Abstract
Background
A number of epidemiological studies have reported inconsistent findings on the association between meat consumption and lung cancer.
Design
We therefore conducted a systematic review and meta-analysis to investigate the relationship between meat consumption and lung cancer risk in epidemiological studies.
Results
Twenty-three case–control and 11 cohort studies were included. All studies adjusted for smoking or conducted in never smokers. The summary relative risks (RRs) of lung cancer for the highest versus lowest intake categories were 1.35 (95% confidence interval (CI) 1.08–1.69) for total meat, 1.34 (95% CI 1.18–1.52) for red meat, and 1.06 (95% CI 0.90–1.25) for processed meat. An inverse association was found between poultry intake and lung cancer (RR = 0.91, 95% CI 0.85–0.97), but not for total white meat (RR = 1.06, 95% CI 0.82–1.37) or fish (RR = 1.01, 95% CI 0.96–1.07).
Conclusions
The relationship between meat intake and lung cancer risk appears to depend on the types of meat consumed. A high intake of red meat may increase the risk of lung cancer by about 35%, while a high intake of poultry decreases the risk by about 10%. More well-designed cohort studies on meat mutagens or heme iron, meat cooking preferences, and doneness level are needed to fully characterize this meat–lung cancer association.
Keywords: case–control study, cohort study, lung cancer, meat consumption, meta-analysis
introduction
Lung cancer is the leading cause of cancer-related mortality worldwide [1]. Cigaret smoking is the principal and an indisputable risk factor for lung cancer; however, numerous studies have shown that diet may also be of etiologic importance. Red meat (beef, pork, lamb, and goat from domesticated animals) and processed meat (meats preserved by smoking, curing, or salting, or by addition of chemical preservatives) have been hypothesized to play a role in carcinogenesis, owing to their high levels of saturated fat and heme iron content, and potent mutagens produced during high temperature cooking and meat processing or preservation, including heterocyclic amines (HCAs) [2, 3], polycyclic aromatic hydrocarbons (PAHs) [4, 5], and N-nitroso compounds (NOCs) [6]. On the contrary, white meat (poultry and fish), particularly fish intake, has been proposed to lower cancer risk [7–9], due to the relatively lower heme iron content in white meat and long-chain omega-3 polyunsaturated fatty acids present in fish.
Nevertheless, a previous report published in 2007 by World Cancer Research Fund and the American Institute for Cancer Research [10] concluded that the epidemiological evidence for a positive association of total fat, red meat, and processed meat intake with lung cancer risk is suggestive but not sufficient, while the evidence for poultry or fish intake in relation to the lung cancer risk is too limited to draw any conclusions. Recently, >30 epidemiological studies on the consumption of meat and the risk of lung cancer have been accumulated [11–44], and to our knowledge, there has not been any quantitative attempt to summarize the results of this possible meat–lung cancer association. We therefore conducted a systematic review and meta-analysis to investigate this relationship in observational studies.
methods
data sources and searches
We comprehensively identified studies through searching Medline (PubMed), EMBASE and Web of Science through November 2011 for both case–control and cohort studies that evaluated the effect of meat consumption on the risk of lung cancer. Our overall search strategy included terms for outcome (pulmonary neoplasm and lung cancer), exposure (meat, red meat, processed meat, white meat, beef, pork, lamb, goat, poultry, and fish), and study design (case–control study, cohort study, follow-up, prospective study, and longitudinal study). In addition, we carried out a broader search on diet and lung cancer so as to identify studies in which the aforementioned terms did not appear in abstracts. The searches were limited to studies of humans and published in English. The reference lists of retrieved articles were also reviewed in order to locate additional relevant studies.
study selection criteria
Published studies were included in the analysis if these (i) had a case–control or cohort design; (ii) evaluated the association between meats (total meat, white meat, red meat, processed meat, poultry, or fish) intake and lung cancer risk, and (iii) presented odds ratio (OR), relative risk (RR), or hazard ratio (HR) estimates with its 95% confidence interval (CI) or standard error (SE). If an article was duplicated or derived from the same study population as previously published, the most recent publication was included.
data extraction and quality assessment
Two investigators (WSY and RQT) independently searched the literature and determined study eligibility and conducted data extraction and quality assessment; discrepancies were settled by consensus or by involving a third reviewer (LX) for adjudication. Data extracted from the included studies are as follows: study name, authors, year of publication, study region, study design (case–control or cohort study), sample size (number of cases and controls or cohort size), length of follow-up for cohort studies, the exposure of meat intake, the study-specific adjusted ORs, RRs, or HRs with their 95% CIs or SEs for the highest category of meat consumption versus the lowest, and variables matched on or adjusted for in the design or data analysis. The total meat definition in our analysis included meat defined in the individual studies as ‘all meat’ without specifying the type, or ‘total meat’. White meat definition in our analysis included meat defined in the individual studies as ‘white meat’, or poultry and fish.
