Abstract
Background
The evaluation of asthma symptoms is a core outcome measure in asthma clinical research. The Asthma Symptom Utility Index (ASUI) was developed to assess frequency and severity of asthma symptoms. The psychometric properties of the ASUI are not well characterized and a minimal important difference (MID) is not established.
Objectives
We assessed the reliability, validity, and responsiveness to change of the ASUI in a population of adult asthma patients. We also sought to determine the MID for the ASUI.
Methods
Adult asthma patients (n = 1648) from two previously completed multicenter randomized trials were included. Demographic information, spirometry, ASUI scores, and other asthma questionnaire scores were obtained at baseline and during follow-up visits. Participants also kept a daily asthma diary.
Results
Internal consistency reliability of the ASUI was 0.74 (Cronbach’s alpha). Test-retest reliability was 0.76 (intra-class correlation). Construct validity was demonstrated by significant correlations between ASUI scores and Asthma Control Questionnaire (ACQ) scores (Spearman correlation r = −0.79, 95% CI [−0.85, −0.75], P<0.001) and Mini Asthma Quality of Life Questionnaire (Mini AQLQ) scores (r = 0.59, 95% CI [0.51, 0.61], P<0.001). Responsiveness to change was demonstrated, with significant differences between mean changes in ASUI score across groups of participants differing by 10% in the percent predicted FEV1 (P<0.001), and by 0.5 points in ACQ score (P < 0.001). Anchor-based methods and statistical methods support an MID for the ASUI of 0.09 points.
Conclusions
The ASUI is reliable, valid, and responsive to changes in asthma control over time. The MID of the ASUI (range of scores 0–1) is 0.09.
Keywords: Asthma Symptom Utility Index, reliability, validity, responsiveness, minimal important difference
INTRODUCTION
Asthma is a chronic disease associated with substantial morbidity1. Recent asthma guidelines highlight the need to achieve and maintain good disease control1, 2. To assess asthma control in research and clinical practice, well validated questionnaires such as the Asthma Control Questionnaire (ACQ) 3 and the Asthma Control Test (ACT) 4 are often used. Asthma-specific quality of life questionnaires such as the mini Asthma Quality of Life Questionnaire (Mini AQLQ) 5 and the Marks Asthma Quality of Life Questionnaire (AQLQ-Marks) 6 gauge the impact of asthma on the patient’s functioning and well-being. The evaluation of asthma symptoms is a recommended core outcome measure in asthma clinical research, yet there is currently no widely accepted instrument for the standardized measurement of asthma symptoms18. A recent National Institutes of Health (NIH) working group found the Asthma Symptom Utility Index (ASUI) to be promising but not adequately validated18. The ASUI was developed in 1998 by Revicki et al. to measure the degree of asthma symptoms and their impact on patients 7. Some items on the ASUI are similar to those on questionnaires that assess asthma control and asthma-related quality of life3, 5, 14, 15. However, composite scores obtained from these questionnaires allocate equal weight to each item even though the impact of different symptoms on patients may vary. The ASUI is unique insofar as it is a weighted scale and thus particularly valuable in cost-utility analyses7. It is increasingly being used in asthma clinical research 8, 9. The initial study by Revicki et al. showed that the ASUI had good reproducibility (intraclass correlation [ICC] = 0.74), good construct validity (Pearson’s correlation coefficient with the AQLQ = 0.77), and good discriminant validity7. Nonetheless, a comprehensive evaluation of the psychometric properties of the ASUI is lacking. In addition, a minimal important difference (MID) for the ASUI has not been established. Our objectives were to assess the reliability, validity, and responsiveness to change of the ASUI in a population of adult asthma patients participating in two multicenter randomized trials. We also sought to determine the MID for the ASUI.
METHODS
The ASUI
The ASUI is a 10-item self-administered questionnaire with four questions on asthma symptoms (cough, wheeze, shortness of breath, and awakening at night) and one question about side effects of asthma medications. For each symptom, there are two dimensions - frequency and severity. The questionnaire is based on a two week patient recall of symptoms and is scored using a previously derived multi-attribute utility function7. The weighting scheme of the ASUI was developed by first constructing health states with single or multiple asthma symptoms at different frequencies and severities7. Next, the participants were asked to attribute a relative value to various health states using a visual analog scale (VAS) and standard gamble methods 7. Finally, these data were used to derive a multi-attribute utility function for scoring individual patient symptoms7. The summary score is a continuous scale from 0 to 1 with lower scores indicating worse asthma symptoms7. Details on the development of the ASUI have been previously published7.
Data collection
Patients
Data from 1648 adult asthma participants (≥ 18 years) enrolled in two completed clinical trials conducted by the American Lung Association-Asthma Clinical Research Centers (ALA-ACRC) were included in this analysis8, 10. The Study of Inactivated Influenza Vaccine in Asthmatics (SIIVA) trial was conducted between September 15 and November 30, 2000 and showed that the inactivated trivalent split-virus influenza vaccine was safe in adults and children with asthma10. The Study of Acid Reflux and Asthma (SARA) trial was conducted between October 2004 and May 20088. It showed that proton pump inhibitors (PPIs) did not improve asthma control in adults whose asthma was not well controlled on inhaled corticosteroids8.
