Abstract
Although inflammatory immune cells clearly contribute to the development of middle cerebral artery occlusion (MCAO) in mice, the failure to block neutrophil-associated injury in clinical stroke trials has discouraged further development of immunotherapeutic approaches. However, there is renewed interest in a possible protective role for regulatory T- and B-cells that can suppress inflammation and limit central nervous system damage induced by infiltrating pro-inflammatory cells. Our failure to implicate CD4+FoxP3+ T-cells in limiting brain lesion volume after MCAO turned our focus towards regulatory B-cells known to mediate protection against other inflammatory CNS conditions. Our results clearly demonstrated that B-cell deficient mice developed larger infarct volumes, higher mortality and more severe functional deficits compared to wild-type mice, and had increased numbers of activated T-cells, macrophages, microglial cells, and neutrophils in the affected brain hemisphere. These MCAO-induced changes were completely prevented in B-cell-restored mice after transfer of highly purified WT B-cells but not IL-10-deficient B-cells. Our novel observations are the first to implicate IL-10-secreting B-cells as a major regulatory cell type in stroke and suggest that enhancement of regulatory B-cells might have application as a novel therapy for this devastating neurologic condition.
Keywords: Experimental stroke, Bregs, IL-10, PD-1, immunotherapy
Human stroke results in brain infarction but also in multi-organ systemic disease. Many patients who survive the initial cerebral ischemic insult will go on to die from stroke-induced immune system suppression and resulting fatal infection [1–3]. We and others have modeled the double edged sword of immune cell action during experimental stroke in animals [4–16]. The systemic consequences of CNS injury are best described as a serial and long-lasting process: first, there is an induction of lymphoid tissue (e.g. spleen and thymus) into a pro-inflammatory activation state; second, one observes apoptotic self-destruction of these same organs and selective loss of many immunocytes nurtured within [8,10]. Splenic activation also leads to expulsion of immune cells into the blood that then target brain. In most cases, these immunocytes exacerbate the evolving brain infarct.
From the perspective of the brain, inflammation after cerebral ischemia has been studied extensively. Inflammation is initiated through the innate immune system through Toll-like receptors on antigen presenting cells (largely microglia) [17]. Both peripheral innate and adaptive immune cells are early responders to injured tissue signals, and blood borne inflammatory cells begin to enter brain much earlier than previously understood. Early infiltration of macrophages, T and B lymphocytes and dendritic cells into injured brain occurs after middle cerebral artery occlusion (MCAO), even before the peak of neutrophilic influx [18]. The cell specificity and mechanisms of these initiating players in damage remains poorly understood. Parenthetically, the role of neutrophils has been well studied in experimental cerebral ischemia, and agents directed toward blocking neutrophil-associated injury have failed in clinical stroke trials [19,20]. The failure of these early trials has somewhat dampened interest in developing immunotherapy for acute stroke.
However, new evidence is again igniting interest in this modality. Specifically, whereas most inflammatory cells arising from the periphery contribute to CNS damage, regulatory lymphocytes of T or B cell lineages suppress inflammation and may limit damage conferred by other infiltrating pro-inflammatory immunocytes. Under such circumstances, T or B regulatory cells (Treg and Breg) might be viewed as a source of natural CNS protection. If so, then stroke research must renew the challenge to understand the mechanisms by which these immunological cells act on injured brain, as a first step in designing novel immunotherapy.
Regulatory T lymphocytes
In our early studies of the effects of MCAO on the peripheral immune system [8,10,11,21], we observed bi-phasic consequences, which we have conceptualized as “brain-spleen cell cycling”. The initial phase (6–22h post-MCAO) occurs in part by brain to spleen signaling via central adrenergic neural and catecholaminergic mechanisms [22,23]. The result is massive, intra-splenic production of inflammatory factors, followed by progressive death of splenic immune cells in situ by apoptotic mechanisms. Remaining cells in the spleen translocate to the blood and then on to brain (spleen to brain signal). By 96h post-MCAO, there is widespread systemic immunosuppression, developed in tandem with the maturing brain infarct.
