Abstract
Background
The clinicopathological characteristics and the prognostic significance of multifocal (MF) and multicentric (MC) breast cancers are not well established.
Patients and Methods
MF and MC were defined as more than one lesion in the same quadrant or in separate quadrants, respectively. The Kaplan–Meier product limit was used to calculate recurrence-free survival (RFS), breast cancer-specific survival (BCSS), and overall survival (OS). Cox proportional hazards models were fit to determine independent associations of MF/MC disease with survival outcomes.
Results
Of 3924 patients, 942 (24%) had MF (n = 695) or MC (n = 247) disease. MF/MC disease was associated with higher T stages (T2: 26% versus 21.6%; T3: 7.4% versus 2.3%, P < 0.001), grade 3 disease (44% versus 38.2%, P < 0.001), lymphovascular invasion (26.2% versus 19.3%, P < 0.001), and lymph node metastases (43.1% versus 27.3%, P < 0.001). MC, but not MF, breast cancers were associated with a worse 5-year RFS (90% versus 95%, P = 0.02) and BCSS (95% versus 97%, P = 0.01). Multivariate analysis shows that MF or MC did not have an independent impact on RFS, BCSS, or OS.
Conclusions
MF/MC breast cancers were associated with poor prognostic factors, but were not independent predictors of worse survival outcomes. Our findings support the current TNM staging system of using the diameter of the largest lesion to assign T stage.
Keywords: breast cancer, multifocal, multicentric, outcomes
introduction
Although multifocal (MF) and multicentric (MC) breast tumors are a common entity, their clinical behavior is not well characterized. The incidence of MF and MC tumors in the literature ranges from 6% to 60%, with the large variability due to differences in definitions used, inclusion or exclusion of in situ disease, and method of pathologic sampling [1, 2]. As advances in preoperative imaging continue, the number of MF and MC tumors identified increases [3–5], and better guidelines for their management are needed. The tumor size has long been recognized as an independent predictor for worse overall survival (OS) [6]. Intuitively, it would seem that the presence of more than one synchronous unilateral tumor would portend a worse prognosis when compared with unifocal (UF) counterparts. However, while studies have consistently shown a correlation between multifocality and multicentricity and the rate and extent of lymph node metastases [1, 7–14], the literature is divided on whether there is a corresponding impact on survival outcomes. In the absence of compelling evidence to dictate otherwise, the convention according to the current TNM-staging guidelines has been to stage and treat MF and MC patients according to the diameter of the largest lesions, without taking other foci of disease into consideration [15]. This assumes that the prognosis is dependent only on the largest lesion and the presence and the extent of lymph node involvement.
The purpose of this study was to evaluate the frequency of MF and MC disease in a large cohort of breast cancers and its correlation with other pathological characteristics and patient outcomes, including recurrence-free survival (RFS), breast cancer-specific survival (BCSS), and OS.
patients and methods
patient selection
Using the Breast Cancer Management System database of The University of Texas MD Anderson Cancer Center (MDACC), we retrospectively identified all patients diagnosed with invasive breast cancer between 1997 and 2010. We excluded patients with metastatic disease at diagnosis and those treated with neoadjuvant chemotherapy, leaving 6735 patients. We excluded an additional 2811 patients who did not have MF and MC information available.
According to the classification system used to maintain the database, MF was defined as two or more separate invasive tumors in the same quadrant of the breast. MC was defined as two or more separate invasive tumors occupying more than one quadrant of the same breast. If a patient had both MF and MC diseases, they were classified as MC. Patients who had MF or MC in situ disease only were not included in the analysis. Determinations were made based on the pathological review only; radiographic data were not considered. All pathology specimens were independently reviewed by dedicated breast pathologists at MDACC at the time of initial treatment. A total of 3924 patients were included in the analysis. Of these, 2982 (76%) patients had UF breast cancer and 942 (24%) patients had MF (n = 695) or MC (n = 247) breast cancer in their pathology specimens. The Institutional Review Board of The University of Texas, MDACC, approved the retrospective study.
statistical analysis
Patients were categorized as UF, MF, or MC. The MF and MC tumors were analyzed as separate entities and as a group. Patient and clinical characteristics including age, menopausal status, tumor size, nodal status, histology, nuclear grade, presence of lymphovascular invasion, and therapy received (surgery type and adjuvant chemotherapy, radiation therapy and endocrine therapy) were compared between groups using the χ2 test. Five-year RFS, BCSS, and OS were calculated from the date of diagnosis to the date of local or distant recurrence, death attributable to breast cancer or death, or last follow-up, respectively. The Kaplan–Meier product limit method was used to estimate the survival outcomes of all patients, and groups were compared with the log-rank statistic. A subgroup analysis was performed by stage: node-negative (T1-2N0), early node-positive (T1-2N1), and locally advanced (T3 or N2-3). Cox proportional hazards models were fit to determine the association of MF, MC, and the combination of the MF and MC groups with survival outcomes. P values <0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.1 (SAS Institute Inc., Cary, NC) and S-Plus 7.0 (Insightful Corporation, Seattle, WA).