Procedures
The protocols for both studies were approved by institutional review boards in each of the participating centers and informed consent was obtained from each participant. The SARA trial was registered on ClinicalTrials.gov (NCT00069823); the SIIVA trial was conducted before NIH registration requirements were instituted. In the SIIVA trial, baseline demographic data and ASUI score were obtained for all participants (N=1236). Baseline spirometry was obtained in a subset of participants (N=704). After administration of either vaccine or placebo, participants were followed for 14 days during which they kept a daily asthma diary with information on asthma related symptoms, peak expiratory flow rate (PEFR), healthcare utilization, and medication use. After crossover, there was another 14 day follow-up period10. In the SARA trial (N=412), baseline demographic data, spirometry, ASUI score, ACQ score, and the Mini AQLQ score were obtained. Patients were then randomized to either esomeprazole 40mg twice daily or placebo in addition to their inhaled corticosteroid regimen for a total of twenty-four weeks. During follow-up clinic visits that occurred every four weeks, ASUI scores, ACQ scores, and Mini AQLQ scores were obtained. Patients also kept an asthma diary that was returned during each clinic visit8.
Assessments
Reliability
To evaluate the internal consistency reliability, the Cronbach’s α coefficient was calculated using baseline ASUI data from both SIIVA and SARA. Cronbach’s alpha coefficient measures the degree to which the items on the questionnaire measure the same unidirectional construct. Test-retest reliability was assessed by calculating the intraclass correlation coefficient (ICC) between the baseline ASUI score and the ASUI score at the next follow-up visit (four weeks apart) using data from participants in the SARA trial with stable asthma. Stable asthma was defined by the absence of an episode of poor asthma control (EPAC) 8, and no clinically significant change in the ACQ scores3 and Mini AQLQ scores5 (change less than 0.5 points). An EPAC was defined by the occurrence of at least one of the following events: an increase in rescue medication use for asthma symptoms by four or more inhalations per day over baseline, the occurrence of an unscheduled contact with a healthcare provider for asthma, use of systemic corticosteroids for asthma, or a decrease of 30% or more in morning PEFR on 2 consecutive days, as compared with the patient’s best PEFR during the run-in period8.
Construct validity
Construct validity of the ASUI was assessed using data from the SARA trial by computing Spearman’s correlations between baseline ASUI scores and (1) baseline ACQ3 scores, and (2) baseline Mini AQLQ5 scores.
Known-groups validity
Known-groups validity was assessed using data from the SIIVA trial by comparing the mean baseline ASUI score across three categories of baseline percent predicted pre-bronchodilator forced expiratory volume in one second (FEV1) values: (1) less than 60%; (2) 60% to 79 %; (3) greater than or equal to 80%. The mean baseline ASUI score was also compared across a four point scale of ascending asthma severity among SIIVA participants based on asthma medication use at baseline (1 = intermittent, 2 = mild, 3 = moderate, 4 = severe) 11. Asthma severity was assessed according to an approximate Global Initiative for Asthma (GINA) medication classification 11. Data on other methodologies for assessing asthma severity such as the National Asthma Education and Prevention Program (NAEPP) and GINA classifications were not available for this post hoc analysis. Previous studies have shown that current asthma medication use complements other classifications of asthma severity20, 21. One-way ANOVA was then used to test the significance of differences in mean ASUI scores across groups of patients who differed by percent predicted FEV1 and asthma severity categories.
Predictive validity
Using data from the SIIVA trial, predictive validity was assessed by comparing the frequency of EPACs and asthma exacerbations over the next two weeks by quartiles of baseline ASUI. An asthma exacerbation was defined by new use of systemic corticosteroids or an unscheduled contact with a health care provider. The ASUI was classified by quartiles because on exploratory data analysis, baseline ASUI scores had a skewed distribution, such that a majority of patients had very high scores and fewer patients had low scores. Using the highest ASUI quartile as the reference, the relative risk (RR) for each quartile of baseline ASUI was then calculated.
Responsiveness
To determine the responsiveness to change of the ASUI, data from the SARA trial was used. For each participant, there were seven clinic visits each separated by four week intervals from randomization to the end of the study. During each clinic visit, ASUI, ACQ3, and Mini AQLQ5 scores were obtained. All participants were instructed to keep a daily asthma diary that was returned to the clinic during subsequent visits. Linear regression with robust variance estimates and exchangeable correlation structure was used to compare mean changes in ASUI scores across groups of participants who differed by more than 10% in percent predicted FEV1 values, and by greater than 0.5 points in ACQ while adjusting for visit period22. The participant groups for each measure were derived as follows:
Percent predicted FEV1 values: Previous studies have used 10% as the cutoff for significant change in percent predicted FEV113–15. In the chronic obstructive pulmonary disease (COPD) population, the MID of the percent predicted FEV1 is about 10%12. The change in percent predicted FEV1 values was derived by subtracting the baseline percent predicted FEV1 values from the follow-up percent predicted FEV1 and dividing by the baseline percent predicted value. Participants were categorized as better if the increase in percent predicted FEV1 was greater than or equal to 10%. They were categorized as worse if the percent predicted FEV1 decreased by greater than or equal to 10%. If the change was less than 10% in either direction, they were categorized as unchanged. The mean changes in ASUI were then compared between the three groups.
ACQ scores: The MID for the ACQ is 0.5 points3. Participants were categorized as better if the decrease in their ACQ score was greater than or equal to 0.5. They were categorized as worse if the increase in ACQ score was greater than or equal to 0.5 and they were categorized as the same or unchanged if the change was between −0.5 and +0.5. The mean changes in the ASUI scores were then compared between the three groups: better, same, or worse.
