Abstract
Background
Radiotherapy for breast cancer may expose the esophagus to ionizing radiation, but no study has evaluated esophageal cancer risk after breast cancer associated with radiation dose or systemic therapy use.
Design
Nested case–control study of esophageal cancer among 289 748 ≥5-year survivors of female breast cancer from five population-based cancer registries (252 cases, 488 individually matched controls), with individualized radiation dosimetry and information abstracted from medical records.
Results
The largest contributors to esophageal radiation exposure were supraclavicular and internal mammary chain treatments. Esophageal cancer risk increased with increasing radiation dose to the esophageal tumor location (Ptrend < 0.001), with doses of ≥35 Gy associated with an odds ratio (OR) of 8.3 [95% confidence interval (CI) 2.7–28]. Patients with hormonal therapy ≤5 years preceding esophageal cancer diagnosis had lower risk (OR = 0.4, 95% CI 0.2–0.8). Based on few cases, alkylating agent chemotherapy did not appear to affect risk. Our data were consistent with a multiplicative effect of radiation and other esophageal cancer risk factors (e.g. smoking).
Conclusions
Esophageal cancer is a radiation dose-related complication of radiotherapy for breast cancer, but absolute risk is low. At higher esophageal doses, the risk warrants consideration in radiotherapy risk assessment and long-term follow-up.
Keywords: breast cancer, esophageal cancer, radiotherapy, second cancer
introduction
Breast cancer is the most frequently diagnosed cancer among women worldwide [1]. In recent decades, the prognosis for breast cancer patients has improved considerably [2–4]. The resulting large population of long-term breast cancer survivors is at risk for subsequent malignancies associated with late effects of treatment.
The occurrence of esophageal cancer among breast cancer survivors is of particular concern because the esophagus is within or near the border of several radiotherapy fields commonly used to treat breast cancer [2]. Previous studies of second cancers in breast cancer survivors have documented increased esophageal cancer risks [2, 5–20], possibly related to radiotherapy [2, 5, 7, 8, 10, 16, 17, 20, 21]. However, no study has quantified the radiation dose–response relation for esophageal cancer, evaluated the risk associated with other breast cancer treatments (chemotherapy, hormonal agents), or investigated modification of the radiation-related risk by chemotherapy, hormonal agents, or other esophageal cancer risk factors [22–24].
We conducted a multi-center nested case–control study within a cohort of 289 748 breast cancer patients (diagnosed 1946–1996), collecting detailed treatment and esophageal cancer risk factor data to provide new insights into esophageal cancer risk following the treatment of breast cancer [25].
methods
study design and patients
Women who survived ≥5 years following first primary, histologically confirmed invasive breast cancer or ductal carcinoma in situ (DCIS) reported to population-based cancer registries in Denmark (1943–1999), Finland (1953–2002), Sweden (1958–2002), Iowa (USA, 1973–2001), or Ontario (Canada, 1964–2003) were potentially eligible (n = 289 748). Registry reports identified cases with second primary invasive esophageal cancer ≥5 years after breast cancer. For cases with available medical records, two controls per case were identified by stratified random sampling from the cohort, individually matching by registry, race (Iowa/Ontario), birth date (±5 years), breast cancer diagnosis date (±5 years), and survival without subsequent cancer at least as long as the matched case's interval from breast cancer to esophageal cancer. The final study population included 252 cases and 488 matched controls (for additional details, see supplementary methods, available at Annals of Oncology online). The study was approved by each study center's institutional review board and exempted from review by the National Cancer Institute because analyses used only existing, de-identified data.
data collection and review
Detailed data from hospital, clinic, radiotherapy, physician, and cancer registry records were abstracted onto standardized forms. Information was collected on demographics, breast cancer diagnosis and treatment, esophageal cancer risk factors (smoking, alcohol, height, weight, and family history of cancer), and, for cases, esophageal cancer diagnosis.
Radiotherapy data were obtained from full treatment records, treatment summaries, radiotherapy notes, and treatment planning information. Detailed data were available for 411/452 (91%) patients receiving radiotherapy. Abstracted radiotherapy details included dates of administration, reason for treatment (primary or recurrence), beam energy, dose delivered, and field location and configuration. Information on chemotherapy and hormonal agents included specific drugs/regimens and dates and the duration of administration or number of cycles, including all treatments given before the date of esophageal cancer diagnosis (comparable date for controls). Drug doses were not collected.
Abstracted data on cigarette smoking and alcohol consumption included amount and status (current use at the time of medical record report or year quit). Former smokers were identified by medical record reports indicating that the patient quit ≥5 years preceding esophageal cancer diagnosis (comparable date for controls). Data on family history of cancer in first-degree relatives included relationship to the patient and cancer type. To minimize potential bias arising from more complete information on smoking, alcohol, and family history of cancer for cases than controls, only data recorded at least one year preceding esophageal cancer diagnosis (comparable date for controls) were used. Body mass index (BMI, kg/m2) was computed at breast cancer diagnosis. Data on smoking, alcohol, family history of cancer, and BMI were available for 42%, 23%, 47%, and 66% of cases, respectively, and 44%, 27%, 42%, and 54% of controls, respectively.
For cases, pathology, radiology, surgery, endoscopy, hospital, clinic, and registry records were reviewed to confirm esophageal cancer diagnoses. Tumor location data (proximal/distal ends, length) were abstracted from endoscopy reports and imaging studies and translated to bony landmarks for dosimetry.
radiation dosimetry
Radiation doses to the esophagus and gastroesophageal (GE) junction were estimated using a custom-designed dose program, based on measurements in water and anthropomorphic phantoms constructed of tissue-equivalent material [26]. Dosimetry was based on individual patient's fields and dose information abstracted from radiotherapy records.
Women in our study population were treated with medial and lateral tangential or direct chest radiotherapy fields (including boosts), with/without supraclavicular, direct internal mammary chain (IMC), and axillary fields (Figure 1), with 73% of women receiving radiation following modified or radical mastectomy. Beam energies included cobalt-60 gamma rays, orthovoltage X-rays (100–400 kVp), photons (4–18 MV), and electrons (6–20 MeV). Cumulative target doses to the breast and/or peripheral regions were typically 40–50 Gy, using conventional fractionation. We reviewed the location of the supraclavicular field relative to midline for each patient due to its variable contribution to the esophagus dose (Figure 1). Doses were calculated for each patient at 24 positions, each located centrally in the esophagus, anterior to the midpoint of each vertebrae and intervertebral disc from C6 (depth of 3.6 cm from the skin surface) to T10 (depth: 11.2 cm), plus the GE junction (T10/T11, 2 cm left of midline, depth: 10.7 cm ). Esophagus depths were derived from CT scans of normal BMI females. The total radiation dose at each position along the esophagus was computed by summing doses from all treatments received ≥5 years preceding esophageal cancer diagnosis (comparable date for controls); only 18 patients received radiotherapy within 5 years of esophageal cancer diagnosis. Analyses of radiotherapy risks used dose to the esophageal tumor midpoint (comparable location for controls); for the 12 (5%) cases with unknown tumor location, analyses used dose to the esophagus midpoint (T6/7–T7/8).