results
clinicopathological characteristics
MF or MC disease was seen in 942 patients, or 24% of the total patient population. Six hundred and ninety-five patients (17.7%) had MF and 247 (6.3%) patients had MC tumors. Compared with patients with UF disease, patients with MF or MC breast cancer were younger and premenopausal (both P < 0.001). They had higher T stages (T2: 26% versus 21.6%; T3: 7.4% versus 2.3%, P < 0.001) and an increased rate of lymph node metastases (43.1% versus 27.3%, P < 0.001). MF and MC tumors were also associated with a higher N stage, with a larger percentage of patients with N2 (3.8% versus 2.3%) and N3 (3.2% versus 1.1%) (P < 0.001) disease. Histologically, MF and MC tumors were associated with more grade 3 disease (44% versus 38.2%, P < 0.001), lymphovascular invasion (26.2% versus 19.3%, P < 0.001), lobular differentiation (15.4% versus 7.7%, P < 0.001), and epidermal growth factor receptor 2 (Her2/Neu) positivity (21.3% versus 15.6%, P = 0.001). In terms of the treatment received, they were more likely to undergo mastectomy (P < 0.001). More patients with MF and MC tumors received adjuvant chemotherapy (P < 0.001), but there was no difference in the proportion of patients who received adjuvant endocrine therapy (supplementary Table S1, available at Annals of Oncology online).
survival estimates
The median follow-up was 51 months (range 1–162 months). One hundred and ninety patients (4.8%) had recurrence: 137 (4.6%) in the UF group, 33 (4.7%) in the MF group, and 20 (8.1%) in the MC group (supplementary Table S2, available at Annals of Oncology online). There was a statistically significant difference in the 5-year RFS between the UF (95%) and MC groups (90%), P = 0.02 (Figure 1B). There was no significant difference between the UF and MF groups (95%), P = 0.9 (Figure 1A). One hundred and three patients (2.6%) died from their breast cancer: 71 (2.4%) in the UF group, 19 (2.7%) in the MF group, and 13 (5.3%) in the MC group (supplementary Table S2, available at Annals of Oncology online). There was a statistically significant difference in the 5-year BCSS between the UF (97%) and MC groups (95%), P = 0.01 (Figure 2B), but no difference between the UF and MF (98%) groups, P = 0.79) (Figure 2A). Two hundred and eighty patients (7.1%) died from any cause: 207 (7.07%) in the UF group, 47 (6.8%) in the MF group, and 26 (7.5%) in the MC group (supplementary Table S2, available at Annals of Oncology online). When analyzed together or as separate entities, MF and MC tumors were not associated with a statistically significant difference in the 5-year OS (Figure 3), although there was a trend toward worse outcomes in the MC group (93% versus 92%, P = 0.08) (Figure 3B).
Figure 1.
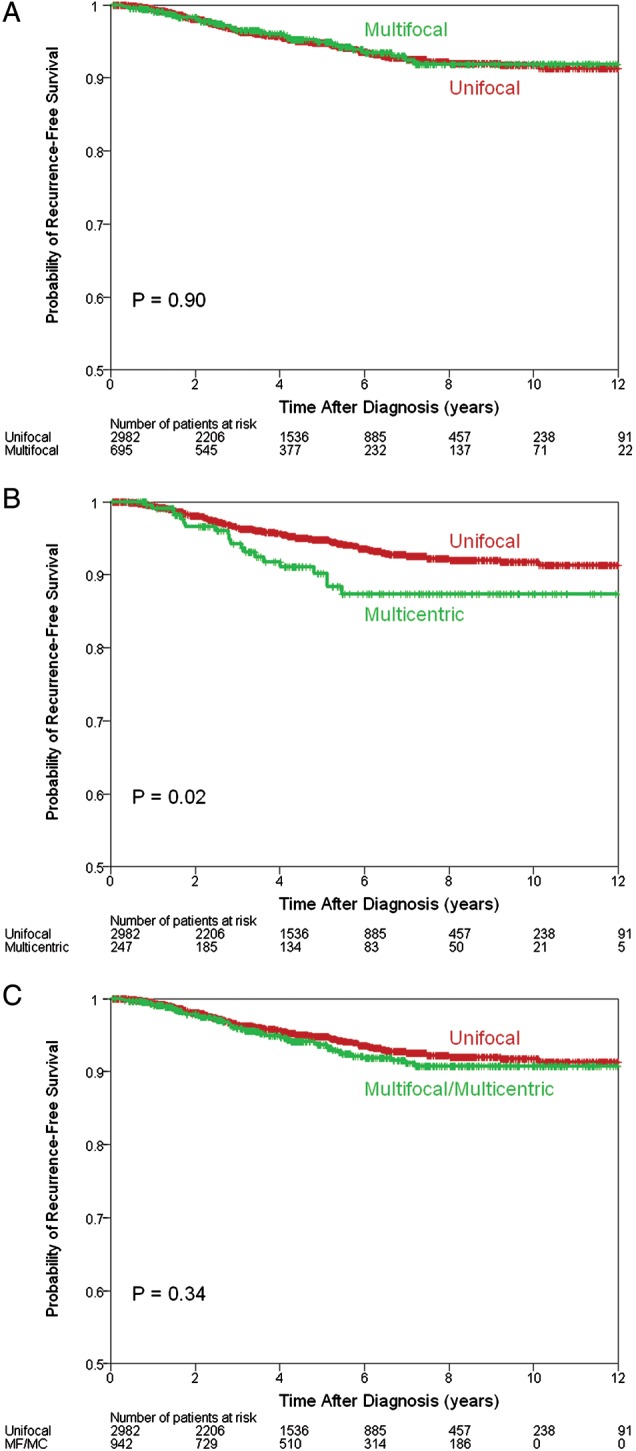
Recurrence-free survival estimates comparing multifocal (MF) with unifocal disease (A), multicentric (MC) with unifocal disease (B) and MF and MC and unifocal disease (C).