Minimal important difference
The MID is the smallest difference in score of an instrument that represents a clinically noticeable change16. In this study, anchor-based and distribution-based methods were used to determine the MID of the ASUI5, 13, 16.
Anchor-based analysis
Two groups of change in percent predicted FEV1 (< 10% versus ≥ 10% improvement from baseline) and seven groups of changes in ACQ scores from baseline (ΔACQ ≥ −1.5, −1.0 ≤ ΔACQ <−1.5, −0.5 ≤ ΔACQ < −1.0, +0.5 > ΔACQ < −0.5 [no change], +1.0 > ΔACQ ≥ +0.5, +1.5 > ΔACQ ≥ +1.0, ΔACQ ≥ +1.5.) were used as “anchors” to evaluate meaningful differences in ASUI score. One-way ANOVA was used to compare mean changes in ASUI scores across the groups in each case. The occurrence of an EPAC as well as individual EPAC components also served as “anchors”. The mean difference in ASUI score between visits with an EPAC in the prior period and those without an EPAC were calculated using repeated measures ANOVA.
Distribution-based analysis
The standard deviations (SD) of the baseline ASUI scores for each study sample were calculated and ½ SD was used as an estimate of the MID16. The standard error of measurement (SEM) of the baseline ASUI scores for each sample was also computed as follows: 13, 16. For the SIIVA sample the Cronbach’s α coefficient was used to estimate the reliability coefficient of the ASUI and for the SARA sample, the test-retest reliability coefficient was used. By convention 1 SEM of baseline ASUI scores is a good estimate of the MID13, 16.
RESULTS
Study population
Data from 1236 study participants ages 18 years and older from the SIIVA trial were included. The mean age of these asthma patients was 42 years (SD, 12). A majority of them were female (75%), and White (67%). Data from 412 participants in the SARA trial were also included. The mean age of asthma patients in SARA was 41 years (SD, 13). The majority were female (68%), fifty percent were White, and 38% were Black. A summary of the baseline characteristics of the study participants from the SIIVA and SARA trials is presented in Table I.
Table I.
Patient characteristics at baseline
| Characteristic | SIIVA (n=1236) | SARA (n= 412) |
|---|---|---|
| Age, year(SD) | 42 (12) | 41 (13) |
| Female(%) | 923 (75) | 279 (68) |
| Race or ethnic group – no. (%) | ||
| White | 828 (67) | 205 (50) |
| Black | 281 (23) | 157 (38) |
| Hispanic | 79 (6) | 41 (10) |
| Other | 44 (4) | 9 (2) |
| Asthma questionnaire scores, mean(SD) | ||
| ASUI↑ (0–1) | 0.82 (0.18) | 0.76 (0.16) |
| ACQ↓ (0–7) | NA | 1.7 (0.9) |
| Mini AQLQ↑ (1–7) | NA | 4.7(1.2) |
| Pulmonary function, mean(SD)* | SIIVA (n=704) | SARA (n=412) |
| Pre-bronchodilator FEV1, Liters | 2.6 (0.9) | 2.4 (0.7) |
| Pre-bronchodilator FEV1, % predicted | 83.4 (21) | 76.7 (15) |
Pulmonary function available for 704 (57%) SIIVA participants
ASUI: Asthma Symptom Utility Index. Scores on the ASUI range from 0 to 1, with higher scores indicating less severe asthma symptoms
ACQ: Asthma Control Questionnaire. Scores on the ACQ range from 0 to 7, with lower scores indicating better asthma control and 0.5 as the minimal clinically important difference
Mini AQLQ: Mini Asthma Quality of Life Questionnaire. Scores on the Mini AQLQ range from 1 to 7, with higher scores indicating better quality of life and 0.5 as the minimal clinically important difference.
FEV1: forced expiratory volume in 1 second and the predicted values are from Hankinson et al.19
SARA: Study of Acid Reflux and Asthma
SIIVA: Safety of Inactivated Influenza Vaccine in Asthma
Reliability
The internal consistency reliability (Cronbach’s alpha) was 0.74 (n = 1223) in the SIIVA sample and 0.71 (n = 412) in the SARA sample. Test-retest reliability (intra class correlation coefficient) among the 55 participants in the SARA trial who had stable asthma over a four week period was 0.76.
Construct validity
Statistically significant Spearman’s correlations were observed between baseline ASUI scores and baseline ACQ scores (r = −0.79, 95% CI [−0.85, −0.75], P<0.001), and baseline Mini AQLQ scores (r = 0.59, 95% CI [0.51, 0.61], P<0.001).
