Abstract
Background
Approximately 15%–23% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2), which leads to the activation of signaling pathways that stimulate cell proliferation and survival. HER2-targeted therapy has substantially improved outcomes in patients with HER2-positive breast cancer. However, both de novo and acquired resistance are observed.
Design
A literature search was performed to identify proposed mechanisms of resistance to HER2-targeted therapy and identified novel targets in clinical development for treating HER2-resistant disease.
Results
Proposed HER2-resistance mechanisms include impediments to HER2-inhibitor binding, signaling through alternative pathways, upregulation of signaling pathways downstream of HER2, and failure to elicit an appropriate immune response. Although continuing HER2 inhibition beyond progression may provide an additional clinical benefit, the availability of novel therapies targeting different mechanisms of action could improve outcomes. The developmental strategy with the most available data is targeting the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (mTOR) pathway. The oral mTOR inhibitor everolimus has shown promising activity in combination with chemotherapy and trastuzumab in trastuzumab-refractory, advanced breast cancer.
Conclusions
Non-HER2-targeted therapy is a promising means of overcoming resistance to HER2-targeted treatment. Ongoing clinical studies will provide additional information on the efficacy and safety of novel targeted therapies in HER2-resistant advanced breast cancer.
Keywords: breast cancer, everolimus, HER2, mTOR, overcoming resistance, restoring sensitivity
introduction
Worldwide, breast cancer is the most common cancer in women and the second most common cancer overall, with 1.4 million incident cases in 2008 [1]. Approximately 458 000 breast cancer-related deaths were reported in 2008, making breast cancer the most common cause of cancer death in women and the fifth most common cause of cancer death overall [1].
Approximately 15%–23% of breast cancers exhibit overexpression of human epidermal growth factor receptor 2 (HER2) caused by amplification of the erb-B2 oncogene [2–5]. Overexpression of HER2, a receptor tyrosine kinase, activates signaling pathways that stimulate cell proliferation and survival, including the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase pathways [6]. Several factors are correlated with HER2 overexpression in breast cancer, including age >50 years, a higher T stage, and a higher histologic grade [2, 7, 8]. HER2 overexpression is also associated with an increased risk of central nervous system (CNS) metastases [9–11]. For example, in a retrospective study of 2441 patients with breast cancer, HER2 overexpression was associated with a 3.4-fold increase in the risk of cerebral metastases [10]. As a marker of aggressive disease, HER2 overexpression is an independent predictor of decreased recurrence-free survival, breast cancer-related survival, and overall survival (OS) [2, 7, 8, 12]. However, the development of HER2-targeted therapy has revolutionized the treatment of HER2-positive breast cancer such that HER2 overexpression can be considered a positive predictor of improved outcomes.
In this article, we briefly review the known efficacy of HER2-targeted therapy and the mechanisms that may lead to resistance. We then evaluate the available literature, drawn from journals and recent congresses, to identify novel targets in current clinical development for treating HER2-resistant disease (i.e. those with ongoing trials according to ClinicalTrials.gov). The main focus of the novel agents section focuses on inhibitors of the PI3K/Akt/mTOR pathway as they are supported by the most preclinical and clinical evidence.
efficacy of HER2-targeted therapy
Recommended first-line treatment for HER2-positive breast cancer includes trastuzumab [13–15], a recombinant humanized monoclonal antibody targeted to the extracellular domain of the HER2 receptor tyrosine kinase [16]. In early-stage breast cancer, adding trastuzumab to neoadjuvant chemotherapy substantially improves OS and reduces the risk of recurrence, both by 33% [17]. Similarly, adjuvant trastuzumab substantially improves disease-free survival by 38% and OS by 34% and substantially reduces the risk of local and distant recurrence by 42% and 40%, respectively [18]. Trastuzumab also provides significant benefit for patients with metastatic breast cancer. Compared with chemotherapy alone, the combination of trastuzumab and chemotherapy substantially increases the time to progression by 49% and the time to treatment failure by 42% and improves OS by 20% [19]. Interestingly, several studies have reported an increased risk of CNS metastases in patients treated with trastuzumab [11, 18, 20, 21]. However, it is unlikely that trastuzumab treatment per se increases the risk of cerebral metastases. Instead, it is likely a multifactorial effect of HER2 overexpression increasing the risk of CNS metastases [9–11], the prolonged survival of trastuzumab-treated patients allowing CNS metastases to become symptomatic [17–19], and the inability of trastuzumab to effectively cross the blood–brain barrier [22].
resistance to HER2-targeted therapy
Although trastuzumab substantially improves outcomes in both early-stage and metastatic breast cancer, not all patients respond to trastuzumab (de novo HER2 resistance), and many progress after realizing an initial response (acquired HER2 resistance) [12]. In early-stage breast cancer, the addition of trastuzumab to neoadjuvant chemotherapy is associated with a complete response (CR) of the breast and lymph nodes in 38%–55% of patients, suggesting a de novo resistance rate of 45%–62% [17]. In patients with metastatic breast cancer treated with trastuzumab and chemotherapy, the median duration of partial or CR is 9.1 months, suggesting that within 1-year patients acquire resistance [19].