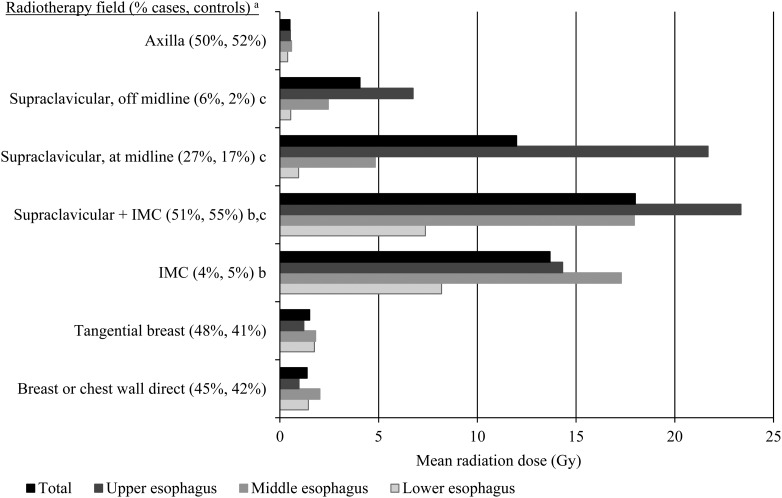
Figure 1.
Mean radiation dose to the esophagus, by region of the esophagus, for specific breast cancer radiotherapy fields. IMC, internal mammary chain. The upper, middle, and lower esophagus were defined as C6-T4, T4/5-T7/8, and T8-T10/11, respectively. aPercentage of cases and controls with radiotherapy who received a particular field, excluding women with unknown fields. Beam energies included cobalt-60 gamma rays (53%), orthovoltage x-rays (100–400 kVp; 36%), photons (4–18 MV; 18%), and electrons (6–20 MeV; 21%). bMost IMC fields were direct anterior fields. cWe categorized the location of each patient's supraclavicular fields relative to midline as at/over midline, off–midline, or unknown due to the variable contribution to the esophagus dose. The supraclavicular field at/over midline was positioned so that the esophagus was directly below the medial field edge on the patient skin surface. The supraclavicular field off-midline was positioned so that the esophagus was approximately 2 cm outside the medial field edge. Supraclavicular fields with unknown medial border were positioned at midline. Supraclavicular fields were simulated as direct beams.
statistical analysis
The relative risk of esophageal cancer was estimated using odds ratios (ORs) derived from conditional logistic regression analyses, comparing cases' exposure histories with those of individually matched controls, using the software package Epicure [27]. The general model for most analyses was as follows: OR = exp(∑jαjxj)[1 + βz], where z is radiation dose in Gy, β is the excess OR per Gray (EOR/Gy), the xj are variables measuring chemotherapy, hormone therapy, or other risk factors, and the OR for xj is given by exp(αjxj). This linear dose–response model has been used extensively in epidemiologic evaluations of radiation risks [28]. Two-sided P-values and 95% confidence intervals (CI) were based on the likelihood ratio statistic. Missing data were handled by including indicator variables.
To calculate absolute excess risk after 25 years, we used non-Hispanic white age-specific esophageal cancer rates from Surveillance, Epidemiology, and End Results (SEER) registries [29] to estimate the number of esophageal cancers expected in the absence of radiation exposure, taking account of competing risks in the US female general population. These estimates were then multiplied by the dose of interest and the EOR/Gy. Results using esophageal cancer rates from the other international cancer registries in this study were similar (results not shown). The attributable risk was calculated by summing the quantities E/(1 + E) over all cases, where E = dose × EOR/Gy.
Additional details on the statistical analysis are provided in supplementary methods, available at Annals of Oncology online.
results
Median age at breast cancer diagnosis was 59 years (range, 28–88 years), and over half the patients were diagnosed in 1975 or later (Table 1). During the study period, the use of breast-conserving surgery, chemotherapy, and hormonal agents increased, whereas the use of radiotherapy decreased. Nearly all women (96% cases, 92% controls) were in clinical remission from breast cancer at the time of esophageal cancer diagnosis (comparable date for controls). The median interval between breast cancer and esophageal cancer was 13 years (range, 5–37 years). The esophageal cancers diagnosed were predominantly squamous cell carcinoma (71%) and occurred more often in the upper or middle than lower esophagus. Overall survival after esophageal cancer diagnosis was poor [232/252 (92%) died; median, 6 months; range, 0–11 years].
Table 1.
Characteristics of women surviving ≥5 years after breast cancer diagnosis who subsequently developed esophageal cancer (cases, n = 252), and matched controls (n = 488)a
| Characteristic | Cases, n (%) | Controlsa, n (%) |
|---|---|---|
| Registrya | ||
| Denmark | 26 (10.3) | 38 (7.8) |
| Finland | 40 (15.9) | 79 (16.2) |
| Iowa | 16 (6.3) | 32 (6.6) |
| Ontario | 90 (35.7) | 179 (36.7) |
| Sweden | 80 (31.7) | 160 (32.8) |
| Age at breast cancer diagnosis (years)* | ||
| 28–49 | 68 (27.0) | 126 (25.8) |
| 50–59 | 60 (23.8) | 122 (25.0) |
| 60–69 | 69 (27.4) | 138 (28.3) |
| 70–88 | 55 (21.8) | 102 (20.9) |
| Calendar year of breast cancer diagnosisa | ||
| 1946–1964 | 22 (8.7) | 40 (8.2) |
| 1965–1974 | 90 (35.7) | 178 (36.5) |
| 1975–1984 | 85 (33.7) | 164 (33.6) |
| 1985–1996 | 55 (21.8) | 106 (21.7) |
| Breast cancer histology | ||
| DCIS | 3 (1.2) | 10 (2.0) |
| Invasive ductal carcinoma | 188 (74.6) | 379 (77.7) |
| Invasive lobular carcinoma | 12 (4.8) | 23 (4.7) |
| Other specified invasive histologyb | 23 (9.1) | 37 (7.6) |
| Unspecified | 26 (10.3) | 39 (8.0) |
| Breast cancer stage | ||
| DCIS | 3 (1.2) | 10 (2.0) |
| I | 98 (38.9) | 193 (39.5) |
| II | 99 (39.3) | 202 (41.4) |
| III/IV | 9 (3.6) | 13 (2.7) |
| Localized/regionalc | 43 (17.1) | 70 (14.3) |
| Initial surgery for breast cancer | ||
| Modified/radical mastectomy | 195 (77.4) | 362 (74.2) |
| Partial mastectomy (breast-conserving surgery) | 34 (13.5) | 78 (16.0) |
| Mastectomy, not otherwise specified | 21 (8.3) | 42 (8.6) |
| Other | 2 (0.8) | 6 (1.2) |
| Non-surgical breast cancer treatment categoryd | ||
| No chemotherapy or radiotherapy | 77 (30.6) | 173 (35.5) |
| Radiotherapy only | 152 (60.3) | 265 (54.3) |
| Chemotherapy and radiotherapy | 15 (6.0) | 20 (4.1) |
| Chemotherapy only | 4 (1.6) | 20 (4.1) |
| Unknown | 4 (1.6) | 10 (2.0) |
| Hormonal agentse | ||
| No | 198 (78.6) | 364 (74.6) |
| Yes | 49 (19.4) | 113 (23.2) |
| Unknown | 5 (2.0) | 11 (2.3) |
| Cigarette smoking statusf | ||
| Non-smoker | 47 (44.8) | 125 (58.4) |
| Former smoker | 12 (11.4) | 25 (11.7) |
| Current smoker <1 pack/day | 23 (21.9) | 46 (21.5) |
| Current smoker ≥1 pack/day | 23 (21.9) | 18 (8.4) |
| Unknown | 147 (—) | 274 (—) |