To assess the study quality, an improved 10-point scoring system based on the Newcastle–Ottawa Scale was used, which has been described in detail elsewhere [45]. Briefly, each study was judged on four broad perspectives: selection of the study groups, comparability of the groups, ascertainment of exposure and outcome, and methods used in data analysis. The maximum score was 10 and a high-quality study was defined as one with a score of ≥7.
data synthesis and analyses
The study-specific most adjusted association estimates were used as the common measure of association across studies and the ORs were considered to be equivalent to RRs or HRs because lung cancer is a rare outcome in humans. The possible heterogeneity in results across studies was examined by using the Cochran Q and I2 statistics [46]. The null hypothesis that the studies are homogeneous was rejected if the P value for heterogeneity was <0.10 or the I2 was ≥50%. When substantial heterogeneity was detected, the summary estimate based on the random effects model (DerSimonian and Laird method) [47] was reported. Otherwise, the summary estimate based on the fixed effects model (the inverse variance method) [48] was reported.
Subgroup analyses were conducted on study quality, study design (case–control compared with cohort studies), sex (men compared with women), histologic subtypes (adenocarcinoma, squamous cell carcinoma, and small cell lung cancer), study adjustments (with compared with without smoking, total energy intake, fruit and vegetable intake, PA, and BMI adjustments), and smoking status (current, ever, and never smokers). Due to the limited number of studies that reported risk estimates according to the smoking status, we only conducted subgroup analysis on smoking status for red meat intake.
To assess the influence of selected studies on the pooled results, sensitivity analysis was conducted firstly by excluding studies that reported the lung cancer mortality rather than incidence as an outcome [17, 18, 20], and then by excluding each study one by one and recalculating the combined estimates on the remaining studies. To assess the potential for misclassification bias of exposure (e.g. the highest category of meat consumption for one study may lie in the lowest category in another study), we repeated analysis in studies with the similar exposure categories as well as the reference group.
Publication bias was evaluated using Egger's linear regression [49] and Begg's rank correlation [50] methods. A P value of <0.05 for the two aforementioned tests was considered representative of significant statistical publication bias. All data analyses were carried out using R 2.13.1 (meta 1.6-1) (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
results
literature search and study characteristics
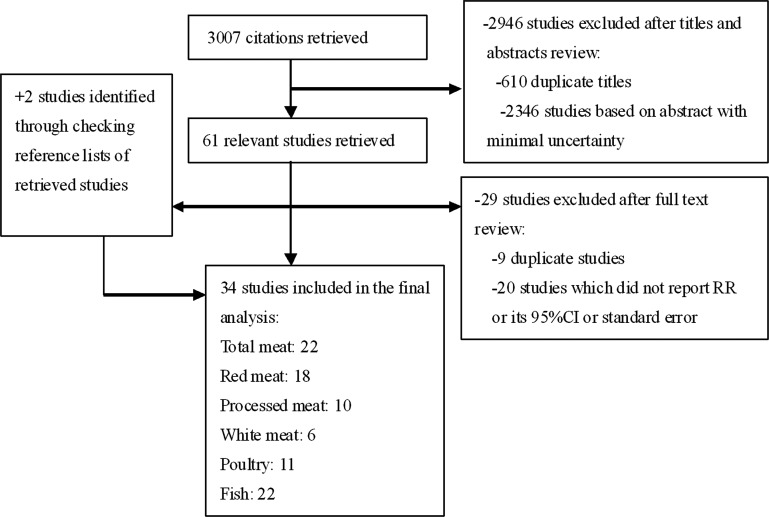
Figure 1 illustrates the search process and the final selection of relevant studies. Our systematic literature search yielded a total of 34 articles on meat intake and lung cancer risk in the final analysis. Descriptive data for the included studies are presented in supplementary Table S1, available at Annals of Oncology online. All of the studies were published from 1989 to 2011, consisting of 11 cohort [11–21] and 23 case–control [22–44] studies. The studies were conducted in Asia {n = 7 [15–17, 22–24, 28]}, North America {n = 10 [11, 12, 14, 18, 20, 21, 34, 36, 39, 43]}, Europe {n = 11 [13, 19, 29–33, 37, 38, 40, 42]}, and others {n = 6 [25–27, 35, 41, 44]}. The total number of participants in this meta-analysis was 1 797 042 including 30 293 lung cancer cases. All studies used food frequency questionnaires for the assessment of meat consumption. Most studies matched or adjusted for a wide range of potential confounders, including age, physical activity (PA), total energy intake, body mass index (BMI), fruit and vegetable intake, and alcohol consumption. All studies adjusted for smoking or conducted in never smokers.
Figure 1.
Search strategy and selection of studies for inclusion in the meta-analysis.
