Abstract
Purpose
MGCD0103 is a novel isotype-selective inhibitor of human histone deaceylases (HDACs) with the potential to regulate aberrant gene expression and restore normal growth control in malignancies.
Patients and Methods
A phase I trial of MGCD0103, given as a three-times-per-week oral dose for 2 of every 3 weeks, was performed in patients with advanced solid tumors. Primary end points were safety, tolerability, pharmacokinetics (PK), pharmacodynamic (PD) assessments of HDAC activity, and histone acetylation status in peripheral WBCs.
Results
Six dose levels ranging from 12.5 to 56 mg/m2/d were evaluated in 38 patients over 99 cycles (median, 2; range, 1 to 11). The recommended phase II dose was 45 mg/m2/d. Dose-limiting toxicities consisting of fatigue, nausea, vomiting, anorexia, and dehydration were observed in three (27%) of 11 and two (67%) of three patients treated at the 45 and 56 mg/m2/d dose levels, respectively. Disease stabilization for four or more cycles was observed in five (16%) of 32 patients assessable for efficacy. PK analyses demonstrated interpatient variability which was improved by coadministration with low pH beverages. Elimination half-life ranged from 6.7 to 12.2 hours, and no accumulation was observed with repeated dosing. PD evaluations confirmed inhibition of HDAC activity and induction of acetylation of H3 histones in peripheral WBCs from patients by MGCD0103.
Conclusion
At doses evaluated, MGCD0103 appears tolerable and exhibits favorable PK and PD profiles with evidence of target inhibition in surrogate tissues.
INTRODUCTION
Histone deacetylation is an important epigenetic event implicated in the development and progression of cancer, by regulating the accessibility of DNA for gene expression and transcription. The basic repeating unit of chromatin is the nucleosome, composed of DNA wrapped around a core of histone proteins.1 Histones of the nucleosome core can be acetylated and deacetylated depending on the opposing activities of enzyme families, histone deacetylases (HDACs), and histone acetyltransferases. Histone acetyltransferases, as transcription coactivators, catalyze the addition of acetyl groups on the ε-amino group of lysine residues in the N-terminal tails of core histones. Conversely, HDACs, as transcription corepressors, remove the acetyl groups from the acetylated lysines in histones which results in gene silencing.2,3 In many cancerous tissues, tumor suppressor genes are silenced through the activity of histone deacetylation. HDAC inhibitors represent a structurally diverse group of molecules whose activity can induce growth arrest, differentiation, apoptosis, and autophagocytic cell death of cancer cells.1
MGCD0103 is a rationally designed, orally available, isotype-specific, benzamide which inhibits HDAC isoforms 1, 2, 3 (class 1), and 11 (class 4), and avoids the class 2 enzymes. MGCD0103 has demonstrated dose-dependent inhibition of neoplastic growth, using an intermittent dosing schedule, in multiple human tumor xenograft models including colon (HCT116, SW48 and Colo205), non–small-cell lung (A549), prostate (DU145), pancreatic (PANC1), and vulval epidermal (A431) cancer models. Preclinical evaluations in beagle dogs revealed no drug-related changes in cardiovascular parameters, and MGCD0103 did not inhibit the human ether-à-go-go related gene (hERG) flux assay (IC50 [median inhibitory concentration] > 50 µM), suggesting minimal QTc prolongation liability.4 Pharmacokinetic (PK) data in animal models showed greater oral bioavailability under fasted conditions, and dose-dependent increases in peak concentrations and plasma exposures.
Based on the relevance of HDACs as cancer therapeutic targets, and the favorable preclinical profile of MGCD0103, a phase I trial was conducted in patients with advanced solid tumors. The primary objective was to determine the safety and tolerability. Secondary objectives included the assessments of PK, pharmacodynamic (PD) changes as measured by whole cell HDAC activity and effects on histone H3 acetylation in blood, and preliminary antitumor efficacy.
PATIENTS AND METHODS
Patient Eligibility
Patients were eligible if they had a histologically or cytologically documented advanced solid malignancy, refractory to standard therapy or for which no standard therapy existed. Other key eligibility criteria included: age ≥ 18 years; Eastern Cooperative Oncology Group performance status 0 to 2; adequate hematologic, hepatic, and renal functions (WBC count ≥ 3 × 109/L, absolute neutrophil count ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, bilirubin ≤ 1.5 mg/dL, AST/ALT ≤ 3 times upper limit of normal or ≤ 5 × upper limit of normal if documented liver metastases, creatinine ≤ 2.0 mg/dL and calculated creatinine clearance > 50 mL/min, and proteinuria < 2+ or ≤ 500 mg protein/24 hours if dipstick ≥ 2+); and unlimited prior cytotoxic therapy but there must be a 4-week interval between study treatment and any prior radiotherapy or chemotherapy. Patients with primary brain malignancies or meningeal metastases were excluded but those with stable and treated brain metastases were allowed on study.