| Alcohol consumptionf,g | ||
| Non-drinker | 16 (27.6) | 61 (45.9) |
| Light drinker | 16 (27.6) | 50 (37.6) |
| Moderate drinker | 12 (20.7) | 16 (12.0) |
| Heavy drinker | 14 (24.1) | 6 (4.5) |
| Unknown | 194 (—) | 355 (—) |
| First-degree relative with cancerf | ||
| No | 75 (48.4) | 163 (62.5) |
| Yes | 80 (51.6) | 98 (37.5) |
| Unknown | 97 (—) | 227 (—) |
| BMI (kg/m2)f | ||
| 13.8–18.4 | 7 (5.9) | 11 (5.4) |
| 18.5–24.9 | 68 (57.1) | 107 (52.5) |
| 25.0–29.9 | 30 (25.2) | 57 (27.9) |
| 30.0–48.4 | 14 (11.8) | 29 (14.2) |
| Unknown | 133 (—) | 284 (—) |
| Interval from breast cancer to esophageal cancer diagnosis or comparable date for controls (years)a | ||
| 5–9 | 87 (34.5) | 169 (34.6) |
| 10–14 | 55 (21.8) | 107 (21.9) |
| 15–24 | 79 (31.3) | 155 (31.8) |
| 25–37 | 31 (12.3) | 57 (11.7) |
| Esophageal cancer locationh | ||
| Upper esophagus | 50 (19.8) | — |
| Overlapping upper/middle esophagus | 17 (6.7) | — |
| Middle esophagus | 69 (27.4) | — |
| Overlapping middle/lower esophagus | 50 (19.8) | — |
| Lower esophagus | 39 (15.5) | — |
| Gastroesophageal junction | 15 (6.0) | — |
| Unknown | 12 (4.8) | — |
| Esophageal cancer histologyi | ||
| Squamous cell carcinoma | 178 (70.6) | — |
| Adenocarcinoma | 34 (13.5) | — |
| Other | 30 (11.9) | — |
| Unknownj | 10 (4.0) | — |
| Esophageal cancer stage | ||
| I/II | 103 (40.9) | — |
| III/IV | 93 (36.9) | — |
| Unknown | 56 (22.2) | — |
DCIS, ductal carcinoma in situ.
aControls were individually matched (2:1) to case patients by registry, race (Iowa only), birth date (within 5 years), breast cancer diagnosis date (within 5 years), and survival without a subsequent cancer at least as long as the period from breast cancer to esophageal cancer for the matched case. Only one matched control could be found for 16 cases.
bOther specified invasive breast cancer histologies included medullary carcinoma (9 cases, 11 controls), mucinous carcinoma (4 cases, 11 controls), tubular carcinoma (1 case, 6 controls), and other rarer or mixed histologies (9 cases, 9 controls).
cPatients were categorized as having localized or regional breast cancer when AJCC stages I–IV could not be assigned due to insufficient information. The category included localized (32 cases, 51 controls), regional (7 cases, 13 controls), and unknown (4 cases, 6 controls).
dBreast cancer treatment category includes radiotherapy and chemotherapy received within the matched time interval, including the first course of therapy as well as treatments for recurrence and/or new primary breast cancer. Patients who received a single cycle of chemotherapy prior to radiotherapy (cyclophosphamide: four cases, eight controls; thiotepa: one control) were categorized as receiving no chemotherapy. Patients with radiotherapy but unknown chemotherapy were categorized as receiving radiotherapy only (two controls). Radiotherapy data included external beam therapy and brachytherapy (n = 1).
eMost patients who received hormonal agents received tamoxifen. Only one case and five controls received non-tamoxifen hormonal agents exclusively.
fData on cigarette smoking, alcohol consumption, and family history of cancer were ascertained up to 1 year prior to esophageal cancer diagnosis (or comparable date for controls). BMI was computed from height and weight data within 5 years of breast cancer diagnosis. Percentages exclude patients with unknown values.
gLight, moderate, and heavy alcohol consumption was defined as <7, 7–20, and ≥21 drinks per week, respectively.
hThe upper, middle, and lower esophagus regions were defined as C6-T4, T4/5-T7/8, and T8-T10/11, respectively.
iOf 252 cases, 224 (89%) were histologically confirmed, 13 (5%) had diagnoses highly consistent with esophageal cancer, and for 15 (6%) the possibility of metastatic cancer to the esophagus could not be excluded.
jUnknown esophageal cancer histology included unspecified (one case) and no histologic confirmation (nine cases).
Radiotherapy was administered to 167 (66%) cases and 285 (58%) controls. The supraclavicular region was the most common radiotherapy target, and the supraclavicular field with a medial border at or over the midline resulted in the highest mean doses to the esophagus, particularly the upper region (>15 Gy, Figure 1). IMC irradiation, which became less frequently used during the study period, resulted in relatively high mean doses to the middle esophagus (>15 Gy). Other commonly used radiotherapy fields, including the axillary, tangential breast, and direct fields to the breast or chest wall, delivered lower mean doses to all regions of the esophagus (<2 Gy).
Esophageal cancer risk increased with increasing radiation dose to the esophageal tumor location (Ptrend < 0.001, Table 2). Compared with women who received no radiation dose to the esophageal tumor location, risk was 8.3-fold increased when doses reached ≥35 Gy (95% CI 2.7–28). There was little evidence of increased risk with doses <20 Gy, though the dose–response relation was compatible with linearity (EOR/Gy = 0.09, 95% CI 0.04–0.16), as evidenced by comparing the linear model with the categorical model in Table 2 (P = 0.39) or a linear-quadratic model (P = 0.08). Radiation-related risks were similar for esophageal squamous cell carcinoma and adenocarcinoma, although the trend for adenocarcinoma was not significant (P = 0.16). Overall, we estimate that 75/252 (30%) of the esophageal cancer cases in this study can be attributed to radiotherapy, but among women with a dose of ≥20 Gy to the esophageal tumor location, 51/71 (72%) can be attributed to radiotherapy. Nevertheless, the absolute risk of esophageal cancer is low: among 1000 women aged 60 at breast cancer diagnosis receiving an esophagus radiation dose of 30 Gy, an excess of five esophageal cancers due to radiation might be expected over 25 years, though this excess would likely vary by other esophageal cancer risk factors.
Table 2.