According to the 10-point scoring system, the study-specific quality scores are summarized in supplementary Table S2, available at Annals of Oncology online. The quality scores ranged from 2 to 9. The average scores (standard deviation) of case–control and cohort studies were 6.2 (1.4) and 7.6 (1.3), respectively. The high-quality studies consisted of 11 case–control studies [24–29, 33, 35, 36, 40, 43] and 9 cohort studies [11–14, 16–20].
total meat intake and lung cancer
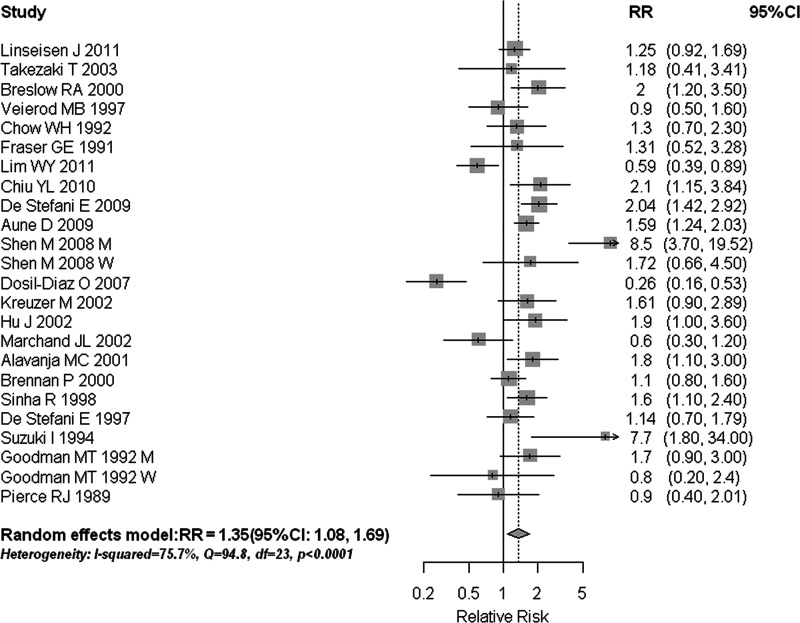
Among the 24 reports from the 22 studies on total meat intake, 18 reported a positive association, with 9 of them being statistically significant. We found that the high consumption of total meat was significantly associated with a 35% increased risk of lung cancer (RR = 1.35, 95% CI 1.08–1.69) (Table 1, Figure 2). Statistically significant heterogeneity was detected (I2 = 75.7%, Q = 94.78, P < 0.001). There was no indication of a publication bias, either from Egger's test (P = 0.857) or from Begg's test (P = 0.960). In subgroup analyses (Table 1), the results were fairly consistent with the overall summary measure when the analyses were restricted to high-quality studies and stratified by study design and histologic type; however, the positive association was not statistically significant in studies that adjusted for fruit and vegetable intake or in those that adjusted for the BMI. Sensitivity analysis by removing each study separately showed that excluding the study by Dosil-Diaz et al. [29] resulted in the highest summary estimate (RR = 1.45, 95% CI 1.19–1.76), while excluding the study by Shen et al. [28] resulted in the lowest summary estimate (RR = 1.26, 95% CI 1.02–1.56); sensitivity analysis where we omitted two studies [18, 20] that reported the risk estimates for lung cancer mortality rather than incidence showed similar results (RR = 1.33, 95% CI 1.05–1.70); sensitivity analysis in studies with the similar categories of total meat [16, 19, 33, 34, 38, 42] revealed that persons consuming meat >3 times per week had a RR of 1.29 (95% CI 1.02–1.63) compared with those consuming <2 times per week.
Table 1.
Summary relative risks (RRs) of the association between meat consumption and lung cancer risk
| No. of studies | RR (95% CI) | Q statistic | P value for heterogeneity | I2 value (%) | |
|---|---|---|---|---|---|
| Overall studies | |||||
| Total meat | 22 | 1.35 (1.08–1.69) | 94.78 | <0.001 | 75.7 |
| Red meat | 18 | 1.34 (1.18–1.52) | 55.35 | <0.001 | 63.9 |
| Processed meat | 10 | 1.06 (0.90–1.25) | 58.42 | <0.001 | 79.5 |
| White meat | 6 | 1.06 (0.82–1.37) | 14.20 | 0.014 | 64.8 |
| Poultry | 11 | 0.91 (0.85–0.97) | 16.85 | 0.112 | 34.7 |
| Fish | 22 | 1.01 (0.96–1.07) | 40.76 | 0.013 | 43.6 |
| Subgroup analyses for total meat | |||||
| High-quality studies (scores ≥7) | 14 | 1.41 (1.06–1.87) | 67.94 | <0.001 | 77.9 |
| Study design | |||||
| Case–control studies | 16 | 1.38 (1.04–1.85) | 90.51 | <0.001 | 81.2 |
| Cohort studies | 6 | 1.30 (1.05–1.60) | 4.12 | 0.532 | 0 |
| Adjustments in models | |||||
| Smoking | |||||
| Yes | 22 | 1.35 (1.08–1.69) | 94.78 | <0.001 | 75.7 |
| No | – | – | – | – | – |
| Total energy intake | |||||
| Yes | 5 | 1.52 (1.27–1.83) | 6.49 | 0.165 | 38.4 |