Figure 2.
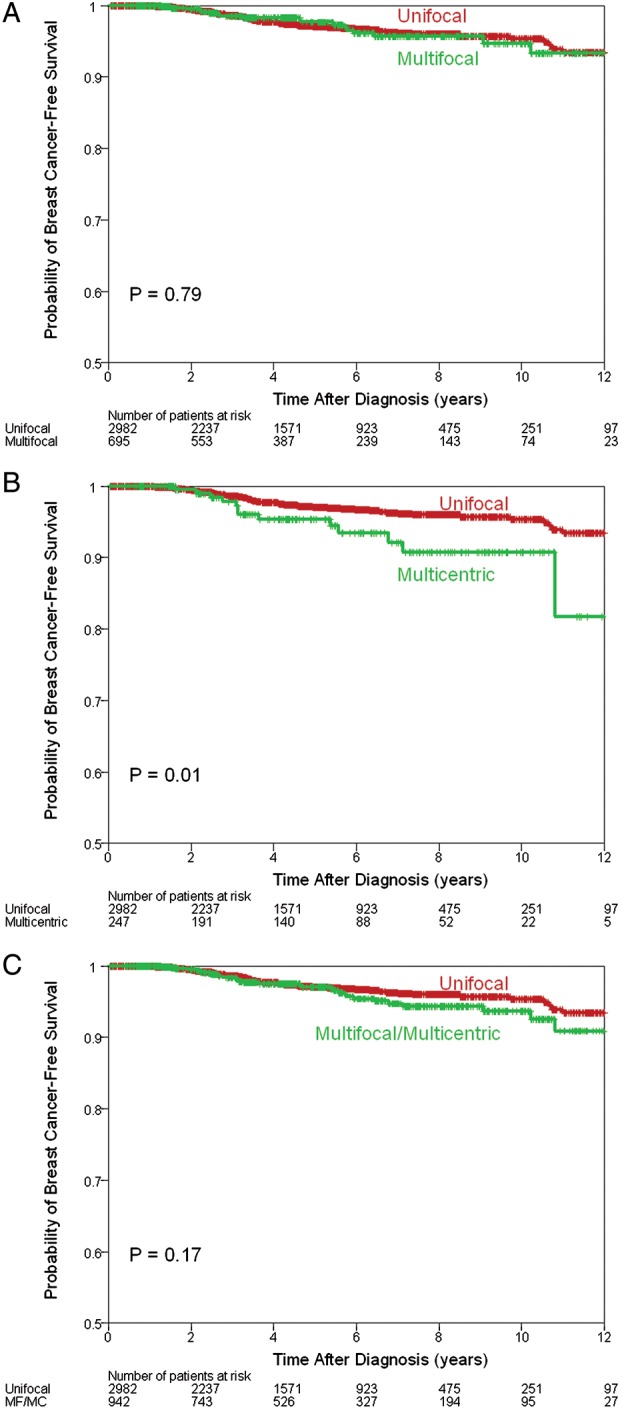
Breast cancer-specific survival estimates comparing multifocal (MF) with unifocal disease (A), multicentric (MC) with unifocal disease (B) and MF and MC with unifocal disease (C).
Figure 3.