Risk of esophageal cancer following breast cancer according to breast cancer treatment, overall and by esophageal cancer histology
| Risk factor | Cases | Controls | OR (95% CI) | By esophageal cancer histology |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Squamous cell carcinoma |
Adenocarcinoma |
||||||||
| Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI) | ||||
| Radiation dose to specific esophageal tumor location (Gy) | |||||||||
| 0a | 84 | 201 | 1.0 (referent) | 55 | 138 | 1.0 (referent) | 17 | 37 | 1.0 (referent) |
| 0.1–4.9 | 49 | 112 | 0.9 (0.6–1.5) | 32 | 73 | 1.0 (0.5–1.7) | 9 | 20 | 1.0 (0.3–2.9) |
| 5.0–9.9 | 21 | 38 | 1.2 (0.6–2.4) | 13 | 25 | 1.2 (0.5–2.7) | 2 | 2 | 1.7 (0.3–8.8) |
| 10.0–19.9 | 15 | 36 | 1.0 (0.5–2.2) | 11 | 27 | 1.0 (0.4–2.4) | 1 | 3 | ∼ |
| 20.0–24.9 | 16 | 19 | 2.4 (1.1–5.5) | 12 | 15 | 2.3 (0.9–6.1) | 1 | 0 | ∼ |
| 25.0–29.9 | 25 | 27 | 3.3 (1.7–6.7) | 20 | 23 | 2.9 (1.3–6.4) | 1 | 0 | ∼ |
| 30.0–34.9 | 19 | 17 | 5.7 (2.3–15) | 19 | 15 | 7.5 (2.8–24) | 0 | 1 | ∼ |
| 35.0–44.5 | 11 | 7 | 8.3 (2.7–28) | 9 | 6 | 8.8 (2.4–37) | 0 | 0 | ∼ |
| Ptrend | <0.001 | <0.001 | 0.16 | ||||||
| Unknown b | 12 | 31 | 0.9 (0.4–2.0) | 7 | 22 | 0.8 (0.3–2.0) | 3 | 4 | 2.8 (0.3–31) |
| Chemotherapyc | |||||||||
| No chemotherapy | 231 | 443 | 1.0 (referent) | 163 | 314 | 1.0 (referent) | 31 | 60 | 1.0 (referent) |
| Any alkylating agent chemotherapy d | 18 | 33 | 1.0 (0.5–1.9) | 14 | 21 | 1.2 (0.5–2.8) | 2 | 6 | 0.7 (0.1–3.4) |
| By duration (cycles) | |||||||||
| 2–6 | 6 | 12 | 0.9 (0.3–2.6) | 6 | 8 | 1.5 (0.4–5.1) | 0 | 2 | ∼ |
| >6 | 12 | 21 | 1.0 (0.5–2.2) | 8 | 13 | 1.1 (0.4–2.9) | 2 | 4 | 0.9 (0.1–5.3) |
| Ptrend | >0.5 | >0.5 | >0.5 | ||||||
| By regimene | |||||||||
| Any CAF/CEF | 6 | 10 | 0.9 (0.3–3.0) | 4 | 7 | 0.8 (0.2–3.4) | 1 | 2 | ∼ |
| CMF | 11 | 19 | 1.0 (0.4–2.4) | 10 | 11 | 1.7 (0.6–4.6) | 0 | 3 | ∼ |
| Melphalan | 1 | 4 | ∼ | 0 | 3 | ∼ | 1 | 1 | ∼ |
| Non-alkylating agent chemotherapy only | 0 | 5 | ∼ | 0 | 4 | ∼ | 0 | 0 | ∼ |
| Unknownf | 3 | 7 | 1.0 (0.2–3.7) | 1 | 5 | ∼ | 1 | 1 | ∼ |
| Hormonal agentsc,g | |||||||||
| No | 198 | 364 | 1.0 (referent) | 145 | 261 | 1.0 (referent) | 22 | 44 | 1.0 (referent) |
| Yes | 49 | 113 | 0.7 (0.4–1.1) | 30 | 77 | 0.6 (0.3–1.0) | 11 | 20 | 1.1 (0.3–3.6) |
| By duration (months) | |||||||||
| 1–23 | 12 | 27 | 0.8 (0.4–1.5) | 5 | 19 | 0.4 (0.1–1.0) | 5 | 4 | 2.9 (0.6–17) |
| 24–35 | 7 | 19 | 0.5 (0.2–1.2) | 5 | 13 | 0.5 (0.1–1.4) | 2 | 4 | 0.8 (0.1–5.0) |
| 36–59 | 11 | 24 | 0.8 (0.3–1.6) | 6 | 17 | 0.6 (0.2–1.4) | 2 | 4 | 1.2 (0.1–8.5) |
| 60–173 | 15 | 32 | 0.8 (0.4–1.6) | 11 | 20 | 0.9 (0.4–2.1) | 2 | 7 | 0.5 (0.0–4.9) |
| Ptrend | >0.5 | >0.5 | 0.36 | ||||||
| Unknown duration | 4 | 11 | 0.6 (0.2–2.0) | 3 | 8 | 0.6 (0.1–2.3) | 0 | 1 | ∼ |
| By time period before esophageal cancer diagnosis (years)h | |||||||||
| 0–4 | 24 | 77 | 0.4 (0.2–0.8) | 14 | 51 | 0.3 (0.1–0.7) | 6 | 16 | 0.6 (0.1–2.4) |
| 5–9 | 30 | 57 | 1.1 (0.5–2.2) | 21 | 34 | 1.5 (0.6–4.1) | 6 | 14 | 0.8 (0.2–3.9) |
| 10–14 | 15 | 18 | 2.5 (0.8–8.4) | 11 | 10 | 3.6 (0.8–27) | 3 | 3 | 1.2 (0.0–46) |
| 15–32 | 6 | 11 | 0.5 (0.1–2.3) | 3 | 7 | 0.3 (0.0–2.2) | 2 | 1 | ∼ |
| Unknown | 5 | 11 | 1.0 (0.3–2.9) | 3 | 6 | 1.1 (0.2–4.3) | 0 | 1 | ∼ |
CI, confidence interval; OR, odds ratio.
∼Risk estimates are not presented for cells containing fewer than two patients.
aIncludes patients whose radiotherapy was received entirely within 5 years of esophageal cancer diagnosis or comparable date for controls (two cases, six controls).
bIncludes patients with unknown radiotherapy (3 cases, 8 controls) and patients who received radiotherapy but had insufficient information for dose estimation (9 cases, 23 controls).
cRisk estimates for chemotherapy and hormonal agents were adjusted for continuous radiation dose.
dPatients who received a single cycle of chemotherapy prior to radiotherapy (cyclophosphamide: four cases, eight controls; thiotepa: one control) were categorized as receiving no chemotherapy.
eChemotherapy categories are mutually exclusive. CAF/CEF includes cyclophosphamide, 5-fluorouracil, and doxorubicin, epirubicin, or mitoxantrone. CMF includes cyclophosphamide, 5-fluorouracil, and methotrexate.
fIncludes patients with unknown chemotherapy (two cases, five controls) and patients who received chemotherapy but had no further information on the agents received (one case, two controls).
gMost patients who received hormonal agents received tamoxifen. Only one case and five controls received non-tamoxifen hormonal agents exclusively.
hAnalyses accounting for time period refer to the use of any hormonal agents during specific time periods prior to esophageal cancer diagnosis for cases or comparable date for controls. For 26 cases and 55 controls, hormonal agents were received in more than one interval.
Alkylating agent-containing chemotherapy was not associated with esophageal cancer risk, regardless of the duration or specific chemotherapy regimen, among the few women in our study who received such chemotherapy (18 cases, 33 controls; Table 2). Women receiving hormonal agents (mainly tamoxifen) had lower risk of esophageal cancer, primarily when treated in the 5 years preceding esophageal cancer diagnosis (comparable date for controls; OR = 0.4, 95% CI 0.2–0.8; Table 2). The duration of hormonal agent use was not associated with esophageal cancer risk (Ptrend > 0.5). Multivariate analyses that included variables for all three treatments gave very similar results (not shown).