| No | 17 | 1.27 (1.13–1.44) | 85.74 | <0.001 | 79.0 |
| Fruit and vegetable intake | |||||
| Yes | 7 | 1.12 (0.74–1.71) | 53.17 | <0.001 | 88.7 |
| No | 15 | 1.49 (1.15–1.94) | 41.02 | <0.001 | 61.0 |
| Body mass index (BMI) | |||||
| Yes | 4 | 1.22 (0.71–2.11) | 21.45 | <0.001 | 86.0 |
| No | 18 | 1.29 (1.08–1.80) | 73.01 | <0.001 | 74.0 |
| Histologic subtypes | |||||
| Adenocarcinoma | 8 | 1.23 (1.04–1.46) | 44.42 | <0.001 | 66.2 |
| Squamous cell carcinoma | 6 | 1.47 (1.31–1.66) | 14.71 | 0.065 | 45.6 |
| Small cell lung cancer | 4 | 1.30 (1.14–1.49) | 5.00 | 0.660 | 0 |
| Subgroup analyses for red meat | |||||
| High-quality studies (scores ≥7) | 9 | 1.32 (1.11–1.57) | 49.84 | <0.001 | 79.9 |
| Study design | |||||
| Case–control studies | 13 | 1.42 (1.16–1.74) | 36.41 | <0.001 | 67.0 |
| Cohort studies | 5 | 1.20 (1.10–1.30) | 2.50 | 0.927 | 0 |
| Smoking status | |||||
| Never smoking | 5 | 1.66 (1.31–2.11) | 8.00 | 0.156 | 37.5 |
| Ever smoking | 3 | 1.52 (1.07–2.16) | 17.43 | <0.001 | 82.8 |
| Current smoking | 5 | 1.41 (1.10–1.80) | 21.82 | <0.001 | 77.1 |
| Gender | |||||
| Men | 6 | 1.30 (1.02–1.66) | 13.47 | 0.019 | 62.9 |
| Women | 9 | 1.23 (1.00–1.50) | 16.29 | 0.038 | 50.9 |
| Subgroup analyses for processed meat | |||||
| High-quality studies (scores ≥7) | 8 | 1.07 (0.90–1.27) | 55.90 | <0.001 | 82.1 |
| Study design | |||||
| Case–control studies | 6 | 1.05 (0.75–1.49) | 42.88 | <0.001 | 86.0 |
| Cohort studies | 4 | 1.05 (0.92–1.19) | 9.84 | 0.080 | 49.2 |
| Gender | |||||
| Men | 5 | 1.13 (0.85–1.49) | 15.52 | 0.003 | 74.2 |
| Women | 4 | 0.95 (0.84–1.07) | 5.42 | 0.147 | 44.7 |
| Subgroup analyses for white meat | |||||
| High-quality studies (scores ≥7) | 3 | 1.27 (0.79–2.03) | 10.02 | 0.007 | 80.0 |
| Study design | |||||
| Case–control studies | 5 | 1.13 (0.78–1.62) | 13.71 | 0.008 | 70.8 |
| Cohort studies | 1 | 0.95 (0.79–1.14) | N/A | N/A | N/A |
| Gender | |||||
| Men | 2 | 1.09 (0.83–1.42) | 0.39 | 0.531 | 0 |
| Women | 2 | 1.74 (0.54–5.58) | 7.76 | 0.005 | 87.1 |
| Subgroup analyses for poultry | |||||
| High-quality studies (scores ≥7) | 5 | 0.89 (0.84–0.95) | 7.76 | 0.170 | 35.5 |
| Study design | |||||
| Case–control studies | 8 | 0.89 (0.75–1.06) | 12.72 | 0.122 | 37.1 |
| Cohort studies | 3 | 0.95 (0.64–1.39) | 4.10 | 0.129 | 51.2 |
| Gender | |||||
| Men | 5 | 0.83 (0.62–1.11) | 8.08 | 0.089 | 50.5 |
| Women | 4 | 0.99 (0.67–1.48) | 4.32 | 0.229 | 30.5 |
| Subgroup analyses for fish | |||||
| High-quality studies (scores ≥7) | 13 | 1.02 (0.96–1.08) | 18.80 | 0.173 | 25.5 |
| Study design | |||||
| Case–control studies | 15 | 0.97 (0.83–1.15) | 31.52 | 0.008 | 52.4 |
| Cohort studies | 7 | 1.02 (0.96–1.08) | 9.17 | 0.241 | 23.7 |
| Gender | |||||
| Men | 6 | 1.03 (0.87–1.22) | 4.98 | 0.419 | 0 |
| Women | 7 | 0.92 (0.66–1.29) | 20.38 | 0.002 | 70.6 |
aCI, confidence interval; N/A, not available; RR, relative risk; –, no available studies.
Figure 2.
Estimates (95% CIs) of total meat consumption and lung cancer risk. Squares represent study-specific estimates [size of the square reflects the study-specific statistical weight (i.e. inverse of the variance)]; horizontal lines represent 95% CIs; and diamonds represent summary estimates with corresponding 95% CIs. M, men; W, women.