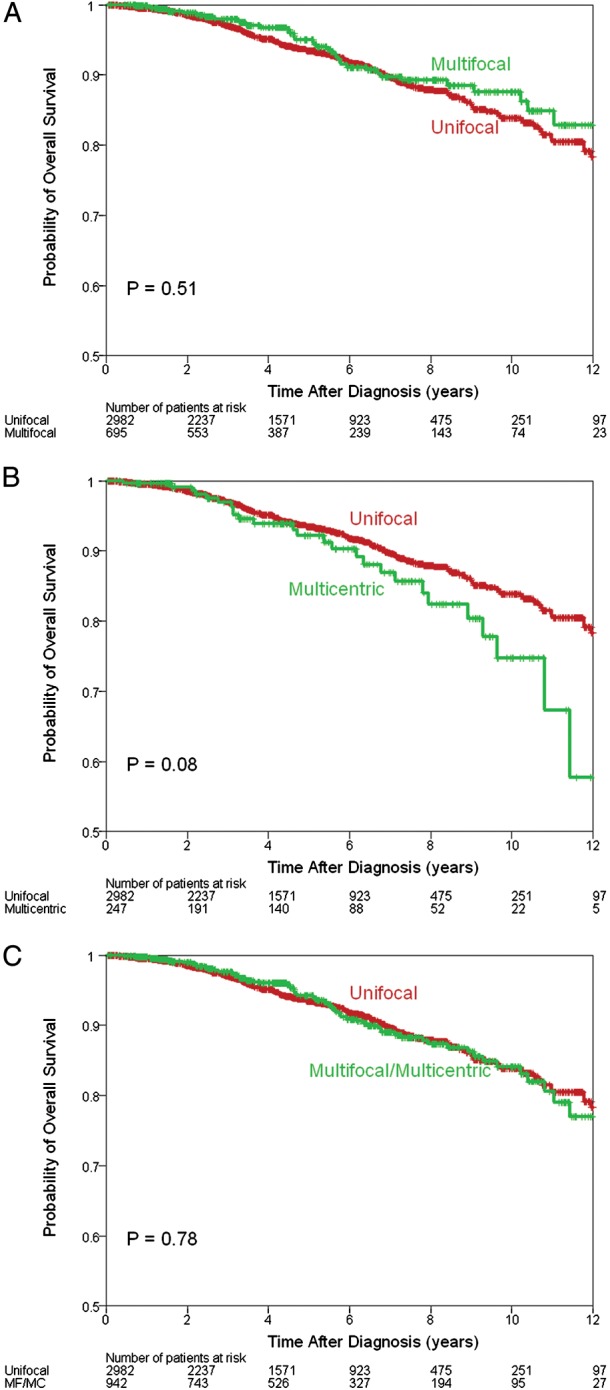
Overall survival estimates comparing multifocal (MF) with unifocal disease (A), multicentric (MC) with unifocal disease (B) and MF and MC with unifocal disease (C).
To evaluate the impact of MF and MC on survival outcomes, a multivariate Cox regression analysis was applied. Risk factors that were significant on univariate analysis were evaluated. The results are shown in Table 1. African–American race, larger tumor size, lymph node metastases, higher tumor grade, and lymphovascular invasion were associated with worse survival. Multifocality and multicentricity were not independent predictors of survival (RFS, BCSS, or OS). Specifically, multicentricity, which had a significant worse outcome on univariate analysis, no longer had a significant impact on RFS (HR = 0.93; 95% CI: 0.56–1.55, P = 0.79) or BCSS (HR = 1.08; 95% CI: 0.57–2.05, P = 0.82) after controlling for other prognostic factors.
Table 1.
Multivariate analysis for RFS, BCSS, and OS
| Recurrence-free survival |
Breast cancer-specific survival |
Overall survival |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Multifocal: yes versus no | 0.85 | 0.58–1.25 | 0.4 | 0.97 | 0.58–1.63 | 0.92 | 0.87 | 0.63–1.2 | 0.39 |
| Age: >50 versus ≤50 | 0.74 | 0.54–1.03 | 0.08 | 0.78 | 0.5–1.23 | 0.29 | 1.70 | 1.24–2.34 | 0.001 |
| Race: African-American versus no | 1.88 | 1.25–2.83 | 0.002 | 1.71 | 0.97–3.04 | 0.06 | 1.63 | 1.11–2.39 | 0.012 |
| Tumor size: T2 versus T1 | 2.48 | 1.76–3.5 | <0.0001 | 3.04 | 1.89–4.9 | <0.0001 | 2.66 | 1.99–3.54 | <0.0001 |
| Tumor size: T3 versus T1 | 4.40 | 2.37–8.15 | <0.0001 | 5.41 | 2.48–11.82 | <0.0001 | 3.51 | 1.98–6.2 | <0.0001 |
| Lymph nodes: N1 versus N0 | 1.76 | 1.2–2.56 | 0.003 | 2.21 | 1.31–3.72 | 0.003 | 2.10 | 1.54–2.87 | <0.0001 |
| Lymph nodes: N2 versus N0 | 1.74 | 0.77–3.91 | 0.18 | 3.35 | 1.24–9.07 | 0.017 | 3.30 | 1.7–6.41 | 0.0004 |
| Lymph nodes: N3 versus N0 | 4.38 | 2.07–9.28 | 0.0001 | 4.45 | 1.65–12.03 | 0.003 | 3.10 | 1.46–6.6 | 0.003 |
| Grade: 3 versus 1 and 2 | 2.51 | 1.74–3.61 | <0.0001 | 2.71 | 1.6–4.6 | 0.0002 | 1.72 | 1.31–2.27 | 0.0001 |
| LVI: present versus absent | 1.96 | 1.39–2.78 | 0.0001 | 1.51 | 0.93–2.44 | 0.09 | 1.54 | 1.14–2.08 | 0.005 |
| Adjuvant chemotherapy: yes versus no | 0.49 | 0.34–0.71 | 0.0002 | 0.52 | 0.31–0.87 | 0.013 | 0.29 | 0.21–0.4 | <0.0001 |
| Adjuvant endocrine therapy: yes versus no | 0.47 | 0.34–0.65 | <0.0001 | 0.39 | 0.25–0.6 | <0.0001 | 0.49 | 0.38–0.64 | <0.0001 |
| Multicentric: yes versus no | 0.93 | 0.56–1.55 | 0.79 | 1.08 | 0.57–2.05 | 0.82 | 1.00 | 0.65–1.55 | 0.99 |