In analyses of other esophageal cancer risk factors, risk was increased for current, heavy cigarette smoking (OR = 2.4, 95% CI 1.1–5.7) and for moderate (OR = 3.1, 95% CI 1.1–8.5) or heavy (OR = 6.9, 95% CI 2.1–25) alcohol consumption (Table 3), with the alcohol excess limited to squamous cell carcinomas. Family history of cancer increased esophageal cancer risk, particularly family history of lung cancer (OR = 6.3, 95% CI 2.0–24; Table 3). As BMI increased, risk of esophageal squamous cell carcinoma decreased (Ptrend = 0.02, Table 3), whereas risk of adenocarcinoma increased (Ptrend = 0.002). There was little indication that esophageal cancer risk factors confounded treatment-related risks: inclusion of these risk factors in multivariate models did not materially alter the estimated treatment-related risks, whether based on all data or based only on patients with data on the risk factor of interest (results not shown).
Table 3.
Risk of esophageal cancer following breast cancer according to esophageal cancer risk factors, overall and by esophageal cancer histology
| Risk factora | Cases | Controls | OR (95% CI)b | By esophageal cancer histology |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Squamous cell carcinoma |
Adenocarcinoma |
||||||||
| Cases | Controls | OR (95% CI)b | Cases | Controls | OR (95% CI)b | ||||
| Cigarette smoking status | |||||||||
| Non-smoker | 47 | 125 | 1.0 (referent) | 29 | 85 | 1.0 (referent) | 11 | 23 | 1.0 (referent) |
| Former smoker | 12 | 25 | 1.0 (0.4–2.3) | 8 | 17 | 0.9 (0.3–2.6) | 1 | 5 | |
| Current smoker <1 pack/day | 23 | 46 | 0.8 (0.4–1.6) | 20 | 33 | 0.9 (0.4–2.1) | 1 | 6 | |
| Current smoker ≥1 pack/day | 23 | 18 | 2.4 (1.1–5.7) | 17 | 14 | 2.6 (0.9–7.5) | 4 | 2 | 4.5 (0.7–40) |
| Ptrend | 0.20 | 0.22 | 0.46 | ||||||
| Unknown | 147 | 274 | 1.0 (0.6–1.7) | 104 | 195 | 1.0 (0.5–2.0) | 17 | 31 | 1.3 (0.4–4.0) |
| Alcohol consumptionc | |||||||||
| Non-drinker | 16 | 61 | 1.0 (referent) | 8 | 43 | 1.0 (referent) | 5 | 14 | 1.0 (referent) |
| Light drinker | 16 | 50 | 1.0 (0.4–2.4) | 12 | 39 | 1.3 (0.4–4.0) | 1 | 2 | |
| Moderate drinker | 12 | 16 | 3.1 (1.1–8.5) | 8 | 13 | 4.4 (1.2–16) | 2 | 2 | 0.9 (0.1–6.5) |
| Heavy drinker | 14 | 6 | 6.9 (2.1–25) | 12 | 4 | 14 (3.1–77) | 0 | 2 | |
| Ptrend | <0.001 | <0.001 | >0.5 | ||||||
| Unknown | 194 | 355 | 2.3 (1.2–4.6) | 138 | 245 | 3.9 (1.6–10) | 26 | 47 | 1.8 (0.5–7.2) |
| First-degree relative with cancer | |||||||||
| By number of relatives | |||||||||
| 0 | 65 | 159 | 1.0 (referent) | 49 | 110 | 1.0 (referent) | 7 | 23 | 1.0 (referent) |
| 1 | 52 | 70 | 1.9 (1.2–3.0) | 40 | 49 | 1.8 (1.0–3.3) | 7 | 9 | 2.6 (0.7–10.4) |
| 2+ | 29 | 31 | 2.8 (1.6–5.2) | 20 | 24 | 2.3 (1.1–4.5) | 3 | 3 | 5.4 (0.7–52) |
| Ptrend | <0.001 | 0.008 | 0.06 | ||||||
| Unknown | 106 | 228 | 1.3 (0.9–1.9) | 69 | 161 | 1.1 (0.7–1.8) | 17 | 32 | 1.8 (0.7–5.0) |
| By cancer typed | |||||||||
| Breast | 22 | 41 | 1.1 (0.6–2.0) | 15 | 28 | 0.9 (0.4–2.0) | 2 | 4 | 1.2 (0.1–8.7) |
| Lung | 13 | 4 | 6.3 (2.0–24) | 8 | 3 | 4.4 (1.1–24) | 4 | 1 | ∼ |
| Upper GI | 13 | 12 | 2.6 (1.0–6.7) | 10 | 10 | 2.2 (0.7–6.5) | 1 | 1 | ∼ |
| Colorectal | 11 | 9 | 2.4 (0.9–6.8) | 8 | 5 | 2.0 (0.6–7.3) | 1 | 2 | ∼ |
| Other | 23 | 39 | 1.1 (0.6–2.0) | 17 | 30 | 1.0 (0.4–2.1) | 4 | 3 | 5.3 (0.8–46) |
| BMI (kg/m2)e | |||||||||
| 13.8–18.4 | 6 | 7 | 1.0 (0.3–3.3) | 0 | 2 | 1.0 (referent) | |||
| 18.5–24.9 | 54 | 75 | 1.0 (referent) | 4 | 19 | ||||
| 25.0–29.9 | 25 | 42 | 0.7 (0.4–1.3) | 2 | 7 | 2.1 (0.2–17) | |||
| 30.0–48.4 | 7 | 22 | 0.4 (0.1–1.0) | 5 | 2 | 20 (2.4–456) | |||
| Ptrend | 0.02 | 0.002 | |||||||
| Unknown | 86 | 198 | 0.5 (0.3–0.9) | 23 | 37 | 4.8 (1.3–23) | |||
BMI, body mass index; CI, confidence interval; OR, odds ratio.
aData on cigarette smoking, alcohol consumption, and family history of cancer were ascertained up to 1 year prior to esophageal cancer diagnosis (or comparable date for controls).
bAll risk estimates were adjusted for continuous radiation dose. Cigarette smoking and alcohol consumption were evaluated in the same analysis, thus each is also adjusted for the other.
cLight, moderate, and heavy alcohol consumption was defined as <7, 7–20, and ≥21 drinks per week, respectively.
dFamily history by cancer type was evaluated in a multivariate analysis. Upper GI cancer includes esophagus, stomach, and pancreas.
eBMI was computed from height and weight data within 5 years of breast cancer diagnosis. Risks are provided by histology only because of the substantially different effects.
Table 4 presents radiation-related esophageal cancer risks (≥20 versus 0–19.9 Gy) for various patient subgroups. Risk peaked 10–24 years after breast cancer and remained non-significantly elevated at ≥25 years. Risk was not strongly related to age at breast cancer or esophageal cancer diagnosis but appeared somewhat higher for women diagnosed at younger ages. Although esophageal cancer risk was slightly higher among women receiving combined alkylating agent-containing chemotherapy and ≥20 Gy radiation, this risk estimate was based on few exposed women. Risks for ≥20 versus 0–19.9 Gy radiation did not differ by type of initial surgery for breast cancer, but patients generally received lower doses to the esophagus following partial mastectomy. Our data were consistent with a multiplicative effect of radiation and other esophageal cancer risk factors. Similar results were observed in analyses considering radiation dose as a continuous rather than categorical variable, with the exception of potential interactions with age at esophageal cancer diagnosis and chemotherapy (Phomogeneity = 0.07 and 0.04, respectively; results not shown).