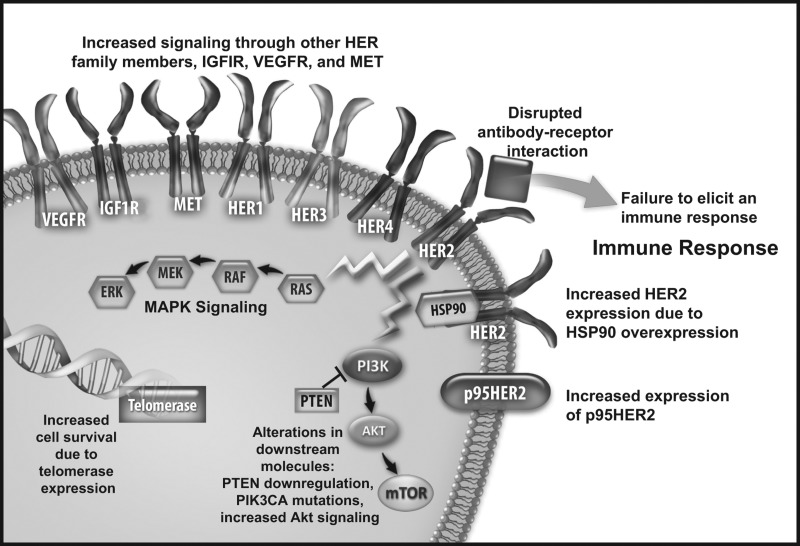
Several mechanisms of resistance to HER2-targeted therapy have been proposed. These include impediments to HER2-trastuzumab binding, signaling through alternative pathways, upregulation of signaling pathways downstream of HER2, and failure to elicit an appropriate immune response (Figure 1) [6, 23]. Specific factors implicated in resistance include the HER2 copy number; HER2 dimerization status; presence of truncated HER2 (p95HER2); Fc receptor status; loss of phosphatase and tensin homolog (PTEN) and p27Kip1 expression; activation of mutations of PI3KCA; amplification of the epidermal growth factor receptor (EGFR) gene or overexpression of the EGFR protein; overexpression of insulin-like growth factor receptor 1 (IGF1R), vascular endothelial growth factor receptor, and heat shock protein 90 (HSP90); activation of the cytoplasmic tyrosine kinase SRC; and mucin 4 glycopeptide expression [6, 12, 23, 24].
Figure 1.
Proposed mechanisms of HER2 resistance. Akt, protein kinase B; HER, human epidermal growth factor receptor; HSP90, heat shock protein 90; IGF1R, insulin-like growth factor receptor 1; MAPK, mitogen-activated protein kinase; MET, mesenchymal epithelial transition factor; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; VEGFR, vascular endothelial growth factor receptor.
current strategies for overcoming resistance: maintaining HER2-targeted therapy
switching chemotherapeutic agent
In the clinical setting, various treatment strategies are used in attempts to overcome trastuzumab resistance [6]. One strategy is to continue trastuzumab therapy but combine it with an alternative chemotherapy regimen [25–29]. Although the optimal duration of trastuzumab therapy remains controversial, data from preclinical studies indicating additive or synergistic effects of trastuzumab with several cytotoxic agents has led many clinicians to continue to administer trastuzumab beyond progression, in combination with second- or third-line chemotherapeutic agents [25, 27]. In one small, retrospective study, some patients experienced benefit with as many as four consecutive trastuzumab-containing regimens [26]. Also, a randomized clinical trial of women with HER2-positive breast cancer that progressed after previous trastuzumab therapy indicated that combination therapy with trastuzumab plus capecitabine provided significant benefit compared with capecitabine alone [28]. Although this phenomenon may be explained by an additive action of trastuzumab and chemotherapy, further prospective randomized studies are needed to evaluate the clinical benefits of continuing trastuzumab beyond progression versus switching to a non-trastuzumab-containing regimen.
switching HER2-targeted therapy
Another strategy for patients with trastuzumab-refractory disease is to switch to a different HER2-targeted therapy. In a phase III study of women with HER2-positive metastatic breast cancer that progressed on trastuzumab, combination therapy with capecitabine and the multityrosine kinase inhibitor lapatinib, which inhibits HER2 and EGFR, substantially extended the time to progression by 4 months over capecitabine alone (8.4 months versus 4.4 months; P < 0.001) [30]. Early clinical data for use of the multityrosine kinase inhibitor neratinib in trastuzumab-refractory disease are also promising [31]. Of note, multityrosine kinase inhibitors may be effective for women who overexpress p95HER2. Expression of p95HER2 leads to de novo trastuzumab resistance [32, 33] and, thus, poorer clinical outcomes [34], because the truncated protein lacks the extracellular domain required for trastuzumab binding. The binding of lapatinib to the intracellular domain of HER2 [35] allows it to inhibit both full-length HER2 and truncated p95HER2 [33]. Indeed, in a pooled analysis, lapatinib as monotherapy or in combination with capecitabine provided the same clinical benefit in patients who did and did not express p95HER2 [36]. An ongoing phase II study is comparing the efficacy of lapatinib plus chemotherapy with that of trastuzumab plus chemotherapy as first-line treatment for women with HER2- and p95HER2-positive metastatic breast cancer (NCT01137994).
The novel antibody-drug conjugate T-DM1, composed of trastuzumab linked to DM1 (a highly potent microtubule inhibitor) may also provide some promise in patients who have developed resistance to trastuzumab alone. In addition to potently inhibiting microtubule assembly, T-DM1 also appears to flag HER2-positive cells for cytotoxic destruction by antibodies [37]. In a phase II study, intravenous T-DM1 showed robust activity in 112 patients with heavily pretreated, trastuzumab resistant HER2-positive breast cancer, recording an objective response rate of 25.9%, and median progression-free survival (PFS) time of 4.6 months [37]. In a second phase II study of 110 extensively pretreated patients, T-DM1 treatment provided an overall response rate of 33%, and a clinical benefit rate of 48% [38]. The median PFS time was 6.9 months [38]. These data suggest that an anti-HER2 antibody may retain the capacity to interact with HER2 in a clinically meaningful way, even after the development of resistance, and indicate that conjugated agents such as T-DM1 may provide a new treatment alternative for those who have previously progressed on native trastuzumab.
combining HER2 inhibitors
Recent data suggest that dual HER2 inhibition provides the clinical benefit in trastuzumab-resistant disease. In a phase III study of patients with metastatic breast cancer exposed to a median of three previous trastuzumab-containing regimens, treatment with trastuzumab plus lapatinib significantly improved median PFS compared with lapatinib alone (12.0 weeks versus 8.1 weeks; P = 0.008) without compromising safety [39]. Preliminary results of the phase III NeoAdjuvant Lapatinib and/or Trastuzumab Treatment Optimization study suggest that neoadjuvant therapy including both trastuzumab and lapatinib provides a significantly greater pathologic CR rate than therapy including either agent alone (P ≤ 0.0001) [40].