| Age: >50 versus ≤50 | 0.78 | 0.55–1.09 | 0.15 | 0.82 | 0.51–1.3 | 0.39 | 1.70 | 1.22–2.38 | 0.002 |
| Race: African-American versus no | 1.45 | 0.91–2.31 | 0.12 | 1.26 | 0.65–2.44 | 0.5 | 1.61 | 1.07–2.4 | 0.021 |
| Tumor size: T2 versus T1 | 2.41 | 1.67–3.46 | <0.0001 | 2.97 | 1.79–4.94 | <0.0001 | 2.60 | 1.91–3.52 | <0.0001 |
| Tumor size: T3 versus T1 | 3.66 | 2.02–6.61 | <0.0001 | 5.02 | 2.38–10.62 | <0.0001 | 3.38 | 1.98–5.76 | <0.0001 |
| Lymph nodes: N1 versus N0 | 1.81 | 1.22–2.69 | 0.003 | 2.25 | 1.3–3.9 | 0.004 | 2.01 | 1.44–2.81 | <0.0001 |
| Lymph nodes: N2 versus N0 | 2.02 | 0.93–4.38 | 0.08 | 3.66 | 1.42–9.42 | 0.007 | 3.67 | 1.92–6.99 | <0.0001 |
| Lymph nodes: N3 versus N0 | 4.32 | 1.95–9.55 | 0.0003 | 4.07 | 1.41–11.69 | 0.009 | 3.37 | 1.59–7.17 | 0.002 |
| Grade: 3 versus 1 and 2 | 2.38 | 1.63–3.49 | <0.0001 | 2.41 | 1.38–4.18 | 0.002 | 1.66 | 1.24–2.22 | 0.0007 |
| LVI: present versus absent | 1.88 | 1.31–2.69 | 0.0006 | 1.56 | 0.95–2.56 | 0.08 | 1.57 | 1.14–2.14 | 0.005 |
| Adjuvant chemotherapy: yes versus no | 0.55 | 0.37–0.82 | 0.004 | 0.59 | 0.34–1.02 | 0.06 | 0.32 | 0.23–0.44 | <0.0001 |
| Adjuvant endocrine therapy: yes versus no | 0.46 | 0.33–0.64 | <0.0001 | 0.36 | 0.22–0.57 | <0.0001 | 0.50 | 0.38–0.66 | <0.0001 |
| Multifocal or multicentric: yes versus no | 0.88 | 0.63–1.21 | 0.42 | 1.01 | 0.66–1.55 | 0.96 | 0.91 | 0.69–1.19 | 0.48 |
| Age: >50 versus ≤50 | 0.75 | 0.55–1.01 | 0.06 | 0.76 | 0.5–1.16 | 0.21 | 1.59 | 1.18–2.13 | 0.002 |
| Race: African-American versus no | 1.94 | 1.32–2.85 | 0.0007 | 1.90 | 1.13–3.2 | 0.016 | 1.72 | 1.21–2.44 | 0.003 |
| Tumor size: T2 versus T1 | 2.33 | 1.68–3.24 | <0.0001 | 3.05 | 1.94–4.8 | <0.0001 | 2.73 | 2.08–3.6 | <0.0001 |
| Tumor size: T3 versus T1 | 3.64 | 2.14–6.19 | <0.0001 | 4.83 | 2.49–9.38 | <0.0001 | 3.64 | 2.26–5.87 | <0.0001 |
| Lymph nodes: N1 versus N0 | 1.83 | 1.28–2.63 | 0.001 | 2.37 | 1.43–3.91 | 0.0008 | 2.17 | 1.61–2.93 | <0.0001 |
| Lymph nodes: N2 versus N0 | 1.78 | 0.83–3.82 | 0.14 | 3.56 | 1.41–9 | 0.007 | 3.42 | 1.81–6.45 | 0.0002 |
| Lymph nodes: N3 versus N0 | 4.24 | 2.12–8.48 | <0.0001 | 5.03 | 2.09–12.1 | 0.0003 | 3.81 | 1.97–7.38 | <0.0001 |
| Grade: 3 versus 1 and 2 | 2.44 | 1.73–3.45 | <0.0001 | 2.52 | 1.54–4.12 | 0.0002 | 1.69 | 1.3–2.2 | <0.0001 |
| LVI: present versus absent | 1.96 | 1.41–2.72 | <0.0001 | 1.61 | 1.03–2.5 | 0.036 | 1.50 | 1.13–2 | 0.005 |
| Adjuvant chemotherapy: yes versus no | 0.49 | 0.34–0.7 | 0.0001 | 0.51 | 0.31–0.83 | 0.007 | 0.29 | 0.21–0.39 | <0.0001 |
| Adjuvant endocrine therapy: yes versus no | 0.46 | 0.34–0.63 | <0.0001 | 0.40 | 0.26–0.6 | <0.0001 | 0.51 | 0.4–0.66 | <0.0001 |
LVI, lymphovascular invasion; CI, confidence interval.