The institutional review board of both participating centers approved the study, which was conducted in accordance with federal and institutional guidelines.
Study Design and Patient Evaluation
This was a two-center, single-agent, open-label, phase I study in which MGCD0103 was administered orally with the requirement of fasting for a minimum of 3 hours before and for 0.5 hours after dosing. Study drug was administered on days 1, 3, 5, 8, 10, and 12 every 21 days. The starting dose of 12.5 mg/m2/d was one tenth of the severely toxic dose to 10% of the rats tested in a 28-day toxicity study (125 mg/m2). Dose escalations occurred in increments of approximately 25% to 50% depending on the toxicity encountered, and the following 6 dose levels were evaluated: 12.5, 20, 27, 36, 45, and 56 mg/m2/d. No intrapatient dose escalation was allowed. Dose escalation followed the standard 3 + 3 rule. The recommended phase II dose (RPTD) was defined as the dose level in which one or fewer of six patients developed dose-limiting toxicity (DLT). Approximately half way through the study, the protocol was amended to evaluate the effect of a low pH beverage (such as soda or carbonated soft drinks) on MGCD0103 solubility instead of water.
Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. DLTs were defined as adverse events occurring during the first cycle of MGCD0103 administration and fulfilling one of the following criteria: absolute neutrophil count lower than 0.5 × 109/L for 5 days or longer; febrile neutropenia or grade 3 or higher neutropenic infection; platelets lower than 25 × 109/L or thrombocytopenic bleeding; any nonhematologic toxicity grade 3 or higher except nausea, vomiting, or diarrhea associated with suboptimal premedication and/or management; AST/ALT elevations of grade 3 or higher for longer than 7 days; any toxic effect leading to missing two or more doses per cycle; and any toxic effect resulting in the delay of the subsequent cycle by longer than 7 days. Response was assessed using the Response and Evaluation Criteria in Solid Tumors (Appendix A1, online only).5
Dose Modification
Patients who experienced any DLT were delayed by 1-week intervals until recovery and then may have continued on study drug with reduction of MGCD0103 dose by one dose level. If no recovery occurred after a delay of 2 weeks from the next scheduled treatment cycle, patients were to discontinue protocol treatment. In addition, patients were required to have absolute neutrophil count ≥ 1 × 109/L and platelets ≥ 75 × 109/L for dosing on day 8 of each cycle.
Duration of Study Treatment
Patients with objective response or stable disease were allowed to remain on study until disease progression. Otherwise, study treatment continued until disease progression, unacceptable adverse event, patient’s decision to withdraw from the study, or changes in the patient’s condition including intercurrent illness rendering the continuation of study treatment unacceptable.
Pharmacokinetic Analysis
Blood samples for evaluation of MGCD0103 PK were collected on cycle 1, day 1, and day 12 before dosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 24 hours postdose, and on cycle 1, day 4 at 24-hour postdose. Whole blood was collected into a 5-mL sodium heparin Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ) and centrifuged at 3,500 rpm for 10 minutes at 4°C. Plasma was aliquoted and stored at ≤ −40°C. Samples were analyzed for MGCD0103 concentrations using a validated high performance liquid chromatograph/mass spectrometry method with a quantitation limit of 0.5 ng/mL (Appendix A2, online only).
Pharmacodynamic Analysis
Whole blood samples for PD evaluations were collected in 10-mL sodium heparin Vacutainer tubes before MGCD0103 dosing on days 1, 3, and 8 of cycle 1, as well as on days 1 and 8 of cycles 2 and 4, and at the end of study. Post-treatment samples were collected at 4 and 20 to 24 hours postdose on day 1 and 24 hours postdose on day 10 (designated as day 11 draw) of cycle 1. Blood samples were shipped at room temperature the day of the PD sample draw in order to arrive for processing within 24 hours of collection. Peripheral WBCs were purified from erythrocytes by treatment with EL lysis buffer (Qiagen, Mississauga, Ontario, Canada), washed, resuspended in RPMI-1640 with 10% fetal bovine serum, and aliquoted for PD analysis assays (whole cell HDAC activity and histone H3 acetylation). Details for whole cell enzyme assay, histone and nuclear extract lysate extraction, measurement of histone H3 acetylation by fluorescence-activated cell sorting or by enzyme-linked immunosorbent assay (ELISA), and serum interleukin-6 (IL-6) expression levels by ELISA, are described in online-only Appendix A3.