In phase II and III studies, the developmental humanized monoclonal antibody pertuzumab, which targets a different HER2 extracellular domain than trastuzumab and inhibits HER2 dimerization, shows promising efficacy when added to trastuzumab in several different settings [41–44]. As shown in cohort 3 of the BO17929 study, combination therapy with trastuzumab and pertuzumab provided the clinical benefit to patients who progressed after sequential trastuzumab and pertuzumab monotherapy [44]. In the phase III Clinical Evaluation of Pertuzumab and Trastuzumab study of patients with HER2-positive metastatic breast cancer, first-line combination therapy with trastuzumab plus pertuzumab and docetaxel prolonged median PFS by 6.1 months compared with trastuzumab and docetaxel alone (18.5 months versus 12.4 months; P < 0.001) [41]. In addition, results of the phase II Trastuzumab plus Pertuzumab in Neoadjuvant HER2-Positive Breast Cancer [42] and phase II Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation [43] demonstrated the potential benefit of adding pertuzumab to trastuzumab and chemotherapy in the neoadjuvant setting.
future strategies for overcoming HER2 resistance: inhibitors of the PI3K/Akt/mTOR pathway
Despite evidence suggesting that continued HER2 inhibition after progression on trastuzumab can provide additional clinical benefit, the availability of novel therapies that target a different mechanism of action could improve outcomes in patients with trastuzumab-resistant disease. Based on various lines of preclinical data, current targets under assessment in clinical trials of HER2-positive, treatment-refractory breast cancer include signal transduction molecules implicated in HER2 resistance (e.g. PI3K, Akt, mTOR, and IGF1R), HSP90, and telomerase (Table 1). The degree of supporting preclinical and clinical evidence for these targets varies.
Table 1.
Ongoing clinical trials of non–HER2-targeted agents in development for the treatment of HER2-resistant advanced breast cancer
| Developmental agent | Design and study treatment | ClinicalTrials.gov identifier |
|---|---|---|
| IGF1R inhibitors | ||
| BMS-754807 | Open-label, phase I/II | NCT00788333 |
| Trastuzumab + BMS-754807 | ||
| Cixutumumab | Randomized, placebo-controlled, phase II | NCT00684983 |
| Lapatinib + capecitabine ± cixutumumab | ||
| OSI-906 | Open-label, phase II | NCT01205685 |
| Erlotinib + letrozole + OSI-906 | ||
| HSP90 inhibitor | ||
| AUY922 | Open-label, phase Ib/II | NCT01271920 |
| Trastuzumab + AUY922 | ||
| Open-label, phase I/II | NCT01361945 | |
| Lapatinib + letrozole + AUY922 | ||
| Ganetespib | Open-label, phase I | NCT01273896 |
| Ganetespib monotherapy | ||
| Telomerase inhibitor | ||
| GRN163L | Open-label, phase I | NCT01265927 |
| Trastuzumab + GRN163L | ||
| PI3K inhibitors | ||
| BKM120 | Open-label, phase I/II | NCT01132664 |
| Trastuzumab + BKM120 | ||
| GDC-0941 | Open-label, phase I | NCT00928330 |
| Trastuzumab or T-DM1 + GDC-0941 | ||
| XL147 | Trastuzumab + XL147 ± paclitaxel | NCT01042925 |
| Open-label, phase I/II | ||
| Akt inhibitor | ||
| MK2206 | Open-label, phase I | NCT01245205 |
| Lapatinib + MK2206 | ||
| Open-label, phase II | NCT01277757 | |
| MK2206 monotherapy | ||
| mTOR inhibitors | ||
| Temsirolimus | Open-label, phase I-II | NCT01111825 |
| Neratinib + temsirolimus | ||
| Everolimus | Open-label, phase II | NCT01305941 |
| Vinorelbine + trastuzumab + everolimus | ||
| Open-label, phase II | NCT01283789 | |
| Lapatinib + everolimus | ||
| Randomized, placebo-controlled, phase III | NCT01007942 | |
| Vinorelbine + trastuzumab ± everolimus | ||
| INK128 | Open-label, phase I | NCT01351350 |
| Paclitaxel + trastuzumab + INK128 | ||
| Dual PI3K/mTOR inhibitor | ||
| BEZ235 | Open-label, phase I/Ib | NCT00620594 |
| Trastuzumab + BEZ235 | ||
| Open-label, phase Ib, randomized phase II | NCT01471847 | |
| Trastuzumab + BEZ235 (versus lapatinib + capecitabine in phase II) | ||
NB: Not all trials are limited to patients with HER2-positive breast cancer that progressed after HER2-targeted therapy.
HSP90, heat shock protein 90; IGF1R, insulin-like growth factor receptor 1; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase.