We also looked at the subgroup of patients with node-negative (T1-2 N0) breast cancer to determine whether MF and MC had a stronger impact on this subset of very early-stage patients. There was no difference in the RFS, BCSS, or OS between the UF and MF and MC tumors in any of the stage groups on either univariate or multivariate analyses (data not shown). Results were also similar when the analysis was limited to patients with ≥5 years of follow-up (data not shown).
discussion
One of the biggest obstacles in interpreting the current literature on MF and MC breast tumors is the lack of a standard definition. MF tumors are classically defined as two or more separate tumors in the same quadrant of the breast, with MC tumors occupying more than one quadrant and separated by normal breast tissue [16]. Alternatively, some studies use definitions based on the distance of uninvolved tissue between lesions [17–19]. In some series, focality is determined by histologic parameters, while others use only clinical and radiographic data. Most of the authors do not differentiate between MF and MC tumors, and those who do almost universally lump the groups together for analysis. In this study, we defined MF and MC as two or more separate tumors in one quadrant of the breast and the multiple quadrants of the breast, respectively, and evaluated the two groups as separate entities, as well as in combination. We did not include tumors that were MF or MC by virtue of in situ disease only. Similar to previous studies, we found a significant association between MF and MC tumors and the rate and the extent of axillary lymph node involvement [10, 12, 20–22]. Our study and others have noted the increased incidence of lymphovascular invasion as well [1, 13, 14]. While both MF and MC tumors were associated with these high-risk features, only MC tumors were associated with a worse RFS and BSSS, and this association was on univariate analysis only. This is consistent with the findings of other recent studies. Vlastos et al. [20] reviewed 60 patients with early-stage (T1-2, N0-1) MC breast cancer, using the same definitions that we used, and found no difference in locoregional recurrence, contralateral breast cancer, distant metastasis, or survival (10-year DFS 84% versus 83%). Pedersen et al. [10] analyzed 158 patients with more than one focus of tumor separated by normal breast tissue, and found worse OS for MF and MC tumors on univariate analysis. As in our study, after adjusting for other prognostic factors with multivariate analysis, MF and MC did not have an independent impact on survival. Cabioglu et al. [12] reviewed 147 patients with MF and MC disease defined as at least two foci of invasive cancer more than 5 mm apart, and found no difference in the 5-year DFS (82% versus 88%, P = 0.14) or OS (93% versus 92%, P = 0.43).
In contrast to our findings, Boyages et al. [23] reported on 94 patients with MF and MC breast cancer using the same definition that we did, although MF and MC tumors were grouped together. For patients with tumors larger than 2 cm, the 10-year survival was 54.7% for MF and MC when compared with 72.1% for UF (P = 0.008), an impact that persisted on multivariate analysis (RR 1.91, P = 0.012). Weissenbacher et al. [11] compared 288 patients with MF and MC tumors based on clinical and radiographic findings with a cohort of matched UF controls and found that these patients had statistically significantly worse RFS (HR 1.74) and BCSS (HR 1.57) on multivariate analysis. Finally, in 1154 patients with two or more invasive lesions anywhere in the same breast, Yerushalmi et al. [1] found a 3.4% decrease in BCSS, but not OS, at 10 years when compared with the UF group. These findings held up on multivariate analysis with a slightly increased relative risk of death from breast cancer of 1.174 which was statistically significant.
There are several explanations for the variability in findings. Boyages et al. found a difference in survival outcomes only in MF and MC tumors >2 cm, suggesting that there may be a difference in the behavior of macroscopically evident MF and MC tumors and those only microscopically apparent. Similarly, all the patients in Weissenbacher et al.'s cohort had MF or MC disease that was large enough to be clinically and/or radiographically detected, again suggesting that only larger MF and MC tumors are clinically significant and affect survival. An alternative explanation is that the lower event rate for smaller tumors resulted in decreased statistical power. Yerushalmi et al. used similar definitions to our study and, in a very large cohort of patients (25 320), found a low incidence of MF and MC tumors (6.1%, in contrast to our 24%). They noted an increase in axillary lymph node involvement and lymphovascular invasion in MF and MC tumors, but in contrast to our study, they did not see an association with increased T stage or grade 3 disease. These differences in the patient population may explain why they detected a very small independent association between MF and MC breast cancer and BCSS.