Known-groups validity
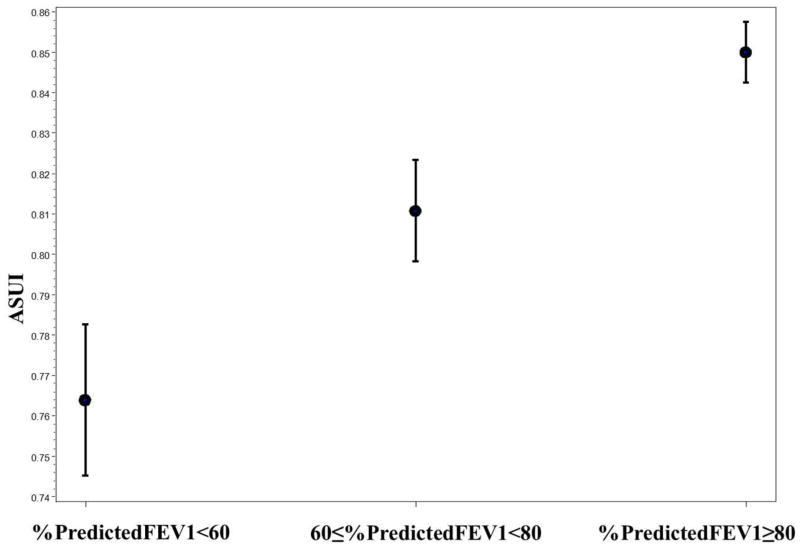
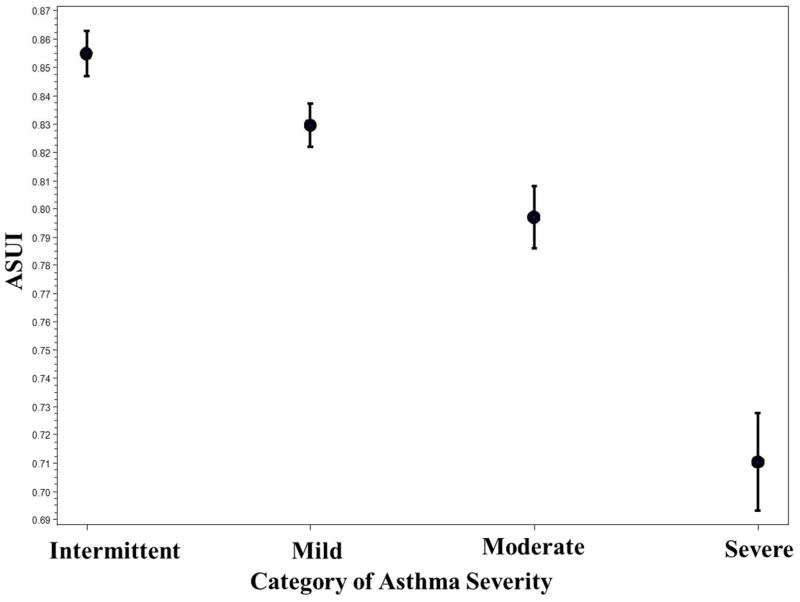
The difference in mean ASUI scores between patients with poor baseline lung function (percent predicted FEV1 <60%) and those with good baseline lung function (percent predicted FEV1 ≥ 80%) was statistically significant (0.76 vs. 0.85, P <0.0001) [Table II]. There was a positive linear relationship between the mean ASUI score and category of percent predicted FEV1 (Figure 1). The difference in mean ASUI scores between patients with severe asthma and those with intermittent asthma was statistically significant (0.71 vs. 0.85, P <0.0001) (Table II). There was a negative linear relationship between mean ASUI score and asthma severity based on asthma medication use at baseline (Figure 2).
Table II.
Known-groups validity tests on mean ASUI scores at baseline (SIIVA)
| Number of participants (N) | Mean (SD) ASUI score | F statistic/P-value | |
|---|---|---|---|
| Percent predicted FEV1 | |||
| < 60% | 97 | 0.76 (0.18) | |
| 60% to 79% | 188 | 0.81 (0.17) | |
| ≥ 80% | 405 | 0.85 (0.15) | 12.5/<0.0001 |
| Asthma severity based on baseline medication use | |||
| Intermittent | 332 | 0.85 (0.14) | |
| Mild | 453 | 0.83 (0.16) | |
| Moderate | 298 | 0.80 (0.19) | |
| Severe | 137 | 0.71 (0.20) | 25.4/<0.0001 |
ASUI: Asthma Symptom Utility Index, SIIVA: Safety of Inactivated Influenza Vaccine in Asthma, FEV1: Forced expiratory volume in the first second and the predicted values are from Hankinson et al.
Figure 1.
Mean of ASUI with Standard Error by Categories of Percent Predicted FEV1
Figure 2.
Mean of ASUI with Standard Error by Category of Asthma Severity
Predictive validity
The frequency of EPACs ranged from 13% in the highest quartile of ASUI to 39% in the lowest quartile. Compared to patients in the highest quartile of baseline ASUI (score >0.95), SIIVA participants with a baseline ASUI score of ≤ 0.73 (lowest quartile) were 40 percent more likely to experience an EPAC over the next two weeks. There was a dose response relationship with increasing likelihood of an EPAC by decreasing quartile of baseline ASUI score (Table III). The overall frequency of asthma exacerbations as defined by new or increased oral corticosteroid use or an unscheduled healthcare contact for asthma, was low (4–11%) across all four groups. Participants in the lowest quartile were 8% more likely to experience an exacerbation compared to those in the highest quartile (Table III).
Table III.