Table 4.
Odds ratios for risk of esophageal cancer following breast cancer associated with radiation dose to the esophageal tumor location of ≥20 Gy (versus 0–19.9 Gy) by categories of time since radiotherapy, age at initial radiotherapy, age at esophageal cancer diagnosis, chemotherapy and hormonal agent use, type of initial surgery for breast cancer, cigarette smoking, alcohol consumption, family history of cancer, and body-mass index
| Risk factor | Radiation dose to esophageal tumor location 0–19.9 Gy (reference) |
Radiation dose to esophageal tumor location ≥20 Gya |
OR (95% CI)b | Phomogeneity | ||
|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | Cases, n (%) | Controls, n (%) | |||
| Total | 169 (100.0) | 387 (100.0) | 71 (100.0) | 70 (100.0) | 3.5 (2.2–5.9) | |
| Time since breast cancer diagnosis (years) | ||||||
| 5–9 | 71 (42.0) | 148 (38.2) | 15 (21.1) | 16 (22.9) | 2.7 (1.1–7.4) | |
| 10–14 | 40 (23.7) | 86 (22.2) | 15 (21.1) | 13 (18.6) | 5.3 (1.6–24) | |
| 15–24 | 38 (22.5) | 110 (28.4) | 32 (45.1) | 31 (44.3) | 4.3 (2.0–9.9) | |
| 25–37 | 20 (11.8) | 43 (11.1) | 9 (12.7) | 10 (14.3) | 2.5 (0.7–10.3) | >0.5 |
| Age at breast cancer diagnosis (years) | ||||||
| 28–49 | 34 (20.1) | 98 (25.3) | 28 (39.4) | 21 (30.0) | 7.6 (3.0–24) | |
| 50–59 | 43 (25.4) | 90 (23.3) | 14 (19.7) | 19 (27.1) | 1.7 (0.7–4.0) | |
| 60–69 | 44 (26.0) | 102 (26.4) | 23 (32.4) | 24 (34.3) | 3.7 (1.5–11) | |
| 70–88 | 48 (28.4) | 97 (25.1) | 6 (8.5) | 6 (8.6) | 3.4 (0.7–25) | 0.14 |
| Year of breast cancer diagnosis | ||||||
| 1946–1964 | 16 (9.5) | 28 (7.2) | 4 (5.6) | 3 (4.3) | 4.2 (0.5–87) | |
| 1965–1974 | 45 (26.6) | 122 (31.5) | 37 (52.1) | 38 (54.3) | 4.2 (2.1–9.4) | |
| 1975–1984 | 56 (33.1) | 135 (34.9) | 27 (38.0) | 26 (37.1) | 3.2 (1.5–7.2) | |
| 1985–1996 | 52 (30.8) | 102 (26.4) | 3 (4.2) | 3 (4.3) | 2.4 (0.4–19) | >0.5 |
| Age at esophageal cancer diagnosis (years) | ||||||
| 43–59 | 13 (7.7) | 34 (8.8) | 8 (11.3) | 4 (5.7) | 10.8 (1.9–203) | |
| 60–69 | 33 (19.5) | 93 (24.0) | 22 (31.0) | 18 (25.7) | 4.9 (2.1–13) | |
| 70–79 | 59 (34.9) | 122 (31.5) | 23 (32.4) | 32 (45.7) | 1.9 (0.9–4.4) | |
| 80–98 | 64 (37.9) | 138 (35.7) | 18 (25.4) | 16 (22.9) | 4.8 (1.6–17) | 0.23 |
| Alkylating agent chemotherapy | ||||||
| No | 155 (92.3) | 356 (93.0) | 65 (92.9) | 66 (95.7) | 3.4 (2.1–5.7) | |
| Yes | 13 (7.7) | 27 (7.0) | 5 (7.1) | 3 (4.3) | 6.9 (1.2–56) | 0.44 |
| Hormonal agent use | ||||||
| No | 129 (78.2) | 289 (76.1) | 59 (83.1) | 54 (79.4) | 3.5 (2.1–6.1) | |
| Yes | 36 (21.8) | 91 (23.9) | 12 (16.9) | 14 (20.6) | 4.3 (1.6–12) | >0.5 |
| Initial surgery for breast cancer | ||||||
| Partial mastectomy | 28 (17.7) | 70 (19.9) | 6 (9.8) | 6 (9.4) | 3.0 (0.8–10.9) | >0.5 |
| Modified or radical mastectomy | 130 (82.3) | 281 (80.1) | 55 (90.2) | 58 (90.6) | 3.4 (2.0–6.1) | |
| Current cigarette smoking ≥1 pack per day | ||||||
| No | 59 (78.7) | 168 (90.8) | 19 (73.1) | 23 (95.8) | 2.9 (1.4–6.4) | |
| Yes | 16 (21.3) | 17 (9.2) | 7 (26.9) | 1 (4.2) | 16.3 (2.2–340) | 0.12 |
| Moderate or heavy alcohol consumption | ||||||
| No | 20 (48.8) | 93 (82.3) | 9 (64.3) | 14 (87.5) | 4.2 (1.4–13) | |
| Yes | 21 (51.2) | 20 (17.7) | 5 (35.7) | 2 (12.5) | 2.7 (0.5–21) | >0.5 |
| First-degree relative with lung, upper GI, or colorectal cancer | ||||||
| No | 65 (76.5) | 184 (90.2) | 28 (70.0) | 29 (85.3) | 4.4 (2.3–9.0) | |
| Yes | 20 (23.5) | 20 (9.8) | 12 (30.0) | 5 (14.7) | 3.9 (1.2–15) | >0.5 |
| BMI ≥ 25.0 kg/m2c | ||||||
| No | 38 (64.4) | 71 (61.2) | 19 (65.5) | 9 (37.5) | 5.3 (2.1–15) | |
| Yes | 21 (35.6) | 45 (38.8) | 10 (34.5) | 15 (62.5) | 2.2 (0.7–6.6) | 0.19 |
BMI, body mass index; CI, confidence interval; OR, odds ratio.
aThe mean radiation dose to the esophageal tumor location for patients with ≥20 Gy was similar for cases and controls within each category.
bRisk estimates compared patients who received ≥20 Gy to the esophagus tumor location with a referent group of patients who received <20 Gy. Analyses included a variable indicating patients with unknown radiation dose, including patients with unknown radiotherapy (3 cases, 8 controls) and patients who received radiotherapy but had insufficient information for dose estimation (9 cases, 23 controls).
cOnly squamous cell carcinomas are included.
Treatment-related risk estimates based on all patients were similar to those obtained in sensitivity analyses excluding case sets without histologically confirmed esophageal cancer (n = 28) or patients with lapses in the follow-up (5 cases, 13 controls). Treatment-related risk estimates were similar in influence analyses systematically excluding each study center, except for suggested elevated risk with alkylating agent-containing chemotherapy when Ontario was excluded (OR = 2.1, 95% CI 1.0–8.8, 14 cases/22 controls received such chemotherapy from the remaining four registries).
discussion
In a large, population-based study of breast cancer survivors, we provide the first risk estimates for treatment-related esophageal cancer, using detailed, individualized radiation dosimetry and data on chemotherapy and hormonal agents. Because radiation dose varied >20-fold along the length of the esophagus, we analyzed doses to the specific tumor location to enable sensitive quantification of radiation-related risk. Esophageal cancer risk increased with increasing radiation dose to the tumor location, reaching 8.3-fold at doses ≥35 Gy. Although the absolute risk of esophageal cancer is low, the risk warrants consideration in radiation therapy risk assessment, and in the long-term follow-up of patients, particularly when the supraclavicular or IMC field has been irradiated.
