Abstract
Aims
Duty-cycled radiofrequency ablation (RFA) has been used for atrial fibrillation (AF) for around 5 years, but large-scale data are scarce. The purpose of this survey was to report the outcome of the technique.
Methods and results
A survey was conducted among 20 centres from seven European countries including 2748 patients (2128 with paroxysmal and 620 with persistent AF). In paroxysmal AF an overall success rate of 82% [median 80%, interquartile range (IQR) 74–90%], a first procedure success rate of 72% [median 74% (IQR 59–83%)], and a success of antiarrhythmic medication of 59% [median 60% (IQR 39–72%)] was reported. In persistent AF, success rates were significantly lower with 70% [median 74% (IQR 60–92%)]; P = 0.05) as well as the first procedure success rate of 58% [median 55% (IQR 47–81%)]; P = 0.001). The overall success rate was similar among higher and lower volume centres and were not dependent on the duration of experience with duty-cycled RFA (r = −0.08, P = 0.72). Complications were observed in 108 (3.9%) patients, including 31 (1.1%) with symptomatic transient ischaemic attack or stroke, which had the same incidence in paroxysmal and persistent AF (1.1 vs. 1.1%) and was unrelated to the case load (r = 0.24, P = 0.15), bridging anticoagulation to low molecular heparin, routine administration of heparin over the long sheath, whether a transoesophageal echocardiogram was performed in every patient or not and average procedure times.
Conclusion
Duty-cycled RFA has a self-reported success and complication rate similar to conventional RFA. After technical modifications a prospective registry with controlled data monitoring should be conducted to assess outcome.
Keywords: Atrial fibrillation duty-cycled radiofrequency
Background
Multielectrodes using duty-cycled bipolar/unipolar radiofrequency ablation (RFA) were introduced for ablation of atrial fibrillation (AF) in 2006. First reports were favourable with a success rate of 83% without complications in paroxysmal AF1 and 66% success with 8% complications including one (2%) neurologic event (stroke) in a multicentre trial of 50 patients with longstanding persistent AF.2 The largest single centre study so far with duty-cycled RFA comprised 209 patients and reported two transient ischaemic attacks (TIAs) (0.96%) with duty-cycled RFA.3 Recently however, concerns about the safety of this method have emerged because of the detection of asymptomatic cerebral microemboli, which were more prevalent in patients with duty-cycled RFA (37 and 38%) compared with irrigated RFA (7.4 and 8.3%) and cryoenergy (4.3 and 5.6%).4,5 Although smaller lesions <1 cm of size disappear from follow-up imaging,6 their clinical importance remains to be defined. As a result, serious concerns have risen about the clinical safety and outcome of duty-cycled RFA.
Conventional RFA for AF has been performed since 1998.7 Safety and efficacy of various methods over a large number of centres and continents have been reported by an international survey in 20058 and updated in 2010.9 The purpose of this survey was to report the outcome of duty-cycled RFA for AF in a similar way.
Methods
A detailed questionnaire was sent to 33 EP laboratories using duty-cycled RFA for AF, which were chosen by volume, personal relation and had a structured follow-up with routine patient registry. The questionnaire comprised 91 questions on demographic, procedural, and outcome data in patients undergoing this procedure (see Supplemental data). Among the 33 centres, 21 provided responses, but 1 had to be excluded because of missing data. Thus, retrospective data of 20 centres from seven countries were used for this analysis. The data were regarded complete if 80% or more of the questions were answered.
Prior to RFA, all patients were anticoagulated or underwent a transoeosphageal echocardiogram to rule out left atrial clot.
Duty-cycled radiofrequency ablation for atrial fibrillation
The ablation system of duty-cycled RFA using multielectrodes (Medtronic AF solutions, Carlsbad, CA, UK) has been described previously in paroxysmal and persistent AF.1,2 Briefly, a circular over the wire electrode is used for mapping and ablation of pulmonary veins (Pulmonary Vein Ablation Catheter PVAC, Medtronic AF Solutions). The catheter can also be used to assess isolation of the pulmonary veins by mapping and pacing. For substrate modification at the left interatrial septum a three-arm pull back electrode is used (Multi-Array Septal Catheter MASC) and for the left atrial free wall a four-arm electrode (Multi-Array Ablation Catheter, MAAC). All catheters are deployed over a transseptal sheath of at least 9.5 Fr inner diameter into the left atrium. Radiofrequency is delivered in unipolar/bipolar mode by a generator (GENius) at maximum of 10 W titrated to a target temperature of 60 °C in five predefined energy modes: bipolar, unipolar, and three ratios (1 : 1, 2 : 1, and 4 : 1) of bipolar-to-unipolar energy. In the case of atrial flutter a linear electrode with duty-cycled energy up to 20–40 W was used (TVAC, Medtronic AF solutions) in the right or left atrium as previously reported10,11 or conventional RF energy.