The strategy with the most available preclinical and clinical data is restoration of trastuzumab/HER2 sensitivity by targeting the PI3K/Akt/mTOR pathway. The PI3K/Akt/mTOR signal transduction pathway is critical to cell growth, proliferation, metabolism, survival, and angiogenesis and has been implicated in several types of cancers [45, 46]. Among patients with HER2-positive breast cancer, those who have high levels of phosphorylated Akt, activating mutations of PI3CKA, and loss of PTEN—a negative regulator of the PI3K/AKT/mTOR pathway—have worse outcomes after trastuzumab-based therapy than patients who do not [47–55]. Importantly, activated PI3K/Akt/mTOR signaling appears to play a role in both acquired and de novo HER2 resistance. Both preclinical and clinical data implicate PTEN loss and PI3CKA mutation in constitutive PI3K/Akt/mTOR signaling and de novo resistance to HER2-targeted therapy [54–59]. For example, in one study of women with HER2-positive breast cancer treated with trastuzumab, patients who showed the loss of PTEN expression or PI3CKA mutation had a significantly shorter PFS than patients who did not (P = 0.007) [54]. In another study, the loss of PTEN was associated with a significantly lower overall response rate (P = 0.028) [55]. In vitro models of HER2-positive breast cancer have shown that the loss of PTEN expression and PI3CKA mutations are also markers of lapatinib resistance [56, 58]. However, another in vitro model showed that resistance to trastuzumab, but not lapatinib, was associated with PTEN loss and PI3CKA mutation [57]. Further, in a series of patients with HER2-positive metastatic breast cancer, PI3CKA mutation and low PTEN expression were associated with trastuzumab resistance, whereas low PTEN expression predicted lapatinib response [59].
Activation of the PI3K/Akt/mTOR pathway is also implicated in acquired resistance to HER2-targeted therapy [57, 60–64]. In vitro experiments showed that the levels of phosphorylated p70S6K1, a downstream effector of the PI3K/Akt/mTOR pathway, were substantially greater in cells with acquired lapatinib resistance than in lapatinib-sensitive cells [60]. In another in vitro model, trastuzumab treatment resulted in an Akt-negative feedback loop that perpetuated HER2 phosphorylation, leading to a decreased response to trastuzumab [61]. Other studies confirm that perturbation of the PI3K/Akt/mTOR pathway and the balance between phosphorylated and nonphosphorylated PTEN after exposure to HER2-targeted therapy leads to acquired resistance [57, 64].
supporting preclinical data
Preclinical evidence supports the potential clinical utility of inhibitors of the PI3K/Akt/mTOR pathway in the setting of resistance to HER2-targeted therapy [56, 60, 63, 65–70]. For example, in an in vitro model of trastuzumab resistance caused by the loss of PTEN, trastuzumab sensitivity was restored by treatment with the mTOR inhibitor everolimus and the Akt inhibitor triciribine [65]. In this same model, everolimus and triciribine also inhibited the growth of trastuzumab-resistant cells. In a mouse xenograft model of HER2 resistance caused by PTEN loss, combination therapy with everolimus and trastuzumab slowed tumor growth more than either agent alone [65]. In another series of in vitro and in vivo experiments carried out in trastuzumab resistance models, combination therapy with an mTOR inhibitor (either rapamycin or everolimus) and trastuzumab was much more effective at inhibiting cell growth than either agent alone [66]. Further, the novel mTOR inhibitor INK128, the PI3K inhibitor GDC-0941, and the dual PI3K/mTOR inhibitor BEZ235 have all been shown to restore sensitivity to HER2-targeted therapy in HER2-resistant cell lines and reduce tumor growth [56, 63, 67–70]. These agents have also been shown to reduce tumor growth in various tumor xenograft models of HER2 resistance [67, 68, 70].
supporting clinical data
All currently available data supporting the clinical efficacy and safety of PI3K/Akt/mTOR inhibition in patients with trastuzumab-refractory advanced or metastatic breast cancer come from phase I and II clinical trials of the oral mTOR inhibitor everolimus (Table 2) [71–76]. In a phase Ib dose-finding study, the maximum tolerated dose of everolimus in combination with trastuzumab and paclitaxel in patients with trastuzumab-refractory, HER2-positive breast cancer was 10 mg/day [71]. Although not powered for efficacy, everolimus treatment showed promising efficacy; of the 27 assessable patients, the overall response rate was 44% and the median PFS was 34 weeks. The therapeutic regimen was relatively well tolerated, with the most common adverse events being stomatitis (82%) and neutropenia (64%) [71]. Based on these results, the phase II portion of the study was initiated (Table 2) [73]. Preliminary results of this study demonstrated an overall response rate of 19%, a median PFS of 26 weeks, and a tolerable safety profile, thus confirming the efficacy and safety of everolimus 10 mg/day in combination with paclitaxel and trastuzumab for patients with trastuzumab-resistant, HER2-positive, metastatic breast cancer [73].
Table 2.
Clinical data of everolimus in patients with trastuzumab-refractory, HER2-positive breast cancer
| Author/phase | Population (N) | Treatment | Efficacy | Most common toxic effects |
|---|---|---|---|---|
| Andre/phase Ib [71] | TRAS-refractory, mBC (33) | EVE + PAC + TRAS | ORR: 44%; CBR: 74%; mPFS: 34 weeks | Any grade: neutropenia (64%) and stomatitis (82%); grade 3/4: neutropenia (52%) |
| Dalenc/phase II [73] | TRAS- and taxane-refractory mBC (55) | EVE + PAC + TRAS | ORR: 19%; CBR: 40%; mPFS: 26 weeks | Grade 3/4: neutropenia (27%) |
| Jerusalem/phase I [75] | TRAS-refractory mBC (50) | EVE + VIN + TRAS | ORR: 19%; CBR: 54%; mPFS: 30.7 weeks | Any grade: neutropenia (92%) and stomatitis (70%); grade 3/4: neutropenia (86%) |
| Campone/phase I and II pooled analysis [74] | TRAS-refractory mBC with (57) or without (77) previous LAP exposure | EVE + TRAS + PAC: n = 84; EVE + TRAS + VIN: n = 50 | ORR, LAP-exposed: 21%; ORR, LAP-nonexposed: 29%; mPFS, LAP-exposed: 29 weeks; mPFS, LAP-nonexposed: 36.1 weeks | Grade 3/4: similar in LAP-exposed and nonexposed patients |
| Jerusalem/phase Ib and II extension [72] | TRAS-refractory mBC (31) | EVE + TRAS | ORR: 15%; CBR: 34%; mPFS: 41 weeks | Grade 3/4: neutropenia, leukopenia, and lymphopenia (n = 2 for each) and stomatitis (n = 1) |
| Morrow/phase I and II pooled analysis [76] | TRAS-refractory mBC (47) | EVE + TRAS | ORR: 15%; CBR: 34%; mPFS: 4.1 months | Grade ≥2: mucositis (34%), fatigue (32%), and lymphopenia (26%); grade 3/4: lymphopenia (13%) and hyperglycemia (11%) |
AE, adverse event; CBR, clinical benefit rate (CR + PR + SD); CR, complete response; EVE, everolimus; LAP, lapatinib; mBC, metastatic breast cancer; mPFS, median progression-free survival; ORR, overall response rate (CR + PR); PAC, paclitaxel; PR, partial response; SD, stable disease; TRAS, trastuzumab; VIN, vinorelbine.