Curiously, despite the drastic loss of immune cells in spleen, there was a pronounced increase in Treg cells within days of the ischemic insult. In normal mice, Treg cells limit inflammation and inhibit autoimmune diseases [21,24–26]. Based on our observed increases in surviving CD3+CD4+FoxP3+ Treg cells [10], it seemed that these cells were relatively resistant to apoptosis or other mechanisms that act to reduce viable immune splenocyte numbers. We and others [27] questioned whether these surviving Treg cells might not only impact peripheral splenic pathology but could limit the early inflammatory infarct in brain. Liesz and colleagues [27] evaluated the functional role of CD25+ cells, including Tregs, in experimental stroke, and found that depletion of the CD25+ population with anti-CD25 mAb significantly increased brain infarct volume and worsened functional outcome. These effects were attributed to CD4+CD25+FoxP3+Treg cells, even though the anti-CD25 mAb only depleted ~50% of this Treg phenotype. CD25, the IL-2 receptor chain-α (IL-2Rα), has a broad expression on early progenitors of the T- and B-cell lineages, as well as on activated mature T-cells and B-cells, thus allowing for the possible contribution of other regulatory cell types besides FoxP3+Treg.
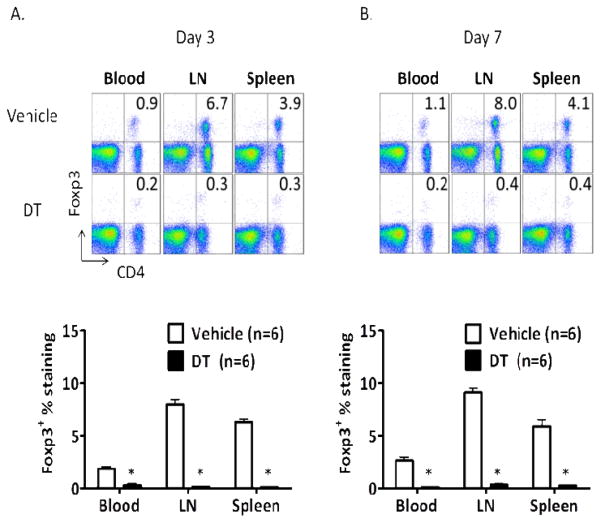
To target Treg more specifically, we employed the FoxP3DTR mouse, in which the coding sequence of diphtheria toxin receptor (DTR) has been inserted into the FoxP3 allele and is co-expressed on FoxP3+ cells [28]. We thus accomplished near complete deletion of FoxP3 expression in the peripheral blood, lymph nodes and spleens (≤0.3%) by treating the mice with two daily i.p. injections of 50μg diphtheria toxin (DT) per kg body weight (Fig. 1A) prior to induction of MCAO for 60min on Day 3, followed by a final DT treatment on Day 4 that maintained deletion of FoxP3+Treg as assessed on Day 7 (Fig. 1B). In comparison, normal levels of FoxP3+Treg cells were observed in vehicle-treated FoxP3DTR mice undergoing MCAO (Fig. 1). Depletion of FoxP3+Treg did not affect infarct volume in cortex or striatum in mice or either sex assessed at 96h after MCAO (timeline in Fig. 2A) compared to vehicle treated mice (Figs. 2B, 2C & 2D). Moreover, there were no significant effects of Treg depletion on behavioral evaluations (data not shown). Thus, we conclude that Treg did not limit brain damage or improve functional outcomes in mice undergoing MCAO. Our failure to implicate CD4+FoxP3+ T-cells in limiting brain lesion volume after MCAO is of general importance to the field because of the increased interest in increasing CD4+FoxP3+Treg as a possible therapeutic approach in stroke. Our results do not support this rationale, but do highlight the need to identify other regulatory pathways that can limit stroke-induced inflammatory damage to the CNS. Accordingly, we focused on an alternative and newly identified B lymphocyte subset that has a crucial regulatory role in neuroimmunology.
