Abstract
We demonstrate a fairly general method for identification of NMR absorption lines of macromolecues extracted from microorganisms, based on nuclear Overhauser effects (NOE). Several NOE in tRNA are observable between resolved imino proton resonances and ring carbon resonances that are either C(2) protons of adenine or C(8) protons of adenine or guanine. Yeast tRNAPhe was deuterated at the purine C(8) positins by heating in 2H2O and also biosynthetically. NOE between imino protons and adenine C(2) protons of standard A . U base pairs would not be affected by such a label, but some other NOE that might be otherwise similar, such as those of reverse Hoogsteen base pairs, should disappear. Six NOE were shown to be from standard A . U pairs by their nondisappearance. Four NOE from methyl resonances to aromatic proton resonances did disappear. The results disagree with previous assignments based on ring-current theories of imino proton NMR shifts.
Full text
PDF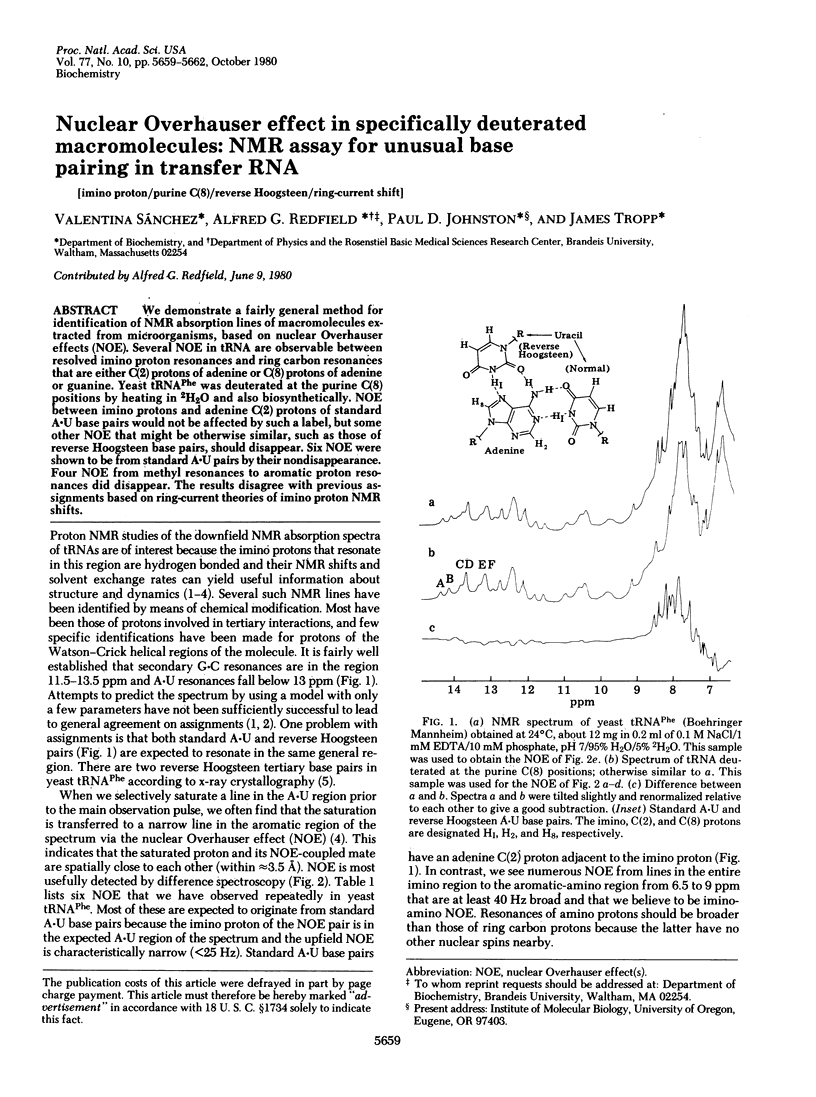



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arter D. B., Schmidt P. G. Ring current shielding effects in nucleic acid double helices. Nucleic Acids Res. 1976 Jun;3(6):1437–1447. doi: 10.1093/nar/3.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Kan L. S., Ts'o P. O. Conformation and interaction of short nucleic acid double-stranded helices. I. Proton magnetic resonance studies on the nonexchangeable protons of ribosyl ApApGpCpUpU. Biochemistry. 1975 Nov 4;14(22):4847–4863. doi: 10.1021/bi00693a012. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Sprinzl M., Cramer F. Proton nuclear magnetic resonance of minor nucleosides in yeast phenylalanine transfer ribonucleic acid. Conformational changes as a consequence of aminoacylation, removal of the Y base, and codon--anticodon interaction. Biochemistry. 1979 Jul 24;18(15):3189–3199. doi: 10.1021/bi00582a001. [DOI] [PubMed] [Google Scholar]
- Geerdes H. A., Hilbers C. W. The iminoproton NMR spectrum of yeast tRNA-Phe predicted from crystal coordinates. Nucleic Acids Res. 1977 Jan;4(1):207–221. doi: 10.1093/nar/4.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd R. E., Azhderian E., Reid B. R. Paramagnetic ion effects on the nuclear magnetic resonance spectrum of transfer ribonucleic acid: assignment of the 15--48 tertiary resonance. Biochemistry. 1979 Sep 4;18(18):4012–4017. doi: 10.1021/bi00585a026. [DOI] [PubMed] [Google Scholar]
- Johnston P. D., Figueroa N., Redfield A. G. Real-time solvent exchange studies of the imino and amino protons of yeast phenylalanine transfer RNA by Fourier transform NMR. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3130–3134. doi: 10.1073/pnas.76.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L. S., Ts'o P. O. 1H NMR studies of transfer RNA III: the observed and the computed spectra of the hydrogen-bonded NH resonances of baker's yeast transfer-RNA Phe. Nucleic Acids Res. 1977;4(5):1633–1647. doi: 10.1093/nar/4.5.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. R., McCollum L., Ribeiro N. S., Abbate J., Hurd R. E. Identification of tertiary base pair resonances in the nuclear magnetic resonance spectra of transfer ribonucleic acid. Biochemistry. 1979 Sep 4;18(18):3996–4005. doi: 10.1021/bi00585a024. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Redfield A. G. Transfer RNA in solution: selected topics. Annu Rev Biophys Bioeng. 1980;9:181–221. doi: 10.1146/annurev.bb.09.060180.001145. [DOI] [PubMed] [Google Scholar]
- Stoesz J. D., Malinowski D. P., Redfield A. G. Nuclear magnetic resonance study of solvent exchange and nuclear Overhauser effect of the histidine protons of bovine superoxide dismutase. Biochemistry. 1979 Oct 16;18(21):4669–4675. doi: 10.1021/bi00588a030. [DOI] [PubMed] [Google Scholar]
- Tropp J., Sigler P. B. Direct iodination of specific residues in crystals of yeast formylatable methionine-accepting transfer ribonucleic acid. Biochemistry. 1979 Nov 27;18(24):5489–5495. doi: 10.1021/bi00591a035. [DOI] [PubMed] [Google Scholar]