In another phase I study, the combination of everolimus with trastuzumab and vinorelbine was assessed (Table 2) [75]. This study determined that everolimus 5 mg/day versus 20 mg/week was the optimal dosing regimen for future studies of this combination. The choice of everolimus 5 mg/day was based on the safety and efficacy results observed in this study [75] and previous studies suggesting that everolimus provides better outcomes with daily versus weekly dosing [77–79]. Preliminary results of a pooled analysis of two phase I studies and one phase II study showed that everolimus treatment provides efficacy in patients who have and have not been exposed to trastuzumab and lapatinib (overall response rate of 21% and 29%, respectively; median PFS of 29 and 36.1 weeks, respectively) [74]. Furthermore, the toxicity profile of patients with previous lapatinib treatment was similar to those without previous exposure. These results suggest that patients exposed to lapatinib in addition to trastuzumab can experience benefit with everolimus- and trastuzumab-based therapies. A pooled analysis of two additional phase I/II studies of everolimus plus trastuzumab in patients with HER2-positive, trastuzumab-refractory, metastatic breast cancer supports the efficacy and safety of everolimus in this patient population (Table 2) [76].
In three of these studies, patients who did not experience disease progression or unacceptable toxicity after six treatment cycles had the option to continue treatment with everolimus and trastuzumab [71, 73, 75]. Of the 135 patients enrolled in these three studies, 31 entered the maintenance phase: 20 who were initially treated with paclitaxel and 11 who were initially treated with vinorelbine [72]. During the maintenance phase (median duration, 15 weeks), two additional complete responses and one additional partial response were achieved, and three patients achieved an unconfirmed 100% decrease in the sum of the target lesions. Overall, between the chemotherapy and maintenance phases, the median PFS was 41 weeks. In addition, long-term treatment was well tolerated with no additional safety issues arising [72].
ongoing clinical trials
There are several ongoing studies of PI3K/Akt/mTOR inhibitors in patients with HER2-positive, trastuzumab-refractory, metastatic breast cancer (Table 1). Of these studies, the international, randomized, placebo-controlled, phase III Breast Cancer Trial of OraL EveROlimus 3 (BOLERO-3), which is analyzing everolimus in combination with vinorelbine and trastuzumab, is the largest (ClinicalTrials.gov identifier NCT01007942). BOLERO-3 is expected to enroll 572 women aged ≥18 years with HER2-positive, trastuzumab-resistant, measurable breast cancer that has recurred locally or metastasized.
Although the BOLERO-3 protocol initially excluded patients with CNS metastases, it was amended in January 2011 to permit the enrollment of patients with previously treated CNS metastases, as long as the last treatment for those metastases was received ≥8 weeks before randomization. The rationale for including patients with CNS metastases in BOLERO-3 included the fact that 25%–36% of patients with HER2-positive metastatic breast cancer treated with trastuzumab developed brain metastases [21, 80], as well as the lack of systemic therapies approved specifically for the treatment of breast cancer-related brain metastases. Further, the ability of everolimus to reduce the volume of subependymal giant-cell astrocytomas and seizure frequency in patients with tuberous sclerosis complex [81] suggests that everolimus may have an impact on brain metastases. The similar response rates reported in studies of vinorelbine plus trastuzumab that did and did not allow the inclusion of patients with asymptomatic brain metastases (44%–68% and 51%–78%, respectively) suggests that vinorelbine plus trastuzumab is an acceptable combination for patients with stable CNS metastases [82–88]. To further characterize the efficacy of adding everolimus to trastuzumab and vinorelbine in patients with HER2-positive breast cancer that metastasized to the CNS, an open-label, phase II study is planned (ClinicalTrials.gov identifier NCT01305941).
In an open-label, phase II study, the efficacy and safety of everolimus in combination with lapatinib in patients with HER2-positive, metastatic breast cancer that progressed during previous HER2-targeted therapy is also being evaluated (ClinicalTrials.gov identifier NCT01283789). In addition to the studies of everolimus being conducted in the treatment-refractory setting in HER2-positive patients, the placebo-controlled, phase III BOLERO-1 study (also known as TRIO 019) is evaluating everolimus in combination with trastuzumab and paclitaxel as first-line therapy in patients with HER2-positive, locally advanced or metastatic breast cancer (ClinicalTrials.gov identifier NCT00876395).