RESULTS
Patient Demographics and Dose Escalation and Recommended Phase II Dose
Thirty-eight patients were enrolled onto this study (Table 1) and completed a total of 99 cycles of MGCD0103 (median, 2; range, 1 to 11). Six dose levels ranging from 12.5 to 56 mg/m2/d were evaluated (Table 2). Some dose levels accrued more than 6 patients, based on the protocol amendment to obtain PK data on MGCD0103 administered with a low-pH beverage. At each of the 20, 27, and 36 mg/m2/d dose levels, dose-limiting grade 3 fatigue was seen in one of six to nine patients. For dose levels 45 and 56 mg/m2/d, DLTs consisting of grade 3 fatigue, nausea, vomiting, anorexia, and dehydration were seen in three (27%) of 11 and two (67%) of three patients, respectively. Asymptomatic grade 3 QTc prolongation was seen in one patient at 45 mg/m2/d, this was unlikely related to study drug as this patient had a borderline pretreatment QTc interval. In addition, asymptomatic and possibly related grade 3 hypophosphatemia was seen in one patient at 45 mg/m2/d, which corrected with phosphate replacement. Based on these findings, the RPTD for MGCD0103 was 45 mg/m2/d when given three times per week for 2 of every 3 weeks.
Table 1.
Patient Characteristics
| Patients | ||
|---|---|---|
| Characteristic | No. | % |
| No. of patients | 38 | |
| Median age, years | 60 | |
| Range | 29–74 | |
| Sex | ||
| Female | 14 | 37 |
| Male | 24 | 63 |
| ECOG PS | ||
| 0 | 12 | 32 |
| 1 | 25 | 66 |
| 2 | 1 | 2 |
| Primary tumor site | ||
| Colorectal | 9 | 24 |
| Kidney | 6 | 16 |
| Lung | 5 | 13 |
| Liver | 3 | 8 |
| Ovary | 2 | 5 |
| Pancreas | 2 | 5 |
| Prostate | 2 | 5 |
| Stomach | 2 | 5 |
| Breast | 1 | 3 |
| Gallbladder | 1 | 3 |
| Skin | 1 | 3 |
| Nasal sinus | 1 | 3 |
| Bone | 1 | 3 |
| Esophagus | 1 | 3 |
| Other (adenocarcinoma unknown origin) | 1 | 3 |
| Prior treatment (n = 38) | ||
| Chemotherapy | 35 | 84 |
| Surgery | 26 | 68 |
| Radiation therapy | 18 | 47 |
| Hormone therapy | 5 | 13 |
| No. of prior chemotherapy regimens | ||
| 0 | 3 | 8 |
| 1 | 6 | 16 |
| 2 | 11 | 29 |
| 3 | 10 | 26 |
| 4 | 5 | 13 |
| 5 | 3 | 8 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performancestatus.
Table 2.
Dose Levels and Frequencies of DLTs
| Dose Level |
Dose of MGCD0103 (mg/m2) |
No. of Patients |
No. of Patients With DLTs (cycle 1) |
Description of DLTs (cycle 1) | No. of Patients With DLTs (all cycles) |
No. of DLTs Reported (all cycles) |
Description of DLTs (all cycles) |
|---|---|---|---|---|---|---|---|
| 1 | 12.5 | 3 | 0 | 3 | 3 | Anorexia, fatigue, vomiting | |
| 2 | 20 | 6 | 1 | Fatigue | 4 | 6 | Abdominal pain, Anorexia, fatigue, nausea, thrombosis, vomiting |
| 3 | 27 | 9 | 1 | Fatigue | 7 | 6 | Abdominal pain, anorexia/decreased appetite, dehydration, fatigue, nausea, vomiting |
| 4 | 36 | 6 | 1 | Fatigue | 6 | 5 | Anorexia/decreased appetite, dehydration, fatigue, nausea, vomiting |
| 5 | 45 | 11 | 3 | Prolonged QTc*, hypophosphatemia, fatigue, nausea, vomiting, anorexia | 10 | 7 | Abdominal pain, Anorexia/decreased appetite, dehydration, prolonged QTc*, fatigue, nausea, vomiting |
| 6 | 56 | 3 | 2 | Abdominal pain, anorexia, dehydration, fatigue, nausea, vomiting | 2 | 6 | Abdominal pain, anorexia, dehydration, fatigue, nausea, vomiting |
Abbreviation: DLT, dose-limiting toxicity.
Prolonged at baseline; no clinical sequelae.
Safety and Compliance
Most frequent adverse events of all grades and those grade 3 or higher, separated by dose levels and of at least possible attribution, are described in Table 3. Fatigue, anorexia, nausea, vomiting, diarrhea, constipation, dehydration, and abdominal pain were the most frequently observed adverse events reported with MGCD0103. Adverse events of grade 3 or higher were rarely encountered at dose levels of MGCD0103 from 12.5 to 36 mg/m2/d. At the 45 and 56 mg/m2/d dose levels, grade 3 or worse fatigue, nausea, vomiting, and dehydration were observed and were dose dependent in frequency. The administration of MGCD0103 was not associated with any hematologic adverse events.
Table 3.