Predictive validity of the ASUI: relationship to frequency of EPACs and exacerbations
| EPACS* | Exacerbations** | |||
|---|---|---|---|---|
| Quartiles of ASUI | Frequency (%) | Relative risk (RR) (95%CI) | Frequency (%) | Relative risk (RR) (95% CI) |
| >0.95 (n=321) | 13 | Reference = 1.00 | 4 | Reference = 1.00 |
| 0.87 to 0.95 (n= 263) | 24 | 1.13 (1.05–1.23) | 4 | 1.00 (0.96–1.03) |
| 0.74 to 0.86 (n= 290) | 30 | 1.24 (1.13–1.35) | 7 | 1.03 (0.99–1.07) |
| ≤ 0.73 (n=304) | 40 | 1.44 (1.30–1.60) | 11 | 1.08 (1.03–1.13) |
EPAC: Episodes of Poor Asthma Control. ASUI: Asthma Symptom Utility Index
EPACs: Any one of the following: 1) peak flow decrease of ≥30% from personal best, 2) increased rescue medication use above the average reported during the two weeks before randomization, 3) new or increased oral corticosteroids for asthma, 4) an unscheduled use of healthcare for treatment of asthma
Exacerbations: Any one of the following: 1) new or increased oral corticosteroids for asthma, 2) an unscheduled healthcare encounter for treatment of asthma
Responsiveness
The ASUI demonstrated good responsiveness to change. As hypothesized, ASUI scores improved significantly among participants whose percent predicted FEV1 improved by greater than or equal to 10% compared to those with no change in percent predicted FEV1 (Table IV). Likewise, there was a significant change in ASUI scores (in the hypothesized direction) when ACQ scores changed by more than the minimally important difference of 0.5 points compared to when the ACQ scores were unchanged (Table IV). After adjusting for visit period, there was a statistically significant difference in mean change in ASUI scores between visits with an EPAC in the prior period and those without an EPAC (P<0.0001) [Table V]. Similar significant differences were seen for all four EPAC components (Table V).
Table IV.
Mean changes in ASUI scores as a function of changes in percent predicted FEV1 values and ACQ scores
| N (pts.)** | Mean change in ASUI (95% CI) | P value | ||
|---|---|---|---|---|
| Changes in percent predicted FEV1 | ||||
| Better (ΔFEV1 ≥ 10%) | 213 (163) | 0.05 (0.03, 0.07) | ||
| Same (−10% ≤ ΔFEV1 <10%) | 1657 (384) | 0.01 (0.00, 0.01) | ||
| Worse (ΔFEV1 ≤−10%) | 222 (170) | −0.03 (−0.05, −0.02) | <0.0001 | |
| Changes in ACQ* (Δ ACQ) | ||||
| Better | ΔACQ ≥ −1.5 | 76 (68) | 0.32 (0.28, 0.35) | |
| −1.0 ≤ ΔACQ < −1.5 | 111 (100) | 0.15 (0.13, 0.18) | ||
| −0.5 ≤ ΔACQ < −1.0 | 255 (198) | 0.09 (0.08, 0.10) | ||
| Same | +0.5 > ΔACQ < −0.5 | 1254 (369) | 0.00 (−0.00, 0.01) | |
| Worse | +1.0 > ΔACQ ≥ +0.5 | 233 (175) | −0.09 (−0.10, −0.07) | |
| +1.5 > ΔACQ ≥ +1.0 | 78 (69) | −0.17 (−0.20, −0.14) | ||
| ΔACQ ≥ + 1.5 | 61 (53) | −0.32 (−0.36, −0.28) | <0.0001 | |
Note: MID for ACQ is 0.5 points
ACQ: Asthma Control Ques ionnaire, ASUI: Asthma Symptom Utility Index, FEV1: Forced expiratoryvolume in the first second
ACQ: Better = decrease by ≥ 0.5 points; same = change by <0.5 points; Worse = increase by ≥ 0.5 points.
N denotes frequency of events and “pts.” indicates the number of patients
Table V.
Mean Difference is ASUI scores by EPAC status for all visits
| ASUI | ||||
|---|---|---|---|---|
| #EPAC(% visits) | Mean difference* | 95% CI | P-value* | |
| Any EPAC | 750(35) | 0.09 | 0.01, 0.10 | <0.0001 |
| EPAC components | ||||
| Peak flow drop | 426(20) | 0.08 | 0.06, 0.10 | <0.0001 |
| Rescue inhalers | 414(19) | 0.10 | 0.01, 0.12 | <0.0001 |
| Oral steroid use | 168(8) | 0.16 | 0.12, 0.20 | <0.0001 |
| Urgent care contact | 103(6) | 0.15 | 0.11, 0.20 | <0.0001 |
2,155 follow-up visit periods evaluated among 390 participants
EPAC: Episodes of Poor Asthma Control.
ASUI: Asthma Symptom Utility Index
CI: Confidence Interval
Mean difference in scores between visits with an EPAC in the prior period and those without an EPAC, adjusted for visit period; repeated measures with independent correlation.
Minimal important difference
Anchor-based results
The absolute mean change in ASUI corresponding to a small change in ACQ (−0.5 ≤ ΔACQ < −1.0 or +1.0 < ΔACQ ≥ +0.5) was 0.09 (Table IV). The mean difference in ASUI score between visits with an EPAC in the prior period and visits without an EPAC was also 0.09 (Table V). For the EPAC components, the mean difference ranged from 0.08 to 0.16 (Table V). The mean differences in ASUI scores for less serious EPACs - peak flow drop and increased use of rescue inhalers were 0.08 and 0.10 respectively. For the more serious EPAC components – oral steroid use and unscheduled urgent care contact, the mean differences in ASUI scores were 0.16 and 0.15. These results support an MID of about 0.09.