Other PI3K/Akt/mTOR inhibitors with active clinical development programs in HER2-resistant breast cancer include the PI3K inhibitor GDC-0941, the mTOR inhibitor INK128, and the dual PI3K/mTOR inhibitor BEZ235 (Table 1). The safety and efficacy of GDC-0941 in combination with trastuzumab or trastuzumab-DM1 is being assessed. In an open-label, dose-escalation, parallel-group, phase I study in patients with HER2-positive, metastatic breast cancer that progressed on previous trastuzumab-based therapy (ClinicalTrials.gov identifier NCT00928330). The safety and efficacy of INK128 in combination with paclitaxel is being assessed as part of an open-label, dose-escalation, phase I study of INK128 plus paclitaxel in advanced or metastatic solid tumors that progressed after standard therapy (ClinicalTrials.gov identifier NCT01351350); this trial includes an expanded cohort of patients with advanced or metastatic breast cancer that progressed after HER2-targeted therapy and will receive trastuzumab in addition to INK128 and paclitaxel. The safety and efficacy of BEZ235 in patients with HER2-resistant breast cancer is being assessed in two ongoing trials. A phase I/Ib trial of patients with advanced solid tumors (ClinicalTrials.gov identifier NCT00620594) will include a HER2-positive metastatic breast cancer patient population who failed trastuzumab treatment and have PIK3CA and/or PTEN alterations. A second phase I study of patients with HER2+ advanced or metastatic breast cancer that failed previous trastuzumab (ClinicalTrials.gov identifier NCT01471847) will evaluate the safety and efficacy of BEZ235 plus trastuzumab. In a planned randomized, open-label, phase II portion of this study, the combination of BEZ235 plus trastuzumab will be compared with lapatinib plus capecitabine in the same patient population.
future strategies for overcoming HER2 resistance: other novel agents
As previously mentioned, there are several agents targeted against other pathways and molecules implicated in HER2 resistance (Figure 1) that are in various stages of clinical development. Aside from PI3K/Akt/mTOR inhibitors, other agents currently being investigated in clinical trials of HER2-resistant breast cancer include inhibitors of IGFR1, HSP90, and telomerase.
The rationale for assessing IGF1R inhibition in HER2-resistant breast cancer is the hypothesis that cross-talk between IGF1R and HER2 may occur in breast cancer cells, leading to receptor heterodimerization, which may, in turn, allow cells to escape trastuzumab cytotoxicity [89]. In preclinical studies, overexpression of IGF1R led to trastuzumab resistance [90], and the addition of an IGF1R inhibitor to trastuzumab resulted in greater cell death than treatment with trastuzumab alone [89]. Currently, there are no clinical data on the efficacy and safety of IGF1R inhibition in HER2-resistant breast cancer. There are, however, ongoing trials of the IGF1R inhibitors BMS-754807, cixutumumab, and OSI-906 (Table 1).
HSP90 acts as a chaperone protein, promoting folding and stabilization of other proteins, and preventing their rapid degradation [91]. Preclinical data have shown that HER2 is chaperoned by HSP90, suggesting that inhibition of HSP90 should be evaluated in HER2-positive breast cancer patients. Indeed, in a phase II study of 31 patients with trastuzumab-refractory, HER2-positive breast cancer, combination therapy with the HSP90 inhibitor tanespimycin and trastuzumab provided a clinical benefit rate of 59% and a median PFS and OS of 6 and 17 months, respectively [91]. Although clinical development of tanespimycin is no longer being pursued, clinical trials of second-generation HSP90 inhibitors in women with HER2-positive breast cancer who progressed on trastuzumab are ongoing (Table 1).
Telomerase expression is essential for cellular proliferation, and telomerase overexpression has been linked to tumorigenesis [92]. Inhibition of telomerase ultimately results in apoptosis or cell senescence, making it a potential target for anticancer therapy. In a trastuzumab-resistant cell line, the telomerase inhibitor GRN163L was able to restore trastuzumab sensitivity [92], suggesting that telomerase inhibition may be a possible strategy for overcoming HER2 resistance in breast cancer. Although no clinical data currently exist, a trial of GRN163L in women with trastuzumab-resistant breast cancer is ongoing (Table 1).
conclusion
The availability of HER2-targeted therapies has substantially improved outcomes for the 15%–23% of patients with breast cancer that overexpresses HER2. However, HER2 resistance, both de novo and acquired, is a real phenomenon that necessitates the development of novel treatment strategies. A large body of preclinical evidence suggests that targeting the PI3K/Akt/mTOR signal transduction pathway can restore sensitivity to trastuzumab. The mTOR inhibitor everolimus, the most advanced of the PI3K/Akt/mTOR inhibitors in development, shows promising efficacy with an acceptable tolerability profile in combination with trastuzumab and paclitaxel or vinorelbine in HER2-positive, trastuzumab-refractory, metastatic breast cancer. Much enthusiasm also surrounds newer PI3K and Akt inhibitors, which inhibit the pathway upstream of mTOR. Ongoing clinical trials, including the everolimus trial program, will provide additional information on the utility of adding PI3K/Akt/mTOR inhibitors to HER2-targeted therapy in patients with treatment-refractory, HER2-positive breast cancer, including those with CNS metastases.
funding
This editorial support was funded by Novartis Pharmaceuticals Corporation.
disclosure
The authors have declared no conflicts of interest.
acknowledgements
Editorial support in the preparation of this manuscript was provided by Melanie Leiby, PhD (ApotheCom, Yardley, PA).