red and processed meat intakes and lung cancer risk
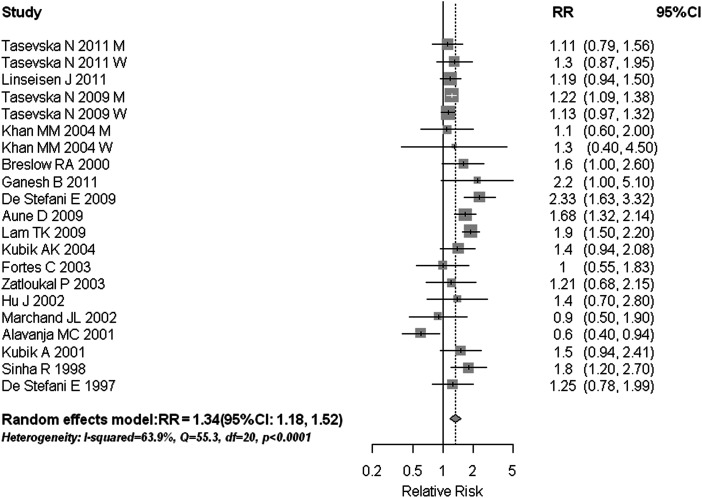
Our analysis of 18 studies on red meat consumption and lung cancer yielded a summary RR of 1.34 (95% CI 1.18–1.52) (Figure 3). This positive association was observed across all subgroup analyses, regardless of study quality, study design, smoking status, sex, histologic subtype (Table 1), and adjustments for total energy intake, fruit and vegetable intake, PA, and BMI (data not shown). We found no evidence of publication bias (Egger's test: P = 0.799; Begg's test: P = 0.952); however, substantial heterogeneity was observed in these studies (I2 = 63.9%, Q = 55.35, P < 0.001). There was no indication of increased risk for lung cancer when we combined the 10 studies of processed meat intake (RR = 1.06, 95% CI 0.90–1.25; I2 = 79.5%, Q = 58.42, P < 0.001) (Table 1, supplementary Figure S1, available at Annals of Oncology online). Like total meat intake, the sensitivity analyses conducted for processed meat did not alter the main results (data not shown).
Figure 3.
Estimates (95% CIs) of red meat consumption and lung cancer risk. Squares represent study-specific estimates [size of the square reflects the study-specific statistical weight (i.e. inverse of the variance)]; horizontal lines represent 95% CIs; and diamonds represent summary estimates with corresponding 95% CIs. M, men; W, women.
white meat, poultry, fish intakes, and lung cancer risk
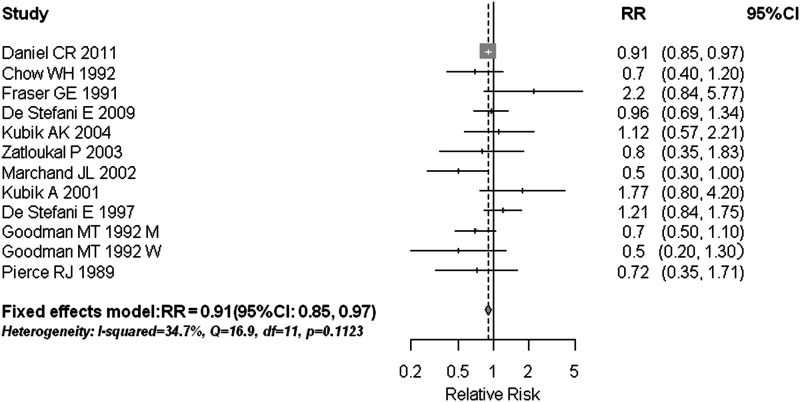
A high poultry intake can weakly decrease the risk of lung cancer (RR = 0.91, 95% CI 0.85–0.97) with an I2 of 34.7% and Q statistic of 16.85 (P = 0.112) (Figure 4). The combined results were consistent among high-quality studies with a RR of 0.89 (95% CI 0.84–0.95), but not statistically significant within either cohort or case–control studies separately, with only few studies of each design (n = 3 and 8, respectively). Intake of fish (supplementary Figure S2, available at Annals of Oncology online) and total white meat (supplementary Figure S3, available at Annals of Oncology online) was not associated with a lower risk of lung cancer, with summary RRs of 1.01 (95% CI 0.96–1.07) and 1.06 (95% CI 0.82–1.37), respectively. These null associations were unchanged in subgroup or sensitivity analyses that accounted for study quality, study design, statistical adjustments, type of outcome reported, and levels of meat consumed (data not shown). No significant publication bias was observed for total white meat (Egger's test: P = 0.310; Begg's test: P = 0.189), poultry (Egger's test: P = 0.913; Begg's test: P = 0.493), or fish (Egger's test: P = 0.594; Begg's test: P = 0.172).
Figure 4.
Estimates (95% CIs) of poultry consumption and lung cancer risk. Squares represent study-specific estimates [size of the square reflects the study-specific statistical weight (i.e. inverse of the variance)]; horizontal lines represent 95% CIs; and diamonds represent summary estimates with corresponding 95% CIs. M, men; W, women.
discussion
This, to our knowledge, is the first meta-analysis to explore the relationship between meat consumption and lung cancer risk. In the present study, we found that the total meat intake is positively associated with the risk of lung cancer. Nonetheless, this link was attenuated by other factors (adjustment for fruit and vegetable intake and BMI), and the association varied by the type of meat consumed. Specifically, a high intake of red meat, but not processed meat, was observed to increase the risk of lung carcinoma; while a higher consumption of poultry, but not total white meat or fish intake, was observed to decrease the risk of lung carcinoma, and these observed results were robust across the subgroup and sensitivity analyses that accounted for study quality, study design, smoking status (for red meat), sex, histologic subtype, statistical adjustments, type of outcome reported (lung cancer incidence and mortality), the influence of each individual study, and the potential for misclassification of meat consumption.