Distribution-based results
The standard deviation of baseline ASUI scores was 0.18 for the SIIVA trial and 0.16 for the SARA trial. Based on the ½ SD criteria as an estimate of MID, the MID of the ASUI is 0.09 and 0.08, respectively. The 1 SEM criteria gave MID estimates of 0.07 for the SARA sample and 0.09 for the SIIVA sample. Overall, from our study samples, we estimate the MID of the ASUI to be about 0.09.
DISCUSSION
The results of this study demonstrate that the ASUI, an asthma-specific utility index designed to summarize the frequency and severity of selected asthma-related symptoms7, has good psychometric properties in two groups of asthma patients. We confirmed the findings of Revicki et al. 7 that ASUI scores have good construct validity, test-retest reliability, and discriminant validity7. We also showed that baseline ASUI scores predict the occurrence of EPACs or asthma exacerbations in the subsequent two weeks. Patients with the lowest baseline ASUI scores were 40% more likely to have an EPAC and 8% more likely to have an asthma exacerbation over the next two weeks compared to those with the highest baseline ASUI scores. The ability to predict EPACs and asthma exacerbations suggests that the ASUI could be useful in guiding asthma therapy in clinical practice. In addition, we have shown that the ASUI is responsive to changes in asthma control. By using well established anchor-based and statistical methods5, 13, 14, 16, we estimated the MID of the ASUI to be about 0.09 points.
Some items on the ASUI are similar to those on other questionnaires that assess asthma control and asthma-related quality of life3, 5, 14, 15. Asthma control as measured by the ACQ is a normative construct developed by physicians and validated against physician assessment of asthma3. Asthma-related quality of life instruments measure the extent to which asthma symptoms interfere with physical functioning in daily life5. The ASUI is complementary to these other tools by focusing on the frequency and severity of asthma symptoms. It is a patient preference weighted scale and thus suitable for economic analyses that incorporate disability-adjusted life years 7.
A key strength of this analysis is that data from two separate trials conducted at different time periods, with different entry criteria, and different interventions were used. However, because of the differing study designs, we were not able to perform the same validation analyses in both trials. It was necessary to use data from both studies in order to fully characterize the psychometric properties of the ASUI and to determine the MID. The SIIVA study included asthma patients with a wide range of clinical severity but ASUI was only administered at baseline10, so test-retest reliability and longitudinal validity could not be assessed in this study population. Also patients in the SIIVA study had only 28 days of follow-up data, which may have limited the number of events, especially exacerbations. Nonetheless, because of the large population, we were able to demonstrate predictive validity based on the frequency of EPACS. In addition, ACQ scores and Mini AQLQ scores were not available for the SIIVA study participants so construct validity could not be determined. The SARA trial which included multiple ASUI measurements as well as ACQ and Mini AQLQ scores provided a good opportunity to determine responsiveness and construct validity8. Even though only patients with poorly controlled asthma were enrolled in the SARA trial, exploratory data analysis showed a skewed distribution of baseline ASUI scores such that a majority of patients had very high scores and only few patients had low scores.
A limitation of this study is the use of percent predicted FEV1, ACQ, and EPACs as “anchors” for determining the MID. Data on other anchors such as physician global rating of asthma severity or control were not available in this post-hoc analysis13–15. Studies evaluating the psychometric properties of other asthma questionnaires have used changes in percent predicted FEV1 and changes in ACQ score as “anchors” to determine the MID13–15.
Generalizability to other patient populations is an important aspect of health utility tools 16. The initial development and validation of the ASUI included asthma patients who were relatively well educated and mostly White7. The current scoring of the ASUI in the United States is based on the multi-attribute utility function that was originally derived by Revicki et al.7. The preference weights, utility functions and mean ASUI scores derived in the United States differ significantly from those obtained in other countries in Europe (Italy, France, and the United Kingdom)17. However, the relative rank ordering of the mean ASUI scores by asthma severity is maintained17. In the current analysis, we included participants with a good representation of women and racial minorities8, 10. Data on education level or socioeconomic status was not available. However, many of the study sites were located in large urban centers in the United States that generally serve patients of low socioeconomic status. This study therefore expands the generalizability of the ASUI to a more diverse population of asthmatics. The ASUI can be complex to calculate for an individual patient in the clinical setting compared to the ACT 4 and this could limit its routine use in clinical practice. However, computers can address this problem.
In summary, we demonstrated that the ASUI has good psychometric properties among adult asthmatics when used in the context of clinical trials in the United States. The MID of the ASUI in our population has been determined to be about 0.09 points. Further studies are needed to determine the psychometric properties of the ASUI in children and other asthma population demographics.
CLINICAL IMPLICATIONS.
The established MID of 0.09 points for the ASUI will aid clinicians in interpreting results of clinical research and improve monitoring of asthma symptoms in clinical practice.
Acknowledgments
Sources of Funding
The SARA trial was supported by: NIH-NHLBI 5U01HL072968 and the American Lung Association and is registered at ClinicalTrials.Gov: NCT00069823
The SIIVA trial was supported by grants from the American Lung Associations of Alabama, Central Florida, Colorado, Delaware, Eastern Missouri, Finger Lakes (New York), Georgia, Gulf coast Florida, Hudson Valley (New York), Illinois, Indiana, Louisiana, Maine, Metropolitan Chicago, Michigan, Mid-Ohio, Minnesota, Nashua–Suffolk (New York), New Hampshire, New York City, North Carolina, Northeast Florida, Ohio, Oklahoma, Pennsylvania, South Florida, Southeast Florida, Texas, Vermont, Western Missouri, Wisconsin, Greater Norfolk County (Massachusetts), Hawaii, Middlesex County (Massachusetts), New York State, Northern Rockies, Queens (New York), Western Massachusetts, and Virginia; Baylor College of Medicine; the Thalheim Family; Duke University; the Ernest N. Morial Asthma, Allergy, and Respiratory Disease Center; the Merck Foundation; and Glaxo–SmithKline.