Adverse Events of Grades ≥ 3 and All Cycles, Separated by Dose Levels of MGCD0103
| Dose Level | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||||||||||||||||
| All Grades |
Grade ≥ 3 |
All Grades |
Grade ≥ 3 |
All Grades |
Grade ≥ 3 |
All Grades |
Grade ≥ 3 |
All Grades |
Grade ≥ 3 |
All Grades |
Grade ≥ 3 |
|||||||||||||
| Adverse Event | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| Dose, mg/m2 | 12.5 | 20 | 27 | 36 | 45 | 56 | ||||||||||||||||||
| Total No. cycles | 6 | 13 | 21 | 21 | 24 | 14 | ||||||||||||||||||
| Constitutional symptoms | ||||||||||||||||||||||||
| Fatigue | 2 | 33 | 0 | 0 | 3 | 23 | 1 | 8 | 8 | 38 | 1 | 5 | 8 | 38 | 2 | 10 | 10 | 42 | 3 | 13 | 3 | 21 | 3 | 21 |
| Weight loss | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 2 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | ||||||||||||||||||||||||
| Anorexia/decreased appetite | 1 | 17 | 0 | 0 | 1 | 8 | 0 | 0 | 3 | 14 | 0 | 0 | 3 | 14 | 0 | 0 | 12 | 50 | 2 | 8 | 2 | 14 | 1 | 7 |
| Nausea | 0 | 0 | 0 | 0 | 3 | 23 | 0 | 0 | 4 | 19 | 0 | 0 | 7 | 33 | 0 | 0 | 7 | 29 | 1 | 4 | 2 | 14 | 2 | 14 |
| Vomiting | 2 | 33 | 0 | 0 | 2 | 15 | 0 | 0 | 2 | 10 | 0 | 0 | 5 | 24 | 0 | 0 | 4 | 17 | 1 | 4 | 2 | 14 | 2 | 14 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 10 | 0 | 0 | 4 | 19 | 0 | 0 | 3 | 13 | 0 | 0 | 1 | 7 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 1 | 8 | 0 | 0 | 1 | 5 | 0 | 0 | 2 | 10 | 0 | 0 | 1 | 4 | 0 | 0 | 1 | 7 | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 0 | 1 | 8 | 1 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 8 | 0 | 0 | 1 | 7 | 1 | 7 |
| Mucositis (functional) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac | ||||||||||||||||||||||||
| QTc prolongation* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 |
| Thrombosis | 0 | 0 | 0 | 0 | 1 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolic | ||||||||||||||||||||||||
| Hypokalemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperkalemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 0 | 0 |
| ALT (increased) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| AST (increased) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| ALP (increased) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 |
| Hypoalbuminemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypophosphatemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 |
| Hematologic | ||||||||||||||||||||||||
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leukopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | ||||||||||||||||||||||||
| Dehydration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 1 | 5 | 1 | 5 | 0 | 0 | 2 | 8 | 0 | 0 | 2 | 14 | 2 | 14 |
Abbreviation: ALP, alkaline phosphatase.
Prolonged at baseline; no clinical sequelae.
Treatment delay occurred at a rate of 8% of cycles (2 of 24) at the 45 mg/m2/d dose level. Dose reductions or omissions occurred with increasing frequency as the MGCD0103 dose was escalated, with 0%, 15%, 10%, 10%, 25%, and 36% of cycles being affected for the six dose levels evaluated, respectively.
Antitumor Activity
Thirty-two of 38 patients were assessable for response. No objective tumor responses were observed. Five patients (15.6%) with previously progressive colorectal (four cycles), renal cell (four, five, and 11 cycles), and lung (7 cycles) cancers had stable disease for four or more cycles.
Pharmacokinetic Analysis
Based on each patient’s body-surface area, the actual dose of MGCD0103 was calculated and then used to determine the amount of water or low pH beverage for coadministration. MGCD0103 was administered with either 100 mL of water with actual doses of 20 to 40 mg of study drug, or 200 mL of water with actual doses of 46 to 66 mg, or 200 mL of low pH beverage with actual doses of 52 to 150 mg. For cycle 1, days 1 and 12, 38, and 14 patients had complete concentration profiles (up to 24 hours), respectively, and these are depicted in Figures A1A and A1B (online only). On both days, for all dose levels, mean maximum concentrations occurred at 0.5 to 1.0 hour postdose, declined biphasically thereafter, and remained detectable at 24 hours.
Mean MGCD0103 PK parameters are presented in Table 4, and individual Cmax and AUC0–24 hour values are plotted in Figures 1A and 1B, respectively. A large interpatient variability was observed, especially in the groups that received the dose with water. Following actual doses ranging from 20 to 66 mg, administered with 100 or 200 mL of water, Cmax and AUC0–24 hour values were not dose-dependent and appeared to remain within the same range of values. However, when administered with 200 mL of a low pH beverage, Cmax and AUC0–24 hours increased proportionately with actual doses between 52 and 150 mg. Median Tmax for all groups on both PK days ranged from 0.5 to 1.1 hours, except for the 12.5 mg/m2/d group (3.0 hours). Elimination terminal half-life (t½) ranged from 6.7 to 12.2 hours across the entire dose range. No signs of accumulation after multiple dose administration were observed.
Table 4.