It has been hypothesized that mutagenic byproducts, including HCAs [2, 3] and PAHs [4, 5], from cooking meat could contribute to lung carcinogenesis. However, evidence from the epidemiological studies of HCAs and PAHs and lung cancer has been inconclusive and limited to a few investigations [12, 14, 22, 25, 27]. Two studies [14, 39] have shown that the intake of well-done meat was significantly associated with the elevated risk of lung cancer, whereas a recent study by Tasevska et al. [12] found no such effect. A second possible mechanism for the adverse effect of red meat, specifically, on lung cancer is via its high content of heme iron which may act as a pro-oxidant and catalyze lipid peroxidation causing DNA damage in tissues [51]. Heme iron has also been shown to induce endogenous formation of NOCs [52]. In addition, high levels of saturated fat present in red meat may be associated with the increased risk of lung cancer, but a study that pooled the raw data from 12 prospective cohorts failed to show such a relation [53]. A possible explanation for the differences in associations between red and processed meats is that cooking red meat is more likely to be over an open grill and produce PAHs than cooking processed meat. However, the mechanism by which poultry intake alone may be associated with a lower lung cancer risk is not well understood, but may be possibly due to its lower content of heme iron compared with red meat. Another explanation is that high poultry eaters often have a healthier overall eating pattern and lifestyle [54].
Strengths of our study include a large sample size (30 293 cases among 1 797 042 participants) and no significant evidence of publication bias. However, several limitations to this meta-analysis should be noted. First, as a meta-analysis of observational data, the possibility of recall and selection biases cannot be ruled out. However, cohort studies, which are less susceptible to bias, showed similar results to case–control studies, indicating that the findings were unlikely to be attributed to recall and selection biases. Second, because of the inability to fully adjust for various confounders, particularly for total energy intake, fruit and vegetable intake, PA, and BMI, which tend to be highly correlated to consumption of most foods and nutrients including meats, and could also be an independent risk factor for lung cancer, may have confounded the reported links making the independent effect of meat intake difficult to determine. For example, the pronounced association between total meat intake and the increased risk of lung carcinoma was no longer observed among studies that adjusted for fruit and vegetable intake [12, 22, 25, 26, 29, 39, 43] or for the BMI [22, 39, 41]. Nonetheless, when we explored the associations for other types of meat (i.e. red meat, processed meat, total white meat, poultry, and fish), the aforementioned variables did not appear to attenuate the findings, given the consistent results observed in each stratum of subgroup analysis. Moreover, since smoking is the most important risk factor for lung cancer, all of included studies adjusted for smoking in statistical models or were conducted among nonsmokers [22, 30, 33, 34, 38, 40]. When we estimated the effect of red meat by smoking status, the results were similar in each stratum. Third, there was a statistically significant heterogeneity across studies with the exception of the studies on poultry intake. For studies on total meat and any other types of meat, the heterogeneity was none or smaller when restricted analysis in cohort studies. The little heterogeneity was also shown in never smokers for red meat, in female sex for processed meat, and in male sex for fish. In addition, studies with data analysis that adjusted for PA or did not adjust for the BMI, or fruit and vegetable intake revealed the homogenous results for any types of meat intake (data not shown). These suggested that the heterogeneity may be partly due to the difference in study design, study populations, and analytic strategies. Although heterogeneity still remained in some subgroups (Table 1), indicating that other unknown factors may also contribute to the aforementioned heterogeneity, results from subgroup analysis cannot alter the main findings in our study. Fourth, because the majority of studies used food frequency questionnaires to collect data regarding the meat consumption, our findings are likely to be influenced by the misclassification of exposure. In cohort studies, this misclassification would likely be non-differential if the exposure variable was dichotomous, and thereby result in an underestimate of the true association, whereas the influence of a misclassification on the results in case–control studies is less predictable. Lastly, due to the different methods used to assess and categorize meat intake among studies (supplementary Table S1, available at Annals of Oncology online), we were unable to evaluate potential dose–response trends between meat intake and lung cancer risk.
In conclusion, our meta-analysis of 34 epidemiological studies suggests that a high intake of red meat may increase the risk of lung cancer by ∼35%, while the high intake of poultry may decrease the risk by about 10%. There was no evidence that the consumption of processed meat, total white meat, or fish was related to lung cancer risk. Cohort studies with long-term follow-up and large sample sizes that fully adjust for potential confounders, such as total energy intake, BMI, PA, and other dietary factors that are highly correlated to meat consumption, are warranted to reach more definitive conclusions. In addition, additional research on meat type, heme iron, cooking method, doneness level, and consumption of meat mutagens should be conducted in order to test the different possible mechanisms for the effect of meat on lung carcinoma.