The authors would like to acknowledge the contribution of the following American Lung Association Asthma Clinical Research Centres towards these studies.
Baylor College of Medicine, Houston: N. A. Hanania (principal investigator), M. Sockrider (co-principal investigator), L. Giraldo (principal clinic coordinator), R. Valdez (coordinator), L. Bertrand (coordinator);
Columbia University–New York University Consortium, New York: J. Reibman (Principal investigator), E. DiMango (co-principal investigator), C Cammarata and K Carapetyan (clinic coordinators at New York University), J. Sormillon and E Simpson (clinic coordinators at Columbia University);
Duke University Medical Center, Durham, N.C.: L. Williams (principal investigator), J. Sundy (co-principal investigator), G. Dudek (principal clinic coordinator), R. Newton and A Dugdale (coordinators);
Emory University School of Medicine, Atlanta: W.G. Teague (principal investigator), R Patel (principal clinic coordinator), J. Peabody, R. Patel, E Hunter;, D Whitlock (coordinators);
Illinois Consortium, Chicago: L. Smith (principal investigator), J. Moy, E Naureckas, C.S. Olopade (co-principal investigators), J. Hixon (principal clinic coordinator), A. Brees, G. Rivera, S. Sietsema, V. Zagaja (coordinators);
Indiana University, Asthma Clinical Research Center, Indianapolis: M. Busk (principal investigator), F. Leickly, C. Williams (co-principal investigators), P. Puntenney (coordinator);
Jefferson Medical College, Philadelphia: F. Leone (principal investigator), M. Hayes-Hampton (principal clinic coordinator);
Louisiana State University Health Sciences Center, Ernest N. Morial Asthma, Allergy, and Respiratory Disease Center, New Orleans: W.R. Summer (principal investigator), C. Glynn and G Meyaski (clinic coordinators);
National Jewish Medical and Research Center, Denver: R. Katial (principal investigator), R. Gibbs (principal clinic coordinator), L. Lopez, C. Ruis, B. Schoen (coordinators);
Nemours Children’s Clinic–University of Florida Consortium, Jacksonville: J. Lima (principal investigator), K. Blake (co-principal investigator), A. Santos (principal clinic coordinator), L. Duckworth, D. Schaeffer, M McRae (coordinators);
North Shore–Long Island Jewish Health System, New Hyde Park, N.Y.: J. Karpel (Principal investigator), R. Cohen (co-principal investigator), R. Ramdeo (principal clinic coordinator);
Northern New England Consortium formerly Vermont Lung Center at the University of Vermont), Colchester, Vt.: C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky, E. Kent, T. Lahiri, P. Shapiro (co-principal investigators), S. Lang (principal clinic coordinator), J. Allen, A. Coote, L.M. Doucette, K. Girard, J. Lynn, L. Moon, T. Viola, S Burns (coordinators);
The Ohio State University Medical Center/Columbus Children’s Hospital, Columbus: J. Mastronarde (principal investigator), K. McCoy (co-principal investigator), J. Parsons (co-investigator), J. Drake (principal clinic coordinator), R. Compton, L. Raterman, D. Cosmar (coordinators);
University of Alabama at Birmingham, Birmingham: L.B. Gerald (principal investigator), W.C. Bailey (co-principal investigator), S. Erwin (principal clinic coordinator), H. Young, A. Kelley, D. Laken, B. Martin (coordinators);
University of Miami, Miami–University of South Florida, Tampa: A. Wanner (principal investigator, Miami), R. Lockey (principal investigator, Tampa), E. Mendes (principal clinic coordinator for University of Miami), S. McCullough (principal clinic coordinator for University of South Florida) B Fimbel, M Grandstaff (coordinators);
University of Minnesota, Minneapolis: M.N. Blumenthal (principal investigator), G. Brottman, J. Hagen (co-principal investigators), A. Decker, D. Lascewski, S. Kelleher (principal clinic coordinators), K. Bachman, M. Sneen (coordinators);
University of Missouri, Kansas City School of Medicine, Kansas City: G. Salzman (principal investigator), D. Pyszczynski (co-principal investigator), P. Haney (principal clinic coordinator);
St. Louis Asthma Clinical Research Center: Washington University, St. Louis University, and Clinical Research Center, St. Louis: M. Castro (principal investigator), L. Bacharier, K. Sumino (co-investigators), M.E. Scheipeter and J. Tarsi (coordinators);
University of California San Diego: S. Wasserman (principal investigator), J. Ramsdell (co-principal investigator). J Vitin and T Tucker (clinic coordinators);
Chairman’s Office, Respiratory Hospital, Winnipeg, Man., Canada: N. Anthonisen (research group chair);
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), D. Amend-Libercci, K. Barry, M. Daniel, G. Leatherman, C. Levine, A. Lears, R. Masih, S. Modak, D. Nowakowski, N. Prusakowski, D. Shade, E. Sugar, C. Shiflett
Esophageal pH Probe Quality Control Center, Temple University School of Medicine: J. Richter (center director)
Data and Safety Monitoring Board: S. Lazarus(chair), W. Calhoun, P. Kahrilas, B. McWilliams, A. Rogatko, C. Sorkness
Project Office, American Lung Association New York: E. Lancet, R. Vento (project officers), N. Edelman (scientific consultant), S. Rappaport, G. Pezza.