For the safety analysis, access vein complication was defined as groin hematoma, AV fistula or any other complication related to vein access prolonging hospitalization.
Follow-up
The follow-up after the intervention was not standardized. A Holter-electrocardiogram (ECG) for 24 h was performed in eight (40%) centres, two centres (10%) performed a 72 h Holter, a 7-day ECG monitoring by either continuous Holter or loop recorder was performed in nine (45%) centres, and one centre (5%) performed 14-day Holter recording. The time interval to the last regularly performed test were 3 months in 10%, 6 months in 50%, 1 year in 40%, and 2 years in 5%. Success was defined as absence of symptoms and AF on ECG recordings and was assessed by each centre individually.
Statistical analysis
Data were weighted based on the number of patients included per centre. To avoid overestimation of the statistical significance of data weighing was arbitrarily done to achieve n = 2 × 20 considering the two groups of paroxysmal and persistent AF (e.g. 146 patients with persistent AF in centre 1 resulted in a weighted n = 2.13; i.e. 146/2748*20*2), since individual data of patients as well as deviations in each centre were not known. In addition to the weighted values, success rate is also given as median (interquartile range [IQR]) of individual centres. Comparison between groups were then done based on weighted data using Student's t-test or Mann–Whitney U test, as appropriate. Correlations were calculated based on raw data using Spearman correlation since variability of data per centre was not known. All calculations were done with the use of the commercially available statistical package SPSS 18.0.
Results
Data on patients treated with duty-cycled RFA over a mean of 2.3 ± 1 years (range 1–4.7) were collected and analysed. A total of 2748 patients were included, of which 683 (25%) were treated in England, 625 (23%) in Germany, 560 (20%) in Switzerland, 524 (19%) in the Netherlands, 176 (6.4%) in France, 98 (4%) in Belgium, and 82 (3%) in Norway. Although the number of patients varied considerably between centres, none treated >20% of all patients (Table 1). Paroxysmal AF (n = 2128, 77%) was targeted more frequently than persistent AF (n = 620, 23%). However, three centres used the technology predominantly in persistent AF. Most centres were highly experienced with conventional AF ablation cases, but two centres began AF ablation by using duty-cycled RFA as their first technique.
Table 1.
Patient distribution across centres
| Centre | Patients total | Paroxysmal AF | % | Persistent AF | % |
|---|---|---|---|---|---|
| 1 | 530 (19.3%) | 384 | 18.0 | 146 | 23.5 |
| 2 | 463 (16.8%) | 374 | 17.6 | 89 | 14.4 |
| 3 | 303 (11.0%) | 269 | 12.6 | 34 | 5.5 |
| 4 | 249 (09.1%) | 249 | 11.7 | 0 | 0.0 |
| 5 | 210 (07.6%) | 152 | 7.1 | 58 | 9.4 |
| 6 | 184 (06.7%) | 76 | 3.6 | 108 | 17.4 |
| 7 | 103 (03.7%) | 58 | 2.7 | 45 | 7.3 |
| 8 | 98 (03.6%) | 73 | 3.4 | 25 | 4.0 |
| 9 | 93 (03.4%) | 79 | 3.7 | 14 | 2.3 |
| 10 | 82 (03.0%) | 75 | 3.5 | 7 | 1.1 |
| 11 | 70 (02.5%) | 44 | 2.1 | 26 | 4.2 |
| 12 | 61 (02.2%) | 57 | 2.7 | 4 | 0.6 |
| 13 | 60 (02.2%) | 55 | 2.6 | 5 | 0.8 |
| 14 | 58 (02.1%) | 58 | 2.7 | 0 | 0.0 |
| 15 | 42 (01.5%) | 26 | 1.2 | 16 | 2.6 |
| 16 | 33 (01.2%) | 29 | 1.4 | 4 | 0.6 |
| 17 | 30 (01.1%) | 11 | 0.5 | 19 | 3.1 |
| 18 | 30 (01.1%) | 30 | 1.4 | 0 | 0.0 |
| 19 | 28 (01.0%) | 25 | 1.2 | 3 | 0.5 |
| 20 | 21 (00.08% | 4 | 0.2 | 17 | 2.7 |
| Total | 2748 (100%) | 2128 | 620 |
AF, atrial fibrillation.
Patients and procedural details
Patients with paroxysmal AF had an average age between 55 and 65 years similar to persistent AF patients and across countries and centres (Tables 2 and 3). In patients with persistent AF, left atrial diameters did not differ significantly compared with paroxysmal AF (45.3 vs. 43.8 mm, P = 0.62), whereas the ejection fraction was lower (54.1 vs. 58.2%, P = 0.02).