references
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Moon YW, Park S, Sohn JH, et al. Clinical significance of progesterone receptor and HER2 status in estrogen receptor-positive, operable breast cancer with adjuvant tamoxifen. J Cancer Res Clin Oncol. 2011;137:1123–1130. doi: 10.1007/s00432-011-0976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowsett M, Houghton J, Iden C, et al. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol. 2006;17:818–826. doi: 10.1093/annonc/mdl016. [DOI] [PubMed] [Google Scholar]
- 4.Parise CA, Bauer KR, Brown MM, et al. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 5.Knoop AS, Bentzen SM, Nielsen MM, et al. Value of epidermal growth factor receptor, HER2, p53, and steroid receptors in predicting the efficacy of tamoxifen in high-risk postmenopausal breast cancer patients. J Clin Oncol. 2001;19:3376–3384. doi: 10.1200/JCO.2001.19.14.3376. [DOI] [PubMed] [Google Scholar]
- 6.Hubalek M, Brunner C, Mattha K, et al. Resistance to HER2-targeted therapy: mechanisms of trastuzumab resistance and possible strategies to overcome unresponsiveness to treatment. Wien Klin Wochenschr. 2010;160:506–512. doi: 10.1007/s10354-010-0838-6. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kuraya K, Schraml P, Torhorst J, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004;64:8534–8540. doi: 10.1158/0008-5472.CAN-04-1945. [DOI] [PubMed] [Google Scholar]
- 8.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–5704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 9.Sanna G, Franceschelli L, Rotmensz N, et al. Brain metastases in patients with advanced breast cancer. Anticancer Res. 2007;27:2865–2869. [PubMed] [Google Scholar]
- 10.Heitz F, Harter P, Lueck HJ, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45:2792–2798. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 12.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 13.Aebi S, Davidson T, Gruber G, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v9–v14. doi: 10.1093/annonc/mdq159. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso F, Senkus-Konefka E, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v15–v19. doi: 10.1093/annonc/mdq160. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™: Breast Cancer. Fort Washington, PA: 2011. [Google Scholar]
- 16.Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrelli F, Borgonovo K, Cabiddu M, et al. Neoadjuvant chemotherapy and concomitant trastuzumab in breast cancer: a pooled analysis of two randomized trials. Anticancer Drugs. 2010;22:128–135. doi: 10.1097/cad.0b013e32834120aa. [DOI] [PubMed] [Google Scholar]
- 18.Dahabreh IJ, Linardou H, Siannis F, et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008;13:620–630. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Montagna E, Cancello G, D'Agostino D, et al. Central nervous system metastases in a cohort of metastatic breast cancer patients treated with trastuzumab. Cancer Chemother Pharmacol. 2009;63:275–280. doi: 10.1007/s00280-008-0737-3. [DOI] [PubMed] [Google Scholar]
- 21.Ono M, Ando M, Yunokawa M, et al. Brain metastases in patients who receive trastuzumab-containing chemotherapy for HER2-overexpressing metastatic breast cancer. Int J Clin Oncol. 2009;14:48–52. doi: 10.1007/s10147-008-0797-8. [DOI] [PubMed] [Google Scholar]
- 22.Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18:23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 23.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res. 2009;15:7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthuswamy SK. Trastuzumab resistance: all roads lead to SRC. Nat Med. 2011;17:416–418. doi: 10.1038/nm0411-416. [DOI] [PubMed] [Google Scholar]
- 25.Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer. 2004;5:52–58. doi: 10.3816/cbc.2004.n.010. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Saenz JA, Martin M, Puente J, et al. Trastuzumab associated with successive cytotoxic therapies beyond disease progression in metastatic breast cancer. Clin Breast Cancer. 2005;6:325–329. doi: 10.3816/CBC.2005.n.035. [DOI] [PubMed] [Google Scholar]
- 27.Fabi A, Metro G, Ferretti G, et al. Do HER-2 positive metastatic breast cancer patients benefit from the use of trastuzumab beyond disease progression? A mono-institutional experience and systematic review of observational studies. Breast. 2008;17:499–505. doi: 10.1016/j.breast.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 28.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 29.Mannocci A, De Feo E, de Waure C, et al. Use of trastuzumab in HER2-positive metastatic breast cancer beyond disease progression: a systematic review of published studies. Tumori. 2010;96:385–391. doi: 10.1177/030089161009600302. [DOI] [PubMed] [Google Scholar]
- 30.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 31.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 32.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325–334. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 34.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–4235. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 35.Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 36.Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16:2688–2695. doi: 10.1158/1078-0432.CCR-09-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burris HA, III, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 38.Krop I, LoRusso P, Miller KD, et al. A phase 2 study of the HER2 anitbody-drug conjugate trastuzumab-DM1 (T-MD1) in patients (PTS) with HER2-positive metastatic breast cancer (MBC) previously treated with tratuzumab, lapatinib, and chemotherapy. Ann Clin Oncol. 2010;21:viii–97. [Google Scholar]
- 39.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 40.Baselga J, Bradbury I, Eidtmann H, et al. First results of the NeoALTTO trial (BIG 01-06 / EGF 106903): a phase III randomized, open label, neoadjuvant study of lapatinib, trastuzumab, and their combination plus paclitaxel in women with HER2-positive primary breast cancer. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–12; San Antonio, TX. 2010. [Google Scholar]
- 41.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012. 366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneeweiss A, Chia S, Hickish T, et al. Neoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline-containing or concurrent with an anthracycline-free standard regimen: a randomized phase II study (TRYPHAENA). Presented at the 34th Annual San Antonio Breast Cancer Symposium; December 6–10, 2011; San Antonio, TX. [Google Scholar]
- 43.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 44.Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:1594–1600. doi: 10.1200/JCO.2011.37.4207. doi:10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 45.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 46.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 47.Tokunaga E, Kimura Y, Mashino K, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13:137–144. doi: 10.2325/jbcs.13.137. [DOI] [PubMed] [Google Scholar]