Because of the long induction period for solid cancers, investigations of late effects of treatment cannot fully address carcinogenic risks associated with current treatment practices. However, our study provides risk estimates for a number of radiotherapy treatments that remain in use for breast cancer, including supraclavicular irradiation, which can deliver high doses to the upper esophagus. The axillary, tangential breast and direct chest fields are commonly used today but deliver relatively low doses to the esophagus (mean < 2 Gy). We found no evidence of increased risk at such doses, but esophageal cancer has been linked with radiation doses <5 Gy in other settings [28, 30, 31], and concerns remain regarding the doses to other organs (e.g. heart, lung) from breast cancer radiotherapy [32]. IMC irradiation also resulted in relatively high doses to the esophagus in our study. The use of more modern radiation techniques (oblique fields, intensity-modulated radiotherapy) and infrequent use of a separate IMC field lower doses to the esophagus [33].
Radiation-related esophageal cancer risk was highest 10–24 years after initial radiotherapy, consistent with previous reports of increased risk among long-term breast cancer survivors [5, 8, 9, 12, 14–18, 20]. Some studies have suggested higher esophageal cancer risks among women diagnosed with breast cancer at a younger age [7–10, 12, 13]. Our data are compatible with those studies and with declining risk with increasing age at esophageal cancer, although these findings were not statistically significant. Similar radiation-related risks were seen for squamous cell carcinomas and adenocarcinomas, but the estimates for adenocarcinomas were based on small numbers. The higher risks for squamous cell carcinomas reported in studies without individual dose estimates may reflect higher radiation doses from breast cancer radiotherapy to the upper and middle esophagus, where squamous cell carcinomas tend to occur, compared with the lower esophagus, where adenocarcinomas typically develop [5, 11, 14, 20]. The lack of a radiation association following partial mastectomy reported previously [19] may also be explained by the lower doses received by these patients; we found no difference in radiation risks by the type of initial surgery when dose was taken into account. We did not observe attenuation of esophageal cancer risk at high doses (≥35 Gy). However, the EOR/Gy in this study was lower than previous reports in populations with lower radiation doses [30, 31], suggesting that the risk per Gy may be lower in the higher dose range, although differences in fractionation or study population (e.g. age at exposure, gender) may also contribute.
Chemotherapy use increased substantially during the study period with the introduction of cyclophosphamide-containing regimens [3]. Alkylating agents such as cyclophosphamide are carcinogenic [34, 35], and animal studies support the sensitivity of esophageal tissue to DNA alkylation (e.g. by nitrosamines) [36, 37]. We observed no increase in esophageal cancer risk among the few women receiving alkylating agent-containing chemotherapy, suggesting that chemotherapy alone is not an important determinant of esophageal cancer risk. However, we did note higher radiation-related esophageal cancer risk among women receiving alkylating agent chemotherapy, supporting further study of chemotherapy-related esophageal cancer risk.
Our data suggested reduced esophageal cancer risk among women receiving hormonal agents (primarily tamoxifen) in the 5 years preceding esophageal cancer diagnosis. Molecular studies demonstrate estrogen receptor expression in some esophageal squamous cell and adenocarcinomas [38], but previous studies of tamoxifen and esophageal cancer have been inconsistent [7, 38, 39]. The time-dependent nature of our observation raises the possibility that tamoxifen may inhibit esophageal cancer progression, but could also be spurious. Further studies are needed to investigate this effect.
Our data were consistent with a multiplicative effect of radiation and other esophageal cancer risk factors (smoking, alcohol consumption, family history of cancer, and BMI). These results support the need for patients receiving chest radiotherapy to avoid cigarette smoking in particular [40, 41]. Although comprehensive data on esophageal cancer risk factors were not available in our medical record-based study, analyses revealed no indication of confounding by these factors, and yielded risk estimates similar to those reported in the literature for de novo esophageal cancer, supporting the validity of our data [22].
A further limitation of our study was lack of centralized pathology review of the esophageal cancers since archived diagnostic materials were not available. However, 89% of cases were histologically confirmed, and breast cancer metastases to the esophagus are rare [42]. In addition, small sample size for certain subgroup analyses (e.g. combined chemotherapy and radiotherapy) and uncertainties in the dosimetry due to insufficient detail in the medical records limited our ability to draw definitive conclusions, and our results may not be generalizable to male or non-Caucasian breast cancer survivors.
Although esophageal cancer is a frequently fatal complication of radiotherapy for breast cancer, the absolute risk of esophageal cancer following breast cancer is low. Nevertheless, patient and clinician education is warranted to heighten suspicion of esophageal cancer at the first sign of dysphagia or other symptoms of esophageal disease, particularly for breast cancer survivors who received IMC or supraclavicular irradiation and have other esophageal cancer risk factors, thus lowering the threshold for endoscopy. In addition, our data suggest the importance of considering the dose to the esophagus in radiation therapy dose planning to further quantify the risks and benefits associated with radiotherapy [2].
funding
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto, Canada (NO1-CP-31157); Danish Cancer Society, Copenhagen, Denmark (NO1-CP-31019); Finnish Cancer Registry, Helsinki, Finland (NO1-CP-31154); Information Management Services, Inc., Silver Spring, USA (N01-CP-31003); Karolinska Institute, Stockholm, Sweden (NO1-CP-31156); University of Iowa, Iowa City, USA (NO1-CP-31155); The University of Texas MD Anderson Cancer Center, Houston, USA (N02-CP-55503); and Westat, Inc., Rockville, USA (N02-CP-31136).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank Diane Fuchs, Janet Lawler-Heavner, and their staff at Westat, Inc. (Rockville, MD) for administrative assistance in conducting the field studies, and Jeremy Miller (Information Management Services, Silver Spring, MD) for computer programming support.