Figure 1. Characterization of FoxP3+Treg cell ablation in FoxP3DTR mice.
(A) On day 3 (after 2 DT or Vehicle treatments) and (B) on day 7 (after 3 DT or Vehicle treatments). Flow cytometric analysis of blood, lymph node (LN) and spleen shows efficient depletion of FoxP3+ cells in DT-treated FoxP3DTR mice vs. Vehicle controls (upper right quadrant). *Vehicle vs. DT treatment, p<0.001.
Figure 2. Depletion of Treg cells does not change ischemic lesion volume.
(A) Scheme for experimental design. (B) Infarct volume at 96h reperfusion after 60min MCAO in male mice, (C) female mice and (D) combined data from both genders.
Regulatory B lymphocytes
Based on recent data from studies of CNS auto-immune disease, we first raised the hypothesis that a small but powerful subset of IL-10-producing CD1dhiCD5+ regulatory B lymphocytes could limit infarction after cerebral ischemia and potentially improve outcomes. Recent studies in models of experimental autoimmune encephalomyelitis (EAE), arthritis and colitis identified at least two phenotypically distinct subsets of regulatory B-cells (Breg) —transitional 2 marginal-zone precursor T2- MZP cells and B10 cells— that can exert immunosuppressive functions in vivo as well as in vitro. Breg cells characteristically produce interleukin IL-10 and express CD19. Bregs act mainly, but not exclusively, via the release of IL-10, a well-recognized anti-inflammatory cytokine that is effective against most forms of CNS damage. For example, IL-10 deficient mice develop larger infarcts after focal cerebral ischemia [29]; moreover, administration of IL-10 to the lateral ventricle [30] or intraperitoneally [31], by adenoviral vectors [32], after induction of mucosal tolerance by IL-10-producing T cells [33] or by transgenic over-expression of IL-10 [34] reduces infarct volume. Clinically, early worsening of stroke is associated with lower IL-10 plasma levels in patients [35]. However, it should also be noted that the requirement for other potential soluble factors and co-inhibitory molecules than IL-10 is an active area of investigation. Figure 3 summarizes a simple set of fundamental mechanisms by which IL-10 expressing B10, MZ, T2-MZ and B-1a Breg-cell subsets might act to inhibit activation of T-cells, macrophages and dendritic cells.
Figure 3.
Summarizes a set of fundamental mechanisms by which IL-10 expressing B10, MZ, T2-MZ and B-1a Breg-cell subsets might act to inhibit activation of T-cells, macrophages and dendritic cells.
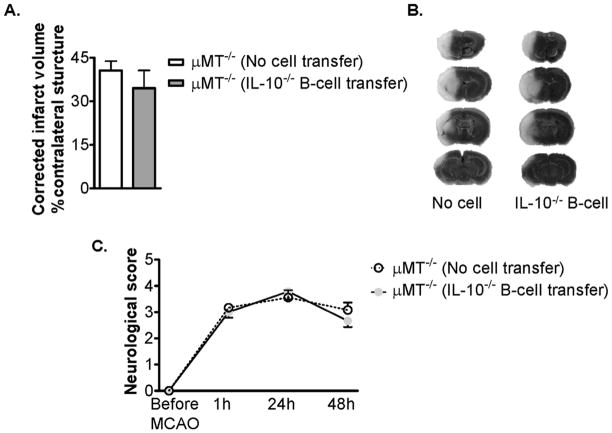
Examining data from models of multiple sclerosis, we noted that depletion of B-cells increases disease severity, as studied in B-cell deficient mice or after cell depletion with anti-CD22 monoclonal antibodies. Further, transfer of CD1dhiCD5+ cell subsets provides protection against disease induction. Consistent with these data, we have recently demonstrated that B-cell deficient mice exhibit increased infarct size after MCAO, implicating protective regulatory effects by B-cells on limiting infarct size and behavioral deficits (Fig. 4). These protective effects were not reconstituted after transfer of B-cells from IL-10 knockout donor mice, indicating that the ability of Breg cells to limit post-MCAO damage was linked to their marked secretion of IL-10 (Fig. 5).