- 48.Tokunaga E, Kataoka A, Kimura Y, et al. The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. Eur J Cancer. 2006;42:629–635. doi: 10.1016/j.ejca.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y, Mohamed H, Chillar R, et al. Clinical significance of Akt and HER2/neu overexpression in African-American and Latina women with breast cancer. Breast Cancer Res. 2008;10:R3. doi: 10.1186/bcr1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Zhang Q, Zhang J, et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razis E, Bobos M, Kotoula V, et al. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128:447–456. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 52.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 53.Fujita T, Doihara H, Kawasaki K, et al. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94:247–252. doi: 10.1038/sj.bjc.6602926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 55.Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eichhorn PJ, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–9230. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Brien NA, Browne BC, Chow L, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 58.Kataoka Y, Mukohara T, Shimada H, et al. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21:255–262. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 59.Dave B, Migliaccio I, Gutierrez MC, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29:166–173. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vazquez-Martin A, Oliveras-Ferraros C, Colomer R, et al. Low-scale phosphoproteome analyses identify the mTOR effector p70 S6 kinase 1 as a specific biomarker of the dual-HER1/HER2 tyrosine kinase inhibitor lapatinib (Tykerb) in human breast carcinoma cells. Ann Oncol. 2008;19:1097–1109. doi: 10.1093/annonc/mdm589. [DOI] [PubMed] [Google Scholar]
- 61.Gijsen M, King P, Perera T, et al. HER2 phosphorylation is maintained by a PKB negative feedback loop in response to anti-HER2 herceptin in breast cancer. PLoS Biol. 2010;8:e1000563. doi: 10.1371/journal.pbio.1000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nencioni A, Cea M, Garuti A, et al. Grb7 upregulation is a molecular adaptation to HER2 signaling inhibition due to removal of Akt-mediated gene repression. PLoS ONE. 2010;5:e9024. doi: 10.1371/journal.pone.0009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunner-Kubath C, Shabbir W, Saferding V, et al. The PI3 kinase/mTOR blocker NVP-BEZ235 overrides resistance against irreversible ErbB inhibitors in breast cancer cells. Breast Cancer Res Treat. 2011;129:387–400. doi: 10.1007/s10549-010-1232-1. [DOI] [PubMed] [Google Scholar]
- 64.Goltsov A, Faratian D, Langdon SP, et al. Compensatory effects in the PI3K/PTEN/AKT signaling network following receptor tyrosine kinase inhibition. Cell Signal. 2011;23:407–416. doi: 10.1016/j.cellsig.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 66.Miller TW, Forbes JT, Shah C, et al. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009;15:7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 68.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 69.Yao E, Zhou W, Lee-Hoeflich ST, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–4156. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Garcia C, Ibrahim YH, Serra V, et al. Dual mTORC1/2 and HER2 Blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin Cancer Res. 2012;18:2603–2612. doi: 10.1158/1078-0432.CCR-11-2750. doi:10.1158/1078-0432.CCR-11-2750. [DOI] [PubMed] [Google Scholar]
- 71.Andre F, Campone M, O'Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 72.Jerusalem G, Fasolo A, Massacesi C, et al. Maintenance with everolimus (RAD001) and trastuzumab (T) after discontinuation of chemotherapy in heavily pre-treated HER-2+ metastatic breast cancer (MBC) patients (pts): pooled data of extension cohorts of phase Ib/II studies. Presented at the American Society of Clinical Oncology Annual Meeting; June 4–8, 2010; Chicago, IL. [Google Scholar]
- 73.Dalenc F, Campone M, Hupperets P, et al. Everolimus in combination with weekly paclitaxel and trastuzumab in patients (pts) with HER-2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab and taxanes: a multicenter phase II clinical trial. Presented at the American Society of Clinical Oncology Annual Meeting; June 4–8, 2010; Chicago, IL. [Google Scholar]
- 74.Campone M, Gianni L, Massacesi C, et al. Trastuzumab (H) and everolimus (RAD001) containing regimens are safe and active when reintroduced in patients (pts) with HER2-overexpressing metastatic breast cancer (MBC) pre-treated with lapatinib [abstract] Eur J Cancer. 2010;8(3):186. [Google Scholar]
- 75.Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 76.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellard SL, Clemons M, Gelmon KA, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27:4536–4541. doi: 10.1200/JCO.2008.21.3033. [DOI] [PubMed] [Google Scholar]
- 78.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka C, O'Reilly T, Kovarik JM, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–1602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 80.Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 82.Jahanzeb M, Mortimer JE, Yunus F, et al. Phase II trial of weekly vinorelbine and trastuzumab as first-line therapy in patients with HER2(+) metastatic breast cancer. Oncologist. 2002;7:410–417. doi: 10.1634/theoncologist.7-5-410. [DOI] [PubMed] [Google Scholar]
- 83.Burstein HJ, Harris LN, Marcom PK, et al. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol. 2003;21:2889–2895. doi: 10.1200/JCO.2003.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Chan A, Martin M, Untch M, et al. Vinorelbine plus trastuzumab combination as first-line therapy for HER 2-positive metastatic breast cancer patients: an international phase II trial. Br J Cancer. 2006;95:788–793. doi: 10.1038/sj.bjc.6603351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papaldo P, Fabi A, Ferretti G, et al. A phase II study on metastatic breast cancer patients treated with weekly vinorelbine with or without trastuzumab according to HER2 expression: changing the natural history of HER2-positive disease. Ann Oncol. 2006;17:630–636. doi: 10.1093/annonc/mdj110. [DOI] [PubMed] [Google Scholar]
- 86.Burstein HJ, Keshaviah A, Baron AD, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 87.De Maio E, Pacilio C, Gravina A, et al. Vinorelbine plus 3-weekly trastuzumab in metastatic breast cancer: a single-centre phase 2 trial. BMC Cancer. 2007;7:50. doi: 10.1186/1471-2407-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schilling G, Bruweleit M, Harbeck N, et al. Phase II trial of vinorelbine and trastuzumab in patients with HER2-positive metastatic breast cancer. a prospective, open label, non-controlled, multicenter phase II trial (to investigate efficacy and safety of this combination chemotherapy) Invest New Drugs. 2009;27:166–172. doi: 10.1007/s10637-008-9166-8. [DOI] [PubMed] [Google Scholar]
- 89.Nahta R, Yuan LX, Zhang B, et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 90.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 91.Modi S, Stopeck A, Linden H, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 92.Goldblatt EM, Erickson PA, Gentry ER, et al. Lipid-conjugated telomerase template antagonists sensitize resistant HER2-positive breast cancer cells to trastuzumab. Breast Cancer Res Treat. 2009;118:21–32. doi: 10.1007/s10549-008-0201-4. [DOI] [PubMed] [Google Scholar]