Mean ± SD (% CV) MGCD0103 PK Parameters on Days 1 and 12 After Administration of Oral Dose, Three Times Per Week for the First 2 Weeks of the Cycle
| Dose (mg/m2) |
Beverage (mL)* |
No. | Day | Cmax (ng/mL) | AUC (0–24h) (ng*hr/mL)† |
t1/2 (hr) | Tmax (hr) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | % CV | Mean ± SD | % CV | Mean ± SD | % CV | Median | Minimum, Maximum | ||||
| 12.5 | 1 | 3 | 1 | 91.4 ± 77.7 | 85.0 | 255 ± 145 | 57.0 | 7.7 ± 1.2 | 15.3 | 0.5 | 0.47, 1.5 |
| 1 | 3 | 12 | 70.1 ± 61.2 | 87.4 | 344 ± 149 | 43.3 | 9.0 ± 0.67 | 7.5 | 3.0 | 1.5, 7.8 | |
| 20 | 1 | 6 | 1 | 116 ± 121 | 105 | 321 ± 119 | 37.0 | 8.05 ± 1.76 | 21.9 | 0.5 | 0.5, 3.9 |
| 1 | 6 | 12 | 108 ± 150 | 139 | 442 ± 203 | 46.0 | 6.7 ± 0.8 | 19.3 | 1.1 | 0.5, 6.0 | |
| 27 | 2 | 6 | 1 | 77 ± 45.7 | 59.4 | 349 ± 192 | 55.1 | 11.2 ± 3.56 | 31.9 | 0.75 | 0.5, 3.0 |
| 2 | 6 | 12 | 87.5 ± 85.4 | 97.6 | 296 ± 172 | 58.1 | 7.7 ± 0.6 | 8.2 | 0.5 | 0.5, 5.0 | |
| 27 | 3 | 3 | 1 | 67.5 ± 58.5 | 86.7 | 316 ± 204 | 64.5 | 10.1 ± 4.79 | 47.6 | 1.0 | 0.45, 1.6 |
| 3 | 3 | 12 | 82.6 ± 55.3 | 67.0 | 251 ± NA | NA | 8.3 ± NA | NA | 0.6 | 0.5, 1.1 | |
| 36 | 2 | 4 | 1 | 74.4 ± 36.6 | 49.3 | 354 ± 94 | 26.5 | 9.6 ± 1.4 | 14.9 | 1.0 | 0.5, 1.1 |
| 2 | 4 | 12 | 122 ± 51.3 | 42.1 | 557 ± 74 | 13.3 | 7.4 ± 0.29 | 3.9 | 1.0 | 0.5, 1.1 | |
| 36 | 3 | 2 | 1 | 92.9 ± 52.0 | 56.0 | 613 ± 80.0 | 13.1 | 12.2 ± 6.1 | 50.0 | 1.1 | 0.5, 1.8 |
| 3 | 1 | 12 | 143 ± NA | NA | NA ± NA | NA | NA ± NA | NA | 1.0 | NA | |
| 45 | 3 | 11 | 1 | 161 ± 74.9 | 46.6 | 901 ± 378 | 42.0 | 9.9 ± 3.6 | 36.9 | 0.6 | 0.5, 4.0 |
| 3 | 7 | 12 | 190 ± 74.4 | 39.3 | 502 ± NA | NA | 9.9 ± NA | NA | 0.57 | 0.48, 1.6 | |
| 56 | 3 | 3 | 1 | 168 ± 21 | 12.5 | 1,500 ± 730 | 48.6 | 8.5 ± 1.8 | 21.4 | 1.0 | 1.0, 1.0 |
| 3 | 1 | 12 | 431 ± NA | NA | NA ± NA | NA | NA ± NA | NA | 1.0 | NA | |
Abbreviations: SD, standard deviation; AUC, area under the curve; NA, not available.
1 = 100 mL of water; 2 = 200 mL of water; 3 = 200 mL of low pH beverage.
On day 12, the 24-hour time point was not collected for 24 of 38 patients, so AUC0–24h was not calculated for those patients.
Fig. 1.
(A) Individual Cmax values versus dose of MGCD0103. (B) Individual AUC0–24 hours values versus dose of MGCD0103.