funding
This work was supported by the fund of the State Key Project Specialized for Infectious Diseases of China (2008ZX10002-015). EV was also supported by the Fogarty International Clinical Research Scholars and Fellows Support Center at the Vanderbilt Institute for Global Health, funded by the Fogarty International Center, NIH, through an R24 Training Grant (Grant number: R24TW007988), and the Cancer Prevention and Control Training Program at the University of Alabama at Birmingham funded through the National Institutes of Health (5R25 CA047888).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. doi:10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Puangsombat K, Gadgil P, Houser TA, et al. Heterocyclic amine content in commercial ready to eat meat products. Meat Sci. 2011;88:227–233. doi: 10.1016/j.meatsci.2010.12.025. doi:10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 3.Sinha R, Knize MG, Salmon CP, et al. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem Toxicol. 1998;36:289–297. doi: 10.1016/s0278-6915(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 4.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 5.Kazerouni N, Sinha R, Hsu CH, et al. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001;39:423–436. doi: 10.1016/s0278-6915(00)00158-7. doi:10.1532/HSF98.20071140. [DOI] [PubMed] [Google Scholar]
- 6.Haorah J, Zhou L, Wang X, et al. Determination of total N-nitroso compounds and their precursors in frankfurters, fresh meat, dried salted fish, sauces, tobacco, and tobacco smoke particulates. J Agric Food Chem. 2001;49:6068–6078. doi: 10.1021/jf010602h. doi:10.1093/eurheartj/ehq445. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Liang J, Zhang L, et al. Fish consumption and the risk of gastric cancer: systematic review and meta-analysis. BMC Cancer. 2011;11:26. doi: 10.1186/1471-2407-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr. 2010;92:1223–1233. doi: 10.3945/ajcn.2010.29530. doi:10.1016/j.ejcts.2008.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. doi:10.1016/j.athoracsur.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 11.Daniel CR, Cross AJ, Graubard BI, et al. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila) 2011;4:1903–1911. doi: 10.1158/1940-6207.CAPR-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tasevska N, Cross AJ, Dodd KW, et al. No effect of meat, meat cooking preferences, meat mutagens or heme iron on lung cancer risk in the prostate, lung, colorectal and ovarian cancer screening trial. Int J Cancer. 2011;128:402–411. doi: 10.1002/ijc.25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linseisen J, Rohrmann S, Bueno-de-Mesquita B, et al. Consumption of meat and fish and risk of lung cancer: results from the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2011;22:909–918. doi: 10.1007/s10552-011-9764-1. [DOI] [PubMed] [Google Scholar]
- 14.Tasevska N, Sinha R, Kipnis V, et al. A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am J Clin Nutr. 2009;89:1884–1894. doi: 10.3945/ajcn.2008.27272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MM, Goto R, Kobayashi K, et al. Dietary habits and cancer mortality among middle aged and older Japanese living in Hokkaido, Japan by cancer site and sex. Asian Pac J Can Prev. 2004;5:58–65. [PubMed] [Google Scholar]
- 16.Takezaki T, Inoue M, Kataoka H, et al. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: with special reference to fish consumption. Nutr Cancer. 2003;45:160–167. doi: 10.1207/S15327914NC4502_04. [DOI] [PubMed] [Google Scholar]
- 17.Ozasa K, Watanabe Y, Ito Y, et al. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC study) in Japan by sex and smoking habit. Jpn J Cancer Res. 2001;92:1259–1269. doi: 10.1111/j.1349-7006.2001.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breslow RA, Graubard BI, Sinha R, et al. Diet and lung cancer mortality: a 1987 national health interview survey cohort study. Cancer Causes Control. 2000;11:419–431. doi: 10.1023/a:1008996208313. [DOI] [PubMed] [Google Scholar]
- 19.Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of lung cancer: a prospective study of 51,452 Norwegian men and women. Eur J Cancer Prev. 1997;6:540–549. doi: 10.1097/00008469-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Chow WH, Schuman LM, McLaughlin JK, et al. A cohort study of tobacco use, diet, occupation, and lung cancer mortality. Cancer Causes Control. 1992;3:247–254. doi: 10.1007/BF00124258. [DOI] [PubMed] [Google Scholar]
- 21.Fraser GE, Beeson WL, Phillips RL. Diet and lung cancer in California seventh-day adventists. Am J Epidemiol. 1991;133:683–693. doi: 10.1093/oxfordjournals.aje.a115943. [DOI] [PubMed] [Google Scholar]
- 22.Lim WY, Chuah KL, Eng P, et al. Meat consumption and risk of lung cancer among never-smoking women. Nutr Cancer. 2011;63:850–859. doi: 10.1080/01635581.2011.589961. [DOI] [PubMed] [Google Scholar]
- 23.Ganesh B, Sushama S, Monika S, et al. A case–control study of risk factors for lung cancer in Mumbai, India. Asian Pac J Cancer Prev. 2011;12:357–362. [PubMed] [Google Scholar]
- 24.Chiu YL, Wang XR, Qiu H, et al. Risk factors for lung cancer: a case–control study in Hong Kong women. Cancer Causes Control. 2010;21:777–785. doi: 10.1007/s10552-010-9506-9. [DOI] [PubMed] [Google Scholar]