references
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. doi:10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. doi:10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. doi:10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 5.Ahsan H, Neugut AI. Radiation therapy for breast cancer and increased risk for esophageal carcinoma. Ann Intern Med. 1998;128(2):114–117. doi: 10.7326/0003-4819-128-2-199801150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ahsan H, Neugut AI, Gammon MD. Association of adenocarcinoma and squamous cell carcinoma of the esophagus with tobacco-related and other malignancies. Cancer Epidemiol Biomarkers Prev. 1997;6(10):779–782. [PubMed] [Google Scholar]
- 7.Andersson M, Jensen MB, Engholm G, et al. Risk of second primary cancer among patients with early operable breast cancer registered or randomised in Danish Breast Cancer cooperative Group (DBCG) protocols of the 77, 82 and 89 programmes during 1977-2001. Acta Oncol. 2008;47(4):755–764. doi: 10.1080/02841860801978921. doi:10.1080/02841860801978921. [DOI] [PubMed] [Google Scholar]
- 8.Berrington de Gonzalez A, Curtis RE, Gilbert E, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102(1):220–226. doi: 10.1038/sj.bjc.6605435. doi:10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown LM, Chen BE, Pfeiffer RM, et al. Risk of second non-hematological malignancies among 376 825 breast cancer survivors. Breast Cancer Res Treat. 2007;106(3):439–451. doi: 10.1007/s10549-007-9509-8. doi:10.1007/s10549-007-9509-8. [DOI] [PubMed] [Google Scholar]
- 10.Curtis RE, Ron E, Hankey BF, et al. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, et al., editors. New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute; 2006. pp. 181–206. NIH Publ. No. 05-5302] [Google Scholar]
- 11.Das A, Thomas S, Zablotska LB, et al. Association of esophageal adenocarcinoma with other subsequent primary cancers. J Clin Gastroenterol. 2006;40(5):405–411. doi: 10.1097/00004836-200605000-00008. doi:10.1097/00004836-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Evans HS, Lewis CM, Robinson D, et al. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84(3):435–440. doi: 10.1054/bjoc.2000.1603. doi:10.1054/bjoc.2000.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KD, Chen SC, Chan CH, et al. Increased risk for second primary malignancies in women with breast cancer diagnosed at young age: a population-based study in Taiwan. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2647–2655. doi: 10.1158/1055-9965.EPI-08-0109. doi:10.1158/1055-9965.EPI-08-0109. [DOI] [PubMed] [Google Scholar]
- 14.Levi F, Handimbison L, Te VC, et al. Increased risk of esophageal cancer after breast cancer. Ann Oncol. 2005;16(11):1829–1831. doi: 10.1093/annonc/mdi363. doi:10.1093/annonc/mdi363. [DOI] [PubMed] [Google Scholar]
- 15.Mellemkjaer L, Friis S, Olsen JH, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118(9):2285–2292. doi: 10.1002/ijc.21651. doi:10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 16.Roychoudhuri R, Evans H, Robinson D, et al. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004;91(5):868–872. doi: 10.1038/sj.bjc.6602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salminen EK, Pukkala E, Kiel KD, et al. Impact of radiotherapy in the risk of esophageal cancer as subsequent primary cancer after breast cancer. Int J Radiat Oncol Biol Phys. 2006;65(3):699–704. doi: 10.1016/j.ijrobp.2006.01.017. doi:10.1016/j.ijrobp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol. 2008;26(8):1239–1246. doi: 10.1200/JCO.2007.11.9081. doi:10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 19.van Halteren HK, Taal BG, van Tinteren H, et al. Risk factors for the development of oesophageal cancer as a second primary tumour. Eur J Cancer. 1995; 31(11):1836–1839. doi: 10.1016/0959-8049(95)00315-a. doi:10.1016/0959-8049(95)00315-A. [DOI] [PubMed] [Google Scholar]
- 20.Zablotska LB, Chak A, Das A, et al. Increased risk of squamous cell esophageal cancer after adjuvant radiation therapy for primary breast cancer. Am J Epidemiol. 2005;161(4):330–337. doi: 10.1093/aje/kwi050. doi:10.1093/aje/kwi050. [DOI] [PubMed] [Google Scholar]
- 21.Maddams J, Parkin DM, Darby SC. The cancer burden in the UK in 2007 due to radiotherapy. Int J Cancer. 2011 doi: 10.1002/ijc.26240. epub ahead of print June 13. [DOI] [PubMed] [Google Scholar]
- 22.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. doi: 10.1056/NEJMra035010. doi:10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 23.Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165(12):1424–1433. doi: 10.1093/aje/kwm051. doi:10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 24.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(5):872–878. doi: 10.1158/1055-9965.EPI-05-0860. doi:10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 25.Breslow NE, Day NE. Statistical Methods in Cancer Research: Volume 1 – The Analysis of Case-Control Studies. Lyon: International Agency for Research on Cancer; 1980. [PubMed] [Google Scholar]
- 26.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1):141–157. doi: 10.1667/RR3525.1. doi:10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 27.Perez CA, Brady LW, Halperin EC, et al. Principles and Practice of Radiation Oncology. 5th edition. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 28.United Nations. Vienna: United Nations; 2008. Effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation - UNSCEAR 2006 Report, Volume 1 - Report to the General Assembly, with Scientific Annexes A and B. [Google Scholar]
- 29.Surveillance, Epidemiology, and End Results (SEER) Program. 2008. SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov. 2007 Sub (2000–2005): National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission www.seer.cancer.gov .
- 30.Weiss HA, Darby SC, Doll R. Cancer mortality following X-ray treatment for ankylosing spondylitis. Int J Cancer. 1994;59(3):327–338. doi: 10.1002/ijc.2910590307. doi:10.1002/ijc.2910590307. [DOI] [PubMed] [Google Scholar]
- 31.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168(1):1–64. doi: 10.1667/RR0763.1. doi:10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 32.Taylor CW, Nisbet A, McGale P, et al. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys. 2007; 69(5):1484–1495. doi: 10.1016/j.ijrobp.2007.05.034. doi:10.1016/j.ijrobp.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Hurkmans CW, Saarnak AE, Pieters BR, et al. An improved technique for breast cancer irradiation including the locoregional lymph nodes. Int J Radiat Oncol Biol Phys. 2000;47(5):1421–1429. doi: 10.1016/s0360-3016(00)00504-6. doi:10.1016/S0360-3016(00)00504-6. [DOI] [PubMed] [Google Scholar]
- 34.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94(3):182–192. doi: 10.1093/jnci/94.3.182. doi:10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 35.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6(11):638–647. doi: 10.1038/nrclinonc.2009.146. doi:10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 36.Gurski R, Schirmer C, Kruel C, et al. Induction of esophageal carcinogenesis by diethylnitrosamine and assessment of the promoting effect of ethanol and N-nitrosonornicotine: experimental model in mice. Dis Esophagus. 1999;12(2):99–105. doi: 10.1046/j.1442-2050.1999.00010.x. doi:10.1046/j.1442-2050.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 37.Zgodzinski W, Zinkiewicz K, Juskiewicz W, et al. Diethylnitrosamine may induce esophageal dysplasia after local intramural administration. Rocz Akad Med Bialymst. 2003;48:48–51. [PubMed] [Google Scholar]
- 38.Hogan AM, Collins D, Baird AW, et al. Estrogen and gastrointestinal malignancy. Mol Cell Endocrinol. 2009;307(1-2):19–24. doi: 10.1016/j.mce.2009.03.016. doi:10.1016/j.mce.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Curtis RE, Boice JD, Jr, Shriner DA, et al. Second cancers after adjuvant tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1996;88(12):832–834. doi: 10.1093/jnci/88.12.832. doi:10.1093/jnci/88.12.832. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert ES, Stovall M, Gospodarowicz M, et al. Lung cancer after treatment for Hodgkin's disease: focus on radiation effects. Radiat Res. 2003;159(2):161–173. doi: 10.1667/0033-7587(2003)159[0161:lcatfh]2.0.co;2. doi:10.1667/0033-7587(2003)159[0161:LCATFH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman EL, Jacobson JS, Hershman DL, et al. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol. 2008;26(3):392–398. doi: 10.1200/JCO.2007.13.3033. doi:10.1200/JCO.2007.13.3033. [DOI] [PubMed] [Google Scholar]
- 42.Rampado S, Ruol A, Guido M, et al. Mediastinal carcinosis involving the esophagus in breast cancer: the "breast-esophagus" syndrome: report on 25 cases and guidelines for diagnosis and treatment. Ann Surg. 2007; 246(2):316–322. doi: 10.1097/01.sla.0000263507.11053.26. doi:10.1097/01.sla.0000263507.11053.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.