Project Office, National Heart Lung and Blood Institute: V. Taggart (project officer), G. Weinmann (DSMB secretary, airway branch chief)
ALA Scientific Advisory Committee: G. Snider (chair), N. Anthonisen, M. Castro, J. Fish, D. Ingbar, S. Jenkinson, D. Mannino, H. Perlstadt, L. Rosenwasser, J. Samet, T. Standiford, J. Smith, L. Smith, D. Schraufnagel, A. Wanner, T. Weaver.
ABBREVIATIONS
- ACT
Asthma Control Test
- ACQ
Asthma Control Questionnaire
- ANOVA
Analysis of Variance
- AQLQ
Asthma Quality of Life Questionnaire
- ASUI
Asthma Symptom Utility Index
- COPD
Chronic Obstructive Pulmonary Disease
- EPAC
Episodes of Poor Asthma Control
- FEV1
Forced Expiratory Volume in One second
- GINA
Global Initiative for Asthma
- ICC
Intraclass correlation
- MID
Minimal Important Difference
- PEFR
Peak Expiratory Flow Rate
- RR
Relative Risk
- SARA
Study of Acid Reflux and Asthma
- SD
Standard Deviation
- SEM
Standard Error of Measurement
- SIIVA
Safety of Inactivated Influenza Vaccine in Asthma
- VAS
Visual Analog Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christian Bime, Email: cbimeny1@jhmi.edu.
Christine Y. Wei, Email: cwei@jhsph.edu.
Janet T. Holbrook, Email: jholbroo@jhsph.edu.
Marianna M. Sockrider, Email: mmsockri@texaschildrens.org.
Dennis A. Revicki, Email: Dennis.Revicki@unitedbiosource.com.
Robert A. Wise, Email: rwise@jhmi.edu.
References
- 1.Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of Asthma–Summary report 2007. J Allergy Clin Immunol. 2007;11:120(5 Supplement 1):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.NHLBI/WHO workshop report. Bethesda (MD): National institutes of health; [accessed April 2008]. 1991. Pub no.95–3659. Global initiative for asthma management and prevention.(update November 2006). 2008. [Google Scholar]
- 3.Juniper E, O’Byrne P, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 4.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;1;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Juniper E, Guyatt G, Cox F, Ferrie P, King D. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14(1):32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 6.Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol. 1992;5;45(5):461–72. doi: 10.1016/0895-4356(92)90095-5. [DOI] [PubMed] [Google Scholar]
- 7.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: The multiattribute asthma symptom utility index. Chest. 1998;114(4):998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 8.Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, Wise RA American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360(15):1487–99. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium step-up therapy in asthma. N Engl J Med. 2011;364(6):578–9. [Google Scholar]
- 10.The American Lung Association Asthma Clinical Research Centers. The safety of inactivated influenza vaccine in adults and children with asthma. N Engl J Med. 2001;345(21):1529–36. doi: 10.1056/NEJMoa011961. [DOI] [PubMed] [Google Scholar]
- 11.McCoy K, Shade DM, Irvin CG, Mastronarde JG, Hanania NA, Castro M, Anthonisen NR. Predicting episodes of poor asthma control in treated patients with asthma. J Allergy Clin Immunol. 2006;118(6):1226–33. doi: 10.1016/j.jaci.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PMA, Celli BR, et al. Outcomes for COPD pharmacological trials: From lung function to biomarkers. European Respiratory Journal. 2008;31(2):416–69. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 13.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the asthma control test. J Allergy Clin Immunol. 2009;124(4):719, 723.e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 14.Schatz M, Zeiger RS, Yang S, Chen W, Kosinski M. Further validation and definition of the psychometric properties of the asthma impact survey. J Allergy Clin Immunol. 2011;128(1):44, 49.e1. doi: 10.1016/j.jaci.2010.12.1112. [DOI] [PubMed] [Google Scholar]
- 15.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, Pendergraft TB, Jhingran P. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Terwee CB, Bot SDM, de Boer MR, van der Windt DAWM, Knol DL, Dekker J, Bouter LM, de Vet HCW. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Flood EM, De CE, Mork A-C, Revicki DA. Evaluating preference weights for the Asthma Symptom Utility Index (ASUI) across countries. Health Qual Life Outcomes. 2006;4:51. doi: 10.1186/1477-7525-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan JA, Lemanske RF, Canino GJ, Elward KS, Kattan M, Matsui EC, Mitchell H, Sutherland ER, Minnicozzi M. Asthma outcomes: Symptoms. J Allergy Clin Immunol. 2012;129(3):S124–S135. doi: 10.1016/j.jaci.2011.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Schatz M, Dombrowski MP, Wise R, Thom EA, Landon M, Mabie W, Newman RB, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112(2):283–288. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- 21.Miller MK, Johnson C, Deniz Y, Bleecker ER, Wenzel SE. Severity assessment in asthma: An evolving concept. J Allergy Clin Immunol. 2005;116(5):990–995. doi: 10.1016/j.jaci.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]