Figure 4. Deficiency of B-cells exacerbates ischemic infarct volume and behavioral outcome after MCAO.
(A) Infarct volume, corrected for the presence of edema, at 48h reperfusion after 60min MCAO given as bar graphs are visualized (Mean ± SEM). Statistical analysis was performed with the Student’s t test. There was a significant difference of infarct volumes between WT and μMT−/− mice; *, P < 0.05. Groups: WT (n = 15); μMT−/− (n = 12). (B) Representative TTC-stained cerebral sections of the MCAO modeled to analyze infarct volume. Localization of the ischemic lesion differed between WT and μMT−/− mice. (C) B-cell deficient μMT−/− mice had no significant functional worsening before reperfusion, but significantly worsened functional outcome at 24h, *, P = 0.02, and 48h, **, P = 0.002, after reperfusion. The statistical analysis was performed using the Mann-Whitney-U-test. Groups: WT (n = 35); μMT−/− (n = 35).
Figure 5. Transfer of IL-10−/− CD19+ B-cells does not alter infarct volume or improve behavioral outcome of μMT−/− mice.
(A) Transfer of 50 million CD19+ B-cells from IL-10−/− mice had no effect on infarct volume of total hemisphere (Mean ± SEM) at 48h reperfusion after 60min MCAO. Statistical analysis was performed using the Student’s t test. There was no significant difference of infarct volumes between no cell (PBS) transferred (n = 9) and IL-10−/− B-cell (n = 8) transferred μMT−/− mice. (B) Representative TTC-stained cerebral sections of the MCAO modeled to analyze infarct volume. Localization of the ischemic lesion did not differ between no cell and IL-10−/− B-cell transferred μMT−/− mice. (C) Transfer of IL-10−/− B-cells did not significantly improve functional outcome after 24h or 48h reperfusion. The statistical analysis was performed using the Mann-Whitney-U-test.
Breg cell activity is also thought to be closely tied to the “Programmed Cell Death 1” receptor (PD-1)/”Programmed Cell Death” ligand (PD-L) co-inhibitory pathway. PD-1, also referred to as CD279, is an Ig superfamily member containing an immunoreceptor tyrosine-based inhibitory motif (ITIM) [36] that is inducibly expressed by activated T-cells, B-cells, NK-cells and monocytes [37,38]. Binding of PD-1 to either of two ligands, PD-L1 (B7-H1 or CD274) or PD-L2 (B7-DC or CD273) that have overlapping expression patterns, induces inhibitory signals that control induction and maintenance of peripheral T cell tolerance and immune homeostasis [22,23,39]. Much work has focused on the autoimmune phenotype of PD-1 deficient mice [40] and linkage of PD-1 genes with autoimmune disorders [41]. Moreover, other inhibitory pathways involving PD-1/PD-L have also been described, including our studies on estrogen mediated suppression via Treg cell activation [42–44] and inhibition of encephalitogenic T cell responses by PD-L-expressing myeloid APC [45]. ‘Reverse signaling’ through PD-L has also been described that induces a suppressive dendritic cell (DC) phenotype [46]. Finally, PD-L1 has been shown to protect against CD8+ T-cell lysis of virally-infected target cells [47] and PD-1-dependent immune-mediated damage of CNS oligodendroglial cells [48]. Specific to our interest in Breg cells and stroke, both PD-1 and PD-L1 are expressed on B-cells, and agents that enhance the CD1dhiCD5+ B-cell numbers also increase expression of PD-L1 on B-cells.
