christiana Care Health Services, Inc., CCOP, Wilmington, DE—Stephen Grubbs, MD, supported by CA45418;
Dana-Farber Cancer Institute, Boston, MA—Harold J. Burstein, MD, supported by CA32291;
Dartmouth Medical School – Norris Cotton Cancer Center, Lebanon, NH—Konstantin Dragnev, MD, supported by CA04326;
Duke University Medical Center, Durham, NC—Jeffrey Crawford, MD, supported by CA47577;
Grand Rapids Clinical Oncology Program, Grand Rapids, MI—Martin J. Bury, MD;
Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY—Jeffrey Kirshner, MD, supported by CA45389;
Illinois Oncology Research Association, Peoria, IL—John W. Kugler, MD, supported by CA35113;
Long Island Jewish Medical Center, Lake Success, NY—Kanti R. Rai, MD, supported by CA35279;
Memorial Sloan-Kettering Cancer Center, New York, NY—Clifford A. Hudis, MD, supported by CA77651;
Missouri Baptist Medical Center, St Louis, MO—Alan P. Lyss, MD, supported by CA114558-02;
Mount Sinai Medical Center, Miami, FL—Rogerio C. Lilenbaum, MD, supported by CA45564;
Mount Sinai School of Medicine, New York, NY—Lewis R. Silverman, MD, supported by CA04457;
North Shore-Long Island Jewish Health System, New Hyde Park, NY—Daniel Budman, MD, supported by CA35279;
Northern Indiana Cancer Research Consortium CCOP, South Bend, IN—Rafat Ansari, MD, supported by CA86726;
Roswell Park Cancer Institute, Buffalo, NY—Ellis Levine, MD, supported by CA59518;
Southeast Cancer Control Consortium, Inc., CCOP, Goldsboro, NC—James N. Atkins, MD, supported by CA45808;
State University of New York Upstate Medical University, Syracuse, NY—Stephen L. Graziano, MD, supported by CA21060;
The Ohio State University Medical Center, Columbus, OH—Clara D. Bloomfield, MD, supported by CA77658;
University of California at San Diego, San Diego, CA—Barbara A. Parker, MD, supported by CA11789;
University of Chicago, Chicago, IL—Hedy L. Kindler, MD, supported by CA41287;
University of Maryland Greenebaum Cancer Center, Baltimore, MD—Martin Edelman, MD, supported by CA31983;
University of Massachusetts Medical School, Worcester, MA—William V. Walsh, MD, supported by CA37135;
University of Minnesota, Minneapolis, MN—Bruce A. Peterson, MD, supported by CA16450;
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO—Michael C. Perry, MD, supported by CA12046;
University of North Carolina at Chapel Hill, Chapel Hill, NC—Thomas C. Shea, MD, supported by CA47559;
University of Vermont, Burlington, VT—Steven M. Grunberg, MD, supported by CA77406;
Washington University School of Medicine, St Louis, MO—Nancy Bartlett, MD, supported by CA77440;
Eastern Cooperative Oncology Group, Philadelphia, PA—Robert L. Comis, MD, Chairman, supported by CA21115;
Southwest Oncology Group, San Antonio, TX—Laurence H. Baker, DO, Chairman, supported by CA32102;
North Central Cancer Treatment Group, Rochester, MN—Jan Buckner, MD, Chairman, supported by CA25224;
National Cancer Institute of Canada, Toronto, ON—Elizabeth Eisenhauer, MD, President; supported by CA77202.
references
- 1.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 3.Zedler P, Kakad P, Colilla S, et al. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clin Ther. 2011;33(1):62–73. doi: 10.1016/j.clinthera.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 5.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19(2):322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 7.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol. 2010;28(14):2418–2422. doi: 10.1200/JCO.2009.26.4671. 7. CTCAE v.2, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weingart SN, Toro J, Spencer J, et al. Medication errors involving oral chemotherapy. Cancer. 2010;116(10):2455–2464. doi: 10.1002/cncr.25027. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1–2):1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 11.Partridge AH. Non-adherence to endocrine therapy for breast cancer. Ann Oncol. 2006;17(2):183–184. doi: 10.1093/annonc/mdj141. [DOI] [PubMed] [Google Scholar]
- 12.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26(4):549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 13.Thompson AM, Johnson A, Quinlan P, et al. Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat. 2011;125(1):279–287. doi: 10.1007/s10549-010-1139-x. [DOI] [PubMed] [Google Scholar]
- 14.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dezentje VO, van Blijderveen NJ, Gelderblom H, et al. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28(14):2423–2429. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 16.Mayer EL, Partridge AH, Harris LN, et al. Tolerability of and adherence to combination oral therapy with gefitinib and capecitabine in metastatic breast cancer. Breast Cancer Res Treat. 2009;117(3):615–623. doi: 10.1007/s10549-009-0366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonadonna MD, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Eng J Med. 1981;304:10–14. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- 18.Waterhouse DM, Calzone KA, Mele C, et al. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11(6):1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 19.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 21.Haynes RB. A critical review of the ‘determinants’ of patient compliance with therapeutic regimens. In: Sackett DL, Haynes RB, editors. Compliance with Therapeutic Regimens. Baltimore, MD: The Johns Hopkins University Press; 1976. pp. 26–39. [Google Scholar]
- 22.Leventhal H, Nerenz D, Leventhal E, et al. The behavioral dynamics of clinical trials. Prev Med. 1991;20:132–146. doi: 10.1016/0091-7435(91)90014-u. [DOI] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Colleoni A, Castiglione-Gertsch M, et al. Adding adjuvant CMF chemotherapy to either sradiotherapy or tamoxifen: are all CMFs alike? Ann Oncol. 1998;9:489–493. doi: 10.1023/a:1008236502420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.