PD Analysis
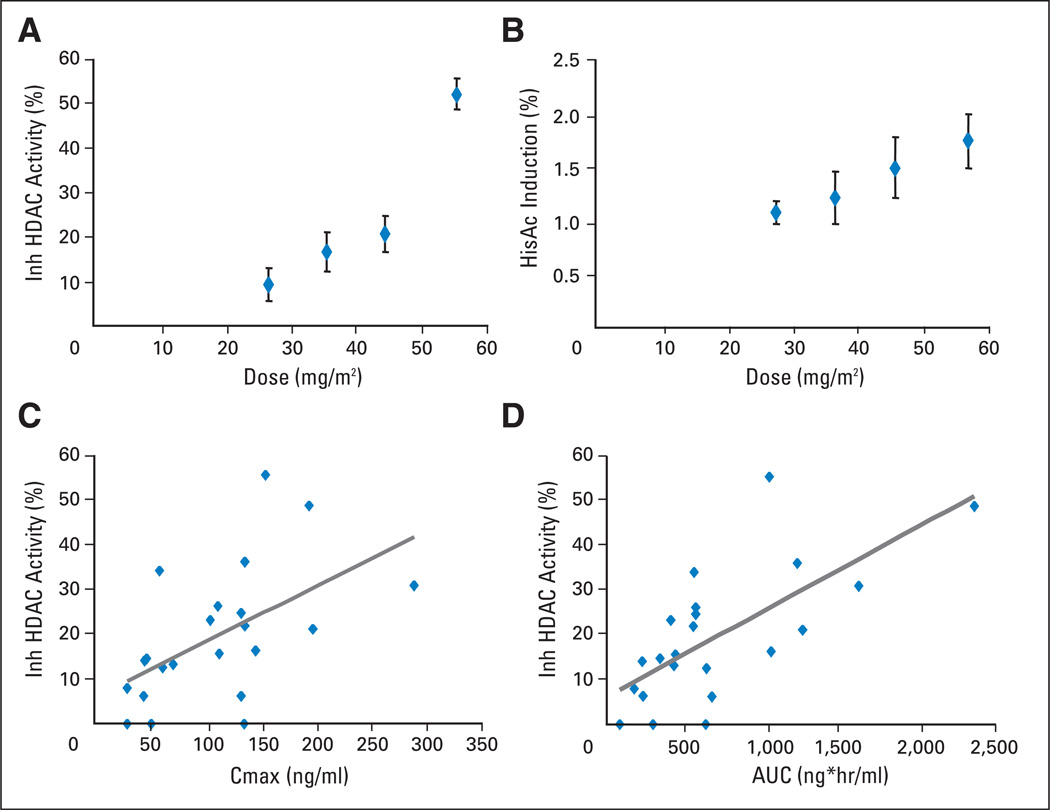
Whole cell HDAC activity was measured in intact peripheral white cells using a cell-permeable deacetylase substrate, Boc-Lys(ε-Ac)-AMC (Bachem, Torrance, CA), before and post-MGCD0103 treatment in dose levels ≥ 27 mg/m2/d. To ensure that an accurate number of cells were used in each well of the assay, cell counts were determined using a hematology analyzer machine (Beckman Coulter Ac•T diff). Earlier samples, from patients treated at a dose lower than 27 mg/m2/d, were counted by eye with a hemacytometer. Because this method of counting is more subjective than the cell counter machine, the data generated from those samples were not included in this study. At 24 hours postdose, MGCD0103 inhibited whole cell HDAC activity in vivo in a dose-dependent manner (Fig 2A). Samples from patients at or above the 45 mg/m2/d dose level showed significant levels of HDAC inhibition when compared with those from the 27 mg/m2/d dose level (n = 6; P = .06 for 45 mg/m2/d, n = 8; and P = .001 for 56 mg/m2/d, n = 2). Induction of histone H3 acetylation was also analyzed as another PD measurement of HDAC inhibitory activity (Fig 2B). When compared with the 27 mg/m2/d-dose level, there was no significant induction of histone H3 acetylation in other dose levels, except in the two patients in the highest dose level (56 mg/m2/d, P = .03). Correlation of HDAC inhibition with PK parameters was analyzed in all patients applicable. As shown in Figures 2C and 2D, there was a general trend of association between higher AUC or Cmax values and a greater degree of HDAC inhibition. No correlation was found between AUC or Cmax with induction of histone acetylation (data not shown).
Fig. 2.
(A) Histone deacetylase (HDAC) inhibition versus dose and (B) induction of histone H3 acetylation versus dose in peripheral white cells from patients 24-hours post-initial dose of MGCD0103. Correlation of HDAC inhibition (24 hours) versus (C) Cmax and (D) area under the curve (AUC) at day 1 is shown.
To investigate the mechanism of fatigue observed in patients treated with MGCD0103, the cytokine IL-6 was measured in plasma using ELISA pre- and post-treatment. Although two patients (one in each of the 36-mg/m2/d and 56-mg/m2/d dose levels) with the most dramatic induction of plasma IL-6 levels (≥ 12-fold) both experienced grade 3 fatigue, there was no clear overall correlation of grade 3 fatigue with the induction of plasma IL-6 or with MGCD0103 dose. There was also no correlation of IL-6 induction with either AUC or Cmax values in patients (data not shown). Thyroid function tests, collected in a limited subset of patients who developed fatigue on study, did not reveal a significant correlation.