- 25.De Stefani E, Boffetta P, Deneo-Pellegrini H, et al. Meat intake, meat mutagens and risk of lung cancer in Uruguayan men. Cancer Causes Control. 2009;20:1635–1643. doi: 10.1007/s10552-009-9411-2. [DOI] [PubMed] [Google Scholar]
- 26.Aune D, De Stefani E, Ronco A, et al. Meat consumption and cancer risk: a case–control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:429–436. [PubMed] [Google Scholar]
- 27.Lam TK, Cross AJ, Consonni D, et al. Intakes of red meat, processed meat, and meat mutagens increase lung cancer risk. Cancer Res. 2009;69:932–939. doi: 10.1158/0008-5472.CAN-08-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen M, Chapman RS, He X, et al. Dietary factors, food contamination and lung cancer risk in Xuanwei, China. Lung Cancer. 2008;61:275–282. doi: 10.1016/j.lungcan.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Dosil-Diaz O, Ruano-Ravina A, Gestal-Otero JJ, et al. Meat and fish consumption and risk of lung cancer: a case–control study in Galicia, Spain. Cancer Lett. 2007;252:115–122. doi: 10.1016/j.canlet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Kubik AK, Zatloukal P, Tomasek L, et al. Dietary habits and lung cancer risk among non-smoking women. Eur J Cancer Prev. 2004;13:471–480. doi: 10.1097/00008469-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Fortes C, Forastiere F, Farchi S, et al. The protective effect of the mediterranean diet on lung cancer. Nutr Cancer. 2003;46:30–37. doi: 10.1207/S15327914NC4601_04. [DOI] [PubMed] [Google Scholar]
- 32.Zatloukal P, Kubik A, Pauk N, et al. Adenocarcinoma of the lung among women: risk associated with smoking, prior lung disease, diet and menstrual and pregnancy history. Lung Cancer. 2003;41:283–293. doi: 10.1016/s0169-5002(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 33.Kreuzer M, Heinrich J, Kreienbrock L, et al. Risk factors for lung cancer among nonsmoking women. Int J Cancer. 2002;100:706–713. doi: 10.1002/ijc.10549. [DOI] [PubMed] [Google Scholar]
- 34.Hu J, Mao Y, Dryer D, White K. Risk factors for lung cancer among Canadian women who have never smoked. Cancer Detect Prev. 2002;26:129–138. doi: 10.1016/s0361-090x(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 35.Marchand JL, Luce D, Goldberg P, et al. Dietary factors and the risk of lung cancer in New Caledonia (south pacific) Nutr Cancer. 2002;42:18–24. doi: 10.1207/S15327914NC421_3. [DOI] [PubMed] [Google Scholar]
- 36.Alavanja MC, Field RW, Sinha R, et al. Lung cancer risk and red meat consumption among Iowa women. Lung Cancer. 2001;34:37–46. doi: 10.1016/s0169-5002(01)00227-6. [DOI] [PubMed] [Google Scholar]
- 37.Kubik A, Zatloukal P, Tomasek L, et al. Diet and the risk of lung cancer among women. A hospital-based case–control study. Neoplasma. 2001;48:262–266. [PubMed] [Google Scholar]
- 38.Brennan P, Fortes C, Butler J, et al. A multicenter case–control study of diet and lung cancer among non-smokers. Cancer Causes Control. 2000;11:49–58. doi: 10.1023/a:1008909519435. [DOI] [PubMed] [Google Scholar]
- 39.Sinha R, Kulldorff M, Curtin J, et al. Fried, well-done red meat and risk of lung cancer in women (United States) Cancer Causes Control. 1998;9:621–630. doi: 10.1023/a:1008805525525. [DOI] [PubMed] [Google Scholar]
- 40.Nyberg F, Agrenius V, Svartengren K, et al. Dietary factors and risk of lung cancer in never-smokers. Int J Cancer. 1998;78:430–436. doi: 10.1002/(sici)1097-0215(19981109)78:4<430::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 41.De Stefani E, Fontham ET, Chen V, et al. Fatty foods and the risk of lung cancer: a case-control study from Uruguay. Int J Cancer. 1997;71:760–766. doi: 10.1002/(sici)1097-0215(19970529)71:5<760::aid-ijc12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki I, Hamada GS, Zamboni MM, et al. Risk factors for lung cancer in Rio de Janeiro, Brazil: a case-control study. Lung Cancer. 1994;11:179–190. doi: 10.1016/0169-5002(94)90538-x. [DOI] [PubMed] [Google Scholar]
- 43.Goodman MT, Hankin JH, Wilkens LR, et al. High-fat foods and the risk of lung cancer. Epidemiology. 1992;3:288–299. doi: 10.1097/00001648-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Pierce RJ, Kune GA, Kune S, et al. Dietary and alcohol intake, smoking pattern, occupational risk, and family history in lung cancer patients: results of a case-control study in males. Nutr Cancer. 1989;12:237–248. doi: 10.1080/01635588909514023. [DOI] [PubMed] [Google Scholar]
- 45.Yang WS, Va P, Wong MY, et al. Soy intake is associated with lower lung cancer risk: results from a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2011;94:1575–1583. doi: 10.3945/ajcn.111.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 48.WOOLF B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 49.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 51.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res. 2003;533:153–171. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 53.Smith-Warner SA, Ritz J, Hunter DJ, et al. Dietary fat and risk of lung cancer in a pooled analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2002;11:987–992. [PubMed] [Google Scholar]
- 54.Flood A, Rastogi T, Wirfalt E, et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr. 2008;88:176–184. doi: 10.1093/ajcn/88.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.