Our study and many others have shown an association between MF and MC breast cancer and the increased incidence and burden of regional lymph node metastases. This association begs the question of whether the overall tumor burden of MF and MC tumors is simply underestimated with the current staging system, or whether MF and MC tumors have an inherently more aggressive biology that causes them to grow and metastasize at a faster rate. Andea et al. [7] and Coombs et al. [9] both found that when T stage was assigned by the diameter of the largest lesion, multifocality and multicentricity was an independent predictor of axillary lymph node involvement. However, re-assigning the T stage based on the combined diameter of all of the foci corrected this disparity and the rate of lymph node metastases between MF and MC and UF tumors became equal. These findings suggest that the increase in lymph node involvement (and any associated worse outcomes) is not due to the inherent nature of MF and MC tumors but to the underestimation of the disease burden by the current staging system. In a later analysis, Andea et al. [8] argued that using the sum of the largest diameters actually overestimates the total tumor burden, and that better measures of a tumor's propensity to metastasize are total tumor volume and surface area, since the surface of the tumor is the region shedding cells for dissemination. When tumors were reclassified according to this model, MF and MC tumors still had an increased rate of lymph node involvement, suggesting that the difference was not due to understaging, but rather to fundamentally more aggressive tumor biology. Two studies have looked at the relationship between different methods of T staging and survival. Boyages et al. [23] found that for tumors larger than 2 cm, MF and MC cancers had a worse 10-year BCSS when compared with UF cancer, but the difference was eliminated by assigning a T stage to MF and MC tumors based on the aggregate diameter. In contrast, Rezo et al. [13] performed a multivariate analysis with three different measurements of T staging (single largest diameter, aggregate diameter, and aggregate volume), and found that using the single largest diameter was the best predictor of PFS and OS. While evaluating other methods of T staging for MF and MC tumors may shed light on their biology, our study suggests that, at this time, there is no justification for changing our current staging system.
In our study, MF and MC tumors were evaluated as separate entities as well as in combination. Patient characteristics between the two groups were similar, but the distinction did reveal a difference in the clinical behavior of MC tumors, with a statistically significantly shorter RFS and BCSS and a trend toward shorter OS when compared with both MF and UF tumors. While this association was no longer evident after controlling for other risk factors on multivariate analysis, it suggests that the underlying pathophysiology of MC tumors may be distinct. Future studies to look at the molecular profiles of separate tumor foci in the same breast may shed light on this issue, and also provide clinically relevant information to guide treatment decisions.
This study is retrospective in nature, and therefore subject to inherent biases. There was some variability in the treatments received by the two cohorts, especially in regard to their local therapy. The MF and MC patients were more likely to undergo mastectomy as opposed to breast-conserving therapy with adjuvant radiation. They were also more likely to receive adjuvant chemotherapy. This may explain why MF and MC had similar survival outcomes despite being associated with a number of more aggressive features; however, the use of adjuvant chemotherapy was included as a prognostic factor on multivariate analysis, and there was still no difference in RFS, BCSS, or OS. Another limitation of this study is the median follow-up of only 4.5 years. About 74.3% of the patients had HR-positive (and Her2/Neu normal) disease and, therefore, many recurrence events may occur after 5 years. An unplanned exploratory analysis of 1634 patients (MF = 302, MC = 106, UF = 1226) with ≥5 years of follow-up (median 88.6 months, range 60–162 months) yielded similar results, with a statistically significantly shorter RFS, BCSS, and OS for MC patients on univariate analysis but no difference in outcomes after the other risk factors were controlled for using multivariate analysis (data not shown). The optimal local therapy for MF and MC tumors is not well defined, and ongoing studies are evaluating the role of breast conserving surgery, sentinel lymph node biopsy, and adjuvant radiation therapy. A standardized method of classifying MF and MC breast cancers and more information about their molecular profiles would help guide their management.
In summary, we report that MF and MC breast cancers are associated with a number of more aggressive features, including an increased rate of regional lymph node metastases. However, when these factors are controlled for, they do not have a worse RFS, BCSS, or OS. This supports the current staging convention of using the diameter of the largest lesion only to assign T stage for MF and MC breast cancers.
funding
National Cancer Institute (1K23CA121994-01); National Cancer Institute through The University of Texas MD Anderson Cancer Center (P30 CA016672). The MD Anderson Breast Cancer Management System and the Breast Tumor Bank is supported in part by the Nelly B. Connally Breast Cancer Research Fund.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Yerushalmi R, Kennecke H, Woods R, et al. Does multicentric/multifocal breast cancer differ from unifocal breast cancer? An analysis of survival and contralateral breast cancer incidence. Breast Cancer Res Treat. 2009;117:365–370. doi: 10.1007/s10549-008-0265-1. doi:10.1007/s10549-008-0265-1. [DOI] [PubMed] [Google Scholar]