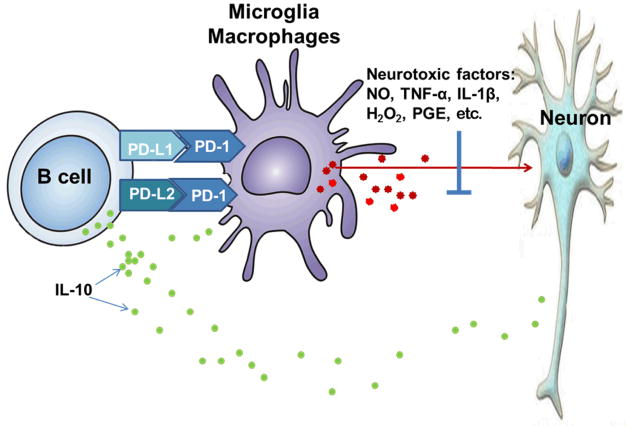
Because of the strong possibility that this pathway would be involved in limiting brain inflammatory damage, we induced MCAO in PD-1-deficient mice. In our mouse MCAO model, infarction in both cortex and striatum were increased in PD-1-deficient mice, implicating the PD-1/PD-L co-inhibitory pathway for the first time in cerebral ischemia. Moreover, we observed increased expression of PD-1 on brain microglial cells and of PD-L on B cells from spleen and blood in mice after MCAO, thus providing insight into interactive cell combinations that likely contribute to limiting infarct size [14]. These novel results suggest a previously undescribed regulatory circuit in which MCAO stimulates circulating PD-L+B-cells to cross into brain, potentially regulating brain inflammation directly through secretion of IL-10 and via suppressive interaction with microglia (Fig. 6).
Figure 6. B-cell regulation of microglial activation may occur both through release of IL-10 and the PD-1/PD-L co-inhibitory pathway.
MCAO causes increased secretion of IL-10 and enhanced expression of PD-L1 and PD-L2 by peripheral B cells. When these B-cells are attracted to the growing infarct, they cross the blood brain barrier and inhibit activation.
The convergence of data from multiple sclerosis and stroke models suggest that Breg cells and the PD-1/PD-L co-inhibitory pathway are important in limiting CNS damage. While further evaluation is strongly needed, one hypothesis is that Breg cells, including the B10 CD1dhiCD5+ B-cell subset, are immunocytes that provide endogenous neuroprotection in many types of CNS pathology. In experimental stroke, Bregs may limit infarct size by controlling immune-mediated inflammation ordinarily triggered both in the CNS and in peripheral immune organs by focal stroke. We further postulate that B10 cells are spared in post-MCAO apoptotic spleen destruction, allowing them to naturally, but partially, limit infarct progression. Of key importance, we predict that either selective induction or passive transfer of B10 cells, so as to augment B10 cell occupation in the injured brain, may provide additional regulatory effects, resulting in smaller infarct and improved functional stroke outcome.
In summary, these studies challenge the prevailing concept that immune cells monolithically damage the post-ischemic brain and propose an alternative. Regulatory lymphocytes, specifically the regulatory B-cell, protect the injured brain and might be harnessed as the first cell-specific immunotherapy for treatment of acute stroke. This change in direction is crucial to obtaining a broader perspective for immune-regulation and selection of immunotherapy for stroke.
Acknowledgments
The authors wish to thank Ms. Eva Niehaus for assistance with manuscript preparation. This work was supported by NIH grants NS075887 (HO) and NR003521 (PDH). This material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center, Portland, OR. The contents do not represent the views of the Department of Veterans Affairs or the United States government.