DISCUSSION
In this patient population, 45 mg/m2/d was the RPTD of MGCD0103 when given on a schedule of three times per week for 2 of every 3 weeks. Subsequent studies of MGCD0103 have used fixed dose dosing, and the RPTD of 45 mg/m2/d would be considered equivalent to 85 to 90 mg of fixed dose. Fatigue was the most common DLT in this study, with other limiting toxicities being nausea, vomiting, anorexia, and dehydration. These toxicities are consistent with class effects observed with other HDAC inhibitors in clinical development.6–10 The etiology of fatigue induced by HDAC inhibitors is poorly understood. Circulating levels of certain cytokines such as IL-6 have been shown to positively correlate with fatigue in cancer patients.11 In our study, some patients treated at higher doses of MGCD0103 had significant elevations of their IL-6 levels, but no clear correlation with grade 3 fatigue was found. Detailed studies are warranted to further investigate whether serum IL-6 release is related to HDAC inhibition.
Despite the lack of objective tumor responses in this phase I trial, the PK and PD evaluations have demonstrated several favorable pharmacologic properties of MGCD0103. The elimination t½ of MGCD0103 is about 10 hours at the RPTD of 45 mg/m2/d, which compares positively against the shorter, 1- to 2-hour half-lives of some of the other HDAC inhibitors such as vorinostat and PXD101.7,12 Furthermore, the PD assays performed in our study revealed that MGCD0103 exerts dose-dependent HDAC inhibitory activity and is able to induce histone acetylation in peripheral WBCs, as a proof of activity on its putative targets using surrogate tissues. Enzyme inhibition readout appeared to be more sensitive than histone acetylation assay to monitor the PD effect, as we did not observe significant induction of histone acetylation at the RPTD. The HDAC enzyme inhibition by MGCD0103 appeared to be sustained, since on day 8 (72 hours after the first three doses) we still observed significant enzyme inhibition at groups treated at RPTD or higher (data not shown). These pharmacologic properties suggest that MGCD0103 may be given with longer rest intervals of up to 72 hours between dosing, such as using a twice-weekly schedule, whereby dose-limiting fatigue may be reduced or ameliorated. In fact, the twice-weekly dosing schedule for MGCD0103 for 3 consecutive weeks, with no recovery period between cycles, has been examined in patients with advanced leukemia or myelodysplastic syndrome, with an RPTD of 66 mg/m2/d.13 In addition to laboratory-based PD assays, because HDAC inhibitors may have antiangiogenic properties, vascular functional imaging tools such as dynamic contrast-enhanced magnetic resonance imaging or ultrasound should be considered as noninvasive methods of PD evaluations in future studies.14–16
The isoform specificity of MGCD0103 has theoretical attributes in comparison to nonspecific HDAC inhibitors. The class I HDAC isoforms (1, 2, 3, and 8) are important in regulating the proliferation and survival of cancer cells. Of all HDAC classes, they are considered by some researchers as having the most clinical relevance in anticancer treatment.17,18 Experimental evidence illustrating this finding includes the following: HDAC1 knockout in mice resulted in embryonic lethality19; HDAC2 knockdown by small interfering RNA in HT29 colonic cancer cells resulted in apoptosis20; and small interfering RNA knockdown of HDACs 1 and 3 (class I), but not of HDACs 4 and 7 (class II), inhibited proliferation in HeLa cells.21 Currently, HDAC inhibitors appear to have limited clinical activity as monotherapy in solid tumors, and their anticancer activity in epithelial malignancies may be manifested predominantly through combinational evaluations with cytotoxic or other targeted therapeutics. Meanwhile, the promising activity of HDAC inhibitors in some lymphoproliferative malignancies has corroborated the therapeutic value of targeting histone deacetylation.22,23 The emergence of isoform-specific HDAC inhibitors, such as MGCD0103, may alter the expression of a more focused, disease-related subset of genes with fewer adverse effects, and thereby optimize the therapeutic index of this class of agents. Ongoing clinical trials of MGCD0103 in combination with cytotoxic agents in solid tumors, such as docetaxel and gemcitabine, with demethylating agents such as azacitidine in hematologic malignancies, and as single-agent in lymphoproliferative disorders will provide further insight into the therapeutic benefit of target specificity.
Supplementary Material
Acknowledgment
We thank Laura Pearce and Tracy Ann Patterson for project management; Claire Bonfils and Marja Dubay for analysis of histone acetylation; and Christiane Maroun, PhD, for interleukin-6 ELISA analysis.