- 2.Egan RL. Multicentric breast carcinomas: clinical-radiographic-pathologic whole organ studies and 10-year survival. Cancer. 1982;49:1123–1130. doi: 10.1002/1097-0142(19820315)49:6<1123::aid-cncr2820490610>3.0.co;2-r. doi:10.1002/1097-0142(19820315)49:6<1123::AID-CNCR2820490610>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson LS, Given-Wilson R, Hall T, et al. Increasing the diagnosis of multifocal primary breast cancer by the use of bilateral whole-breast ultrasound. Clin Radiol. 2005;60:573–578. doi: 10.1016/j.crad.2004.10.015. doi:10.1016/j.crad.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in Fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol. 2004;183:1149–1157. doi: 10.2214/ajr.183.4.1831149. [DOI] [PubMed] [Google Scholar]
- 5.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. doi:10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 6.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. doi:10.1002/1097-0142(19890101)63:1<181::AID-CNCR2820630129>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Andea AA, Wallis T, Newman LA, et al. Pathologic analysis of tumor size and lymph node status in multifocal/multicentric breast carcinoma. Cancer. 2002;94:1383–1390. doi: 10.1002/cncr.10331. doi:10.1002/cncr.10331. [DOI] [PubMed] [Google Scholar]
- 8.Andea AA, Bouwman D, Wallis T, et al. Correlation of tumor volume and surface area with lymph node status in patients with multifocal/multicentric breast carcinoma. Cancer. 2004;100:20–27. doi: 10.1002/cncr.11880. doi:10.1002/cncr.11880. [DOI] [PubMed] [Google Scholar]
- 9.Coombs NJ, Boyages J. Multifocal and multicentric breast cancer: does each focus matter? J Clin Oncol. 2005;23:7497–7502. doi: 10.1200/JCO.2005.02.1147. doi:10.1200/JCO.2005.02.1147. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen L, Gunnarsdottir KA, Rasmussen BB, et al. The prognostic influence of multifocality in breast cancer patients. Breast. 2004;13:188–193. doi: 10.1016/j.breast.2003.11.004. doi:10.1016/j.breast.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Weissenbacher TM, Zschage M, Janni W, et al. Multicentric and multifocal versus unifocal breast cancer: is the tumor-node-metastasis classification justified? Breast Cancer Res Treat. 2010;122:27–34. doi: 10.1007/s10549-010-0917-9. doi:10.1007/s10549-010-0917-9. [DOI] [PubMed] [Google Scholar]
- 12.Cabioglu N, Ozmen V, Kaya H, et al. Increased lymph node positivity in multifocal and multicentric breast cancer. J Am Coll Surg. 2009;208:67–74. doi: 10.1016/j.jamcollsurg.2008.09.001. doi:10.1016/j.jamcollsurg.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Rezo A, Dahlstrom J, Shadbolt B, et al. Tumor size and survival in multicentric and multifocal breast cancer. Breast. 2011;20:259–263. doi: 10.1016/j.breast.2011.01.005. doi:10.1016/j.breast.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Tot T, Gere M, Pekar G, et al. Breast cancer multifocality, disease extent, and survival. Hum Pathol. 2011;42:1761–1769. doi: 10.1016/j.humpath.2011.02.002. doi:10.1016/j.humpath.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC, Fritz AG, et al. AJCC Cancer Staging Manual; New York: Springer. 2009. [Google Scholar]
- 16.Fisher ER, Gregorio R, Redmond C, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol no. 4). I. Observations concerning the multicentricity of mammary cancer. Cancer. 1975;35:247–254. doi: 10.1002/1097-0142(197501)35:1<247::aid-cncr2820350130>3.0.co;2-s. doi:10.1002/1097-0142(197501)35:1<247::AID-CNCR2820350130>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Rakovitch E, Pignol JP, Hanna W, et al. Significance of multifocality in ductal carcinoma in situ: outcomes of women treated with breast-conserving therapy. J Clin Oncol. 2007;25:5591–5596. doi: 10.1200/JCO.2007.11.4686. doi:10.1200/JCO.2007.11.4686. [DOI] [PubMed] [Google Scholar]
- 18.Katz A, Strom EA, Buchholz TA, et al. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735–742. doi: 10.1016/s0360-3016(01)01500-0. doi:10.1016/S0360-3016(01)01500-0. [DOI] [PubMed] [Google Scholar]
- 19.Gupta D, Nath M, Layfield LJ. Utility of four-quadrant random sections in mastectomy specimens. Breast J. 2003;9:307–311. doi: 10.1046/j.1524-4741.2003.09411.x. doi:10.1046/j.1524-4741.2003.09411.x. [DOI] [PubMed] [Google Scholar]
- 20.Vlastos G, Rubio IT, Mirza NQ, et al. Impact of multicentricity on clinical outcome in patients with T1–2, N0–1, M0 breast cancer. Ann Surg Oncol. 2000;7:581–587. doi: 10.1007/BF02725337. [DOI] [PubMed] [Google Scholar]
- 21.Joergensen LE, Gunnarsdottir KA, Lanng C, et al. Multifocality as a prognostic factor in breast cancer patients registered in Danish Breast Cancer Cooperative Group (DBCG) 1996–2001. Breast. 2008;17:587–591. doi: 10.1016/j.breast.2008.06.004. doi:10.1016/j.breast.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Litton JK, Eralp Y, Gonzalez-Angulo AM, et al. Multifocal breast cancer in women ≤35 years old. Cancer. 2007;110:1445–1450. doi: 10.1002/cncr.22928. doi:10.1002/cncr.22928. [DOI] [PubMed] [Google Scholar]
- 23.Boyages J, Jayasinghe UW, Coombs N. Multifocal breast cancer and survival: each focus does matter particularly for larger tumours. Eur J Cancer. 2010;46:1990–1996. doi: 10.1016/j.ejca.2010.03.003. doi:10.1016/j.ejca.2010.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