REFERENCES CITED
- 1.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a minifestation of brain-induced immunodepression. Stroke. 2007;38:1097–103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–3. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 3.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nature Neuroscience Rev. 2005;6:775–86. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 4.Akiyoshi K, Dziennis S, Palmateer J, et al. Recombinant T cell receptor ligands improve outcome after experimental cerebral ischemia. Transl Stroke Res. 2011;2:404–10. doi: 10.1007/s12975-011-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyoshi K, Ren X, Dziennis S, et al. Regulatory B-cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–63. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dziennis S, Kozaburo A, Subramanian S, Offner H, Hurn PD. Role of dihydrotestosterone in post-stroke peripheral immunosuppression after cerebral ischemia. Brain Behav & Immun. 2011;25:685–95. doi: 10.1016/j.bbi.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dziennis S, Mader S, Akiyoshi K, et al. Therapy with recombinant T cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metab Brain Dis. 2011;26:123–33. doi: 10.1007/s11011-011-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurn PD, Subramanian S, Parker SM, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cerebral Blood Flow & Metab. 2007;27:1798–805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cerebral Blood Flow & Metab. 2006;26:654–65. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 10.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–31. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 11.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ishecmia induces profound immunosuppression. Neuroscience. 2009;158:1098–111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren X, Akiyoshi K, Grafe MR, et al. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab Brain Dis. 2012;27:7–15. doi: 10.1007/s11011-011-9267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011;26:87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. Programmed death-1 pathway limits CNS inflammation and neurologic deficits in murine experimental stroke. Stroke. 2011;42:2578–83. doi: 10.1161/STROKEAHA.111.613182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramanian S, Zhang B, Kosaka Y, et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009 doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Subramanian S, Dziennis S, et al. Estradiol an dG1 reduce infarct size and improve immunosuppression after experimental stroke. Journal of Immunology. 2010;184:4087–94. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh BJ, Williams-Karnesky RL, Stenzel-Pore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2008;158:1007–20. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelderblom M, Leypoldt F, Steinbach K. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–57. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 19.Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F26) and Enlimomab (R6. 5) in acute stroke. Current Med Res and Opinion. 2002;19:18–22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- 20.Krams M, Lees KR, Hacke W. Acute stroke therapy by inhibition of neutrophils (ASTIN): an adaptive dose- response study of UK-279. 276 in acute ischemic stroke. Stroke. 2003;34:2543–8. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- 21.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nature Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 22.Dinesh RK, Hahn BH, Singh RP. PD-1, gender, and autoimmunity. Autoimmunity Reviews. 2010;9:583–7. doi: 10.1016/j.autrev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnus T, Schreiner B, Korn T, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. Journal of Neuroscience. 2005;25:2537–46. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649–55. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 25.Roncarolo MG, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000;12:676–83. doi: 10.1016/s0952-7915(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 26.Shevach EM. Regulatory T cells in autoimmunity. Ann Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 27.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature Medicine. 2009;15:192–9. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, Rasmussen FP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 29.Grilli M, Barbieri I, Basudev H, et al. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. European Journal of Neuroscience. 2000;12:2265–72. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- 30.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neuroscience Letters. 1998;251:189–92. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich WD, Busto R, Bethea JR. Postiscchemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Experimental Neurology. 1999;158:444–50. doi: 10.1006/exnr.1999.7115. [DOI] [PubMed] [Google Scholar]
- 32.Ooboshi H, Ibayashi S, Shichita T, et al. Postischemic gene transfer of intrleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–9. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. Journal of the Neurological Sciences. 2005;233:125–32. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 34.de Bilbao FDA, Moll T, et al. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. Journal of Neurochemistry. 2009;110:12–22. doi: 10.1111/j.1471-4159.2009.06098.x. [DOI] [PubMed] [Google Scholar]
- 35.Vila N, Castillo J, Davalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–5. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 36.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Current Opinion in Immunology. 2007;19:309–14. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1-deficient mice: implication of PD-1 as a negative regulator for B cell responses. International Immunology. 1998;10:1563–72. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 39.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature Immunology. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed eath-1 (PD-1) International Immunology. 2007;19:337–43. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Dehghani B, Li Y, et al. Member estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. Journal of Immunology. 2008;182:3294–303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–35. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T cell responses in EAE. European Journal of Immunology. 2008;38:2706–17. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuipers H, Muskens F, Willart M, et al. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. European Journal of Neuroimmunology. 2006;36:2472–82. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 47.Phares TW, Ramakrishna C, Parra GI, et al. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. Journal of Immunology. 2009;182:5430–8. doi: 10.4049/jimmunol.0803557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroner A, Schwab N, Ip CW, et al. PD-1 regulates neural damage in oligodendroglia-induced inflammation. PLoS One. 2009;4:e4405. doi: 10.1371/journal.pone.0004405. [DOI] [PMC free article] [PubMed] [Google Scholar]