Footnotes
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, Atlanta, GA, June 2–6, 2006.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Gregory K. Reid, MethylGene Inc (C); Zuomei Li, MethylGene Inc (C); Ann M. Kalita, MethylGene Inc (C); Eric J. Laille, Pharmion Inc (C); Jeffrey M. Besterman, MethylGene Inc (C); Robert E. Martell, MethylGene Inc (C) Consultant or Advisory Role: Roberto Pili, Pharmion Inc (C); Michael A. Carducci, MethylGene Inc (C), MGI Pharma Inc (C), Merck (C), Abbott Laboratories (C) Stock Ownership: Zuomei Li, MethylGene Inc; Eric J. Laille, Pharmion Inc; Jeffrey M. Besterman, MethylGene Inc Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Lillian L. Siu, Gregory K. Reid, Zuomei Li, Jeffrey M. Besterman, Robert E. Martell, Michael A. Carducci
Financial support: Gregory K. Reid, Jeffrey M. Besterman, Robert E. Martell
Administrative support: Gregory K. Reid, Jeffrey M. Besterman
Provision of study materials or patients: Lillian L. Siu, Roberto Pili, Ignacio Duran, Wells A. Messersmith, Eric X. Chen, Gregory K. Reid, Jeffrey M. Besterman, Robert E. Martell, Michael A. Carducci
Collection and assembly of data: Roberto Pili, Wells A. Messersmith, Eric X. Chen, Rana Sullivan, Martha MacLean, Serina King, Shirley Brown, Zuomei Li, Ann M. Kalita, Robert E. Martell, Michael A. Carducci
Data analysis and interpretation: Lillian L. Siu, Gregory K. Reid, Zuomei Li, Ann M. Kalita, Eric J. Laille, Robert E. Martell, Michael A. Carducci
Manuscript writing: Lillian L. Siu, Gregory K. Reid, Zuomei Li, Eric J. Laille, Robert E. Martell, Michael A. Carducci
Final approval of manuscript: Lillian L. Siu, Roberto Pili, Ignacio Duran, Wells A. Messersmith, Eric X. Chen, Rana Sullivan, Martha MacLean, Serina King, Shirley Brown, Gregory K. Reid, Zuomei Li, Ann M. Kalita, Eric J. Laille, Jeffrey M. Besterman, Robert E. Martell, Michael A. Carducci
REFERENCES
- 1.Dokmanovic M, Marks PA. Prospects: Histone deacetylase inhibitors. J Cell Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- 2.Hassig CA, Schreiber SL. Nuclear histone acetylases and deacetylases and transcriptional regulation: HATs off to HDACs. Curr Opin Chem Biol. 1997;1:300–308. doi: 10.1016/s1367-5931(97)80066-x. [DOI] [PubMed] [Google Scholar]
- 3.Kouzarides T. Histone acetylases and deacetylases in cell proliferation. Curr Opin Genet Dev. 1999;9:40–48. doi: 10.1016/s0959-437x(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 4.MethylGene Inc. Investigator’s brochure of MGCD0103 [Google Scholar]
- 5.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 6.Marshall JL, Rizvi N, Kauh J, et al. A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J Exp Ther Oncol. 2002;2:325–332. doi: 10.1046/j.1359-4117.2002.01039.x. [DOI] [PubMed] [Google Scholar]
- 7.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandor V, Bakke S, Robey RW, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8:718–728. [PubMed] [Google Scholar]
- 9.Ryan QC, Headlee D, Acharya M, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 10.Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 11.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Steele N, Vidal L, Plumb J, et al. A phase 1 pharmacokinetic (PK) and pharmacodynamic (PD) study of the histone deacetylase (HDAC) inhibitor PXD101 in patients (pts) with advanced solid tumours. J Clin Oncol. 2005;23:200s. doi: 10.1158/1078-0432.CCR-07-1786. (abstr 3035) [DOI] [PubMed] [Google Scholar]
- 13.Lancet JE, Nichols G, Assouline S, et al. A phase I study of MGCD0103 given as a twice weekly oral dose in patients with advanced leukemias or myelodysplastic syndromes (MDS) J Clin Oncol. 2007;25:101s. (abstr 2516) [Google Scholar]
- 14.Mehnert JM, Kelly WK. Histone deacetylase inhibitors: Biology and mechanism of action. Cancer J. 2007;13:23–29. doi: 10.1097/PPO.0b013e31803c72ba. [DOI] [PubMed] [Google Scholar]
- 15.Torigian DA, Huang SS, Houseni M, et al. Functional imaging of cancer with emphasis on molecular techniques. CA Cancer J Clin. 2007;57:206–224. doi: 10.3322/canjclin.57.4.206. [DOI] [PubMed] [Google Scholar]
- 16.Atri M. New technologies and directed agents for applications of cancer imaging. J Clin Oncol. 2006;24:3299–3308. doi: 10.1200/JCO.2006.06.6159. [DOI] [PubMed] [Google Scholar]
- 17.Glaser KB. HDAC inhibitors: Clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Karagiannis TC, El-Osta A. Will broad-spectrum histone deacetylase inhibitors be superseded by more specific compounds? Leukemia. 2007;21:61–65. doi: 10.1038/sj.leu.2404464. [DOI] [PubMed] [Google Scholar]
- 19.Lagger G, O’Carroll D, Rembold M, et al. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. Embo J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu P, Martin E, Mengwasser J, et al. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5:455–463. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 21.Glaser KB, Li J, Staver MJ, et al. Role of class I and class II histone deacetylases in carcinoma cells using siRNA. Biochem Biophys Res Commun. 2003;310:529–536. doi: 10.1016/j.bbrc.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Piekarz RL, Robey R, Sandor V, et al. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: A case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- 23.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.