Table 2.
Paroxysmal atrial fibrillation patients (n = 2128)
| Patients (nr) | m/f (%) | Age (years) | LAD (mm) | EF (%) | Procedure duration (min) | X-ray (min) | Success % (first procedure) | Success % off AAD | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 384 | 74/26 | 61 | 41 | 61 | 115 | 29 | 67 | 77 |
| 2 | 374 | 76/24 | 59 | 40 | 56 | 86 | 18 | 64 | 58 |
| 3 | 269 | 82/18 | 58 | 172 | 57 | 80 | 80 | ||
| 4 | 249 | 76/24 | 48 | 60 | 57 | 130 | 20 | 81 | |
| 5 | 152 | 77/23 | 56 | 105 | 21 | 66 | 71 | ||
| 6 | 76 | 63/37 | 60 | 109 | 26 | 77 | 72 | ||
| 7 | 58 | 67/33 | 56 | 40 | 50 | 101 | 19 | 79 | 83 |
| 8 | 73 | 71/29 | 57 | 42 | 61 | 136 | 44 | 86 | 71 |
| 9 | 79 | 67/33 | 55 | 45 | 61 | 100 | 30 | 70 | 40 |
| 10 | 75 | 71/29 | 53 | 41 | 60 | 193 | 58 | 68 | 29 |
| 11 | 44 | 59/41 | 63 | 43 | 60 | 160 | 38 | 75 | 36 |
| 12 | 57 | 93/7 | 56 | 40 | 60 | 60 | |||
| 13 | 55 | 65/35 | 58 | 60 | 110 | 9 | 74 | 26 | |
| 14 | 58 | 64/36 | 60 | 42 | 58 | 78 | 13 | 64 | 52 |
| 15 | 26 | 50/50 | 65 | 43 | 50 | 130 | 85 | 65 | |
| 16 | 29 | 76/24 | 62 | 46 | 55 | 142 | 35 | 75 | 75 |
| 17 | 11 | 100/0 | 59 | 37 | 61 | 154 | 44 | 100 | 73 |
| 18 | 30 | 33/67 | 55 | 29 | 57 | 125 | 13 | 83 | 83 |
| 19 | 25 | 56/44 | 61 | 35 | 60 | 150 | 35 | 85 | 70 |
| 20 | 4 | 100/0 | 65 | 70 | 80 | 27 | 100 | 100 | |
| Total | 2128 | Mean | 57 | 44 | 58 | 122 | 29 | Weighted 72% | 59 |
| SD | 4 | 8 | 3 | 31 | 15 | IQR 59–83% | 39–72% |
LAD, left atrial diameter; EF, ejection fraction; AAD, antiarrhythmic drugs classes 1c or 3, excluding beta-blockers, m, male; f, female.
Data represent raw data except for success data, which is weighted.
Table 3.
Persistent atrial fibrillation patients (n = 620)
| Number of patients | m/f (%) | Age (years) | LAD (mm) | EF (%) | prc duration (min) | X-ray (min) | Success % (first procedure) | Success off AAD | PVAC (%) | MASC (%) | MAAC (%) | TVAC (%) | CTI (%) | 3D (%) | Conventional RFA % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 146 | 83/7 | 62 | 47 | 54 | 154 | 41 | 58 | 52 | 100 | 39.7 | 21.9 | 18.5 | 38.3 | 20 | |
| 2 | 89 | 75/25 | 59 | 42 | 56 | 112 | 21 | 52 | 58 | 100 | 100 | 100 | 0 | 0 | 0 | 0 |
| 3 | 34 | 88/12 | 56 | 45 | 270 | 89 | 60 | 65 | 100 | 2.9 | 2.9 | 100 | 14.7 | 0 | 5.9 | |
| 4 | 0 | 0/0 | ||||||||||||||
| 5 | 58 | 57 | 57 | 117 | 25 | 50 | 60 | 100 | 37.9 | 25.9 | 1.7 | 0 | 1.7 | 0 | ||
| 6 | 108 | 75/25 | 64 | 118 | 35 | 61 | 100 | 100 | 0 | 25 | 21.3 | 0 | 0 | |||
| 7 | 45 | 84/16 | 64 | 45 | 43 | 154 | 42 | 46 | 57 | 100 | 95.5 | 88.9 | 8.9 | 0 | 0 | 0 |
| 8 | 25 | 80/20 | 58 | 45 | 60 | 173 | 67 | 84 | 60 | 100 | 44 | 56 | 0 | 12 | 0 | |
| 9 | 14 | 57/43 | 56 | 47 | 61 | 143 | 43 | 35 | 10 | 100 | 21.4 | 7.1 | 7.1 | 14.3 | 50 | 50 |
| 10 | 7 | 100/0 | 51 | 46 | 60 | 261 | 82 | 50 | 0 | 100 | 100 | 71.4 | 0 | 14.3 | 0 | 0 |
| 11 | 26 | 69/31 | 65 | 46 | 59 | 151 | 43 | 80 | 16 | 100 | 15.4 | 3.8 | 11.5 | 11.5 | 0 | 0 |
| 12 | 4 | 100/0 | 53 | 43 | 25 | 25 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 13 | 5 | 60/40 | 58 | 50 | 50 | 130 | 12 | 100 | 20 | 20 | 0 | 100 | 0 | 100 | ||
| 14 | 0 | 0/0 | ||||||||||||||
| 15 | 16 | 44/56 | 65 | 48 | 47 | 140 | 47 | 31 | 100 | 6.2 | 0 | 0 | 0 | 0 | 6.2 | |
| 16 | 4 | 50/50 | 61 | 54 | 45 | 142 | 29 | 100 | 100 | 50 | 0 | 0 | 0 | 0 | ||
| 17 | 19 | 84/26 | 64 | 44 | 57 | 172 | 17 | 74 | 53 | 100 | 100 | 100 | 100 | 0 | 0 | 5.3 |
| 18 | 0 | 0/0 | ||||||||||||||
| 19 | 3 | 82/18 | 58 | 40 | 50 | 180 | 40 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 0 | 0 |
| 20 | 17 | 71/29 | 51 | 50 | 108 | 34 | 100 | 100 | 100 | 70.6 | 5.9 | 5.9 | 0 | 0 | 5.9 | |
| Total | 620 | mean | 61 | 45 | 54 | 145 | 39 | 70 | 56 | 100 | 62 | 36 | 19 | 15 | 2 | 3 |
| SD | 4 | 2 | 5 | 41 | 18 | 14 | 19 | 0 | 37 | 40 | 28 | 27 | 9 | 12 |
LAD, left atrial diameter; EF, ejection fraction; AAD, antiarrhythmic drugs classes 1c or 3, excluding beta–blockers; PVAC, pulmonary vein ablation catheter; MASC, Multi array septal catheter; MAAC, multiarray atrial catheter; TVAC, tip versatile ablation catheter (linear multielectrode).
Prior to the procedure, anticoagulation was continued in 11 centres (55%) and bridged to low molecular heparin in 9 (45%). During the procedure, 18 centres gave intravenous heparin to achieve a target ACT of 300 s, one targeted 250 s, and one gave empirically 10 000 IU without measurement of ACT levels. In only 7 (35%) centres the heparin was administered via the long transseptal sheath, which was routinely flushed in 18 (90%) centres. A transoesophageal echocardiogram (TEE) was performed in every patient in 13 (65%) centres and selectively in the remaining 7 (35%) in the case of subtherapeutic international normalized ratio (INR) (n = 4), TIA (n = 4), or with persistent AF (n = 1). In two centres, no TEE was performed routinely and only patients with therapeutic anticoagulation treated.
Additional three-dimensional mapping with NavX (SJM, St Paul, MN, USA) was performed routinely in four centres, all others used fluoroscopy only. The mean procedure time was 122 min for paroxysmal and 145 min for persistent AF (P = 0.08). Fluoroscopy times (29.4 vs. 38.6 min, P = 0.13) and RF duration (28.3 vs. 42.6 min, P < 0.001) were shorter in paroxysmal AF.
In patients with paroxysmal AF, the pulmonary veins were isolated using the PVAC in all centres. Additional substrate modification was performed in nine centres using the septal multielectrode (MASC in 0.4–20% of cases) and left atrial multielectrode (MAAC 1.6–12% of cases). In patients with persistent AF, the PVAC was used in all cases, whereas the septal electrode (MASC) was used in 6/16 (38%) centres in all patients and left atrial electrode (MAAC) only in 3/16 (19%) centres for all patients. A linear multielectrode with duty-cycled RFA (TVAC) was used in nine centres in 1–20% of the patients in the place of the left atrial MAAC electrode.
Efficacy
Of the 2748 patients, 2225 (81%) had a structured follow-up defined as routine Holter-ECG or after a mean of 11.2 months. Among the 2128 patients with paroxysmal AF, 1669 (78%) had a structured follow-up reporting an overall success rate of 82% [median success rate 80% (IQR 74–90%)], a first procedure success rate of 72% [median 74% (IQR 59–83%)], and a success of antiarrhythmic medication of 59% [median 60% (IQR 39–72%)]; Table 2 and Figure 1).
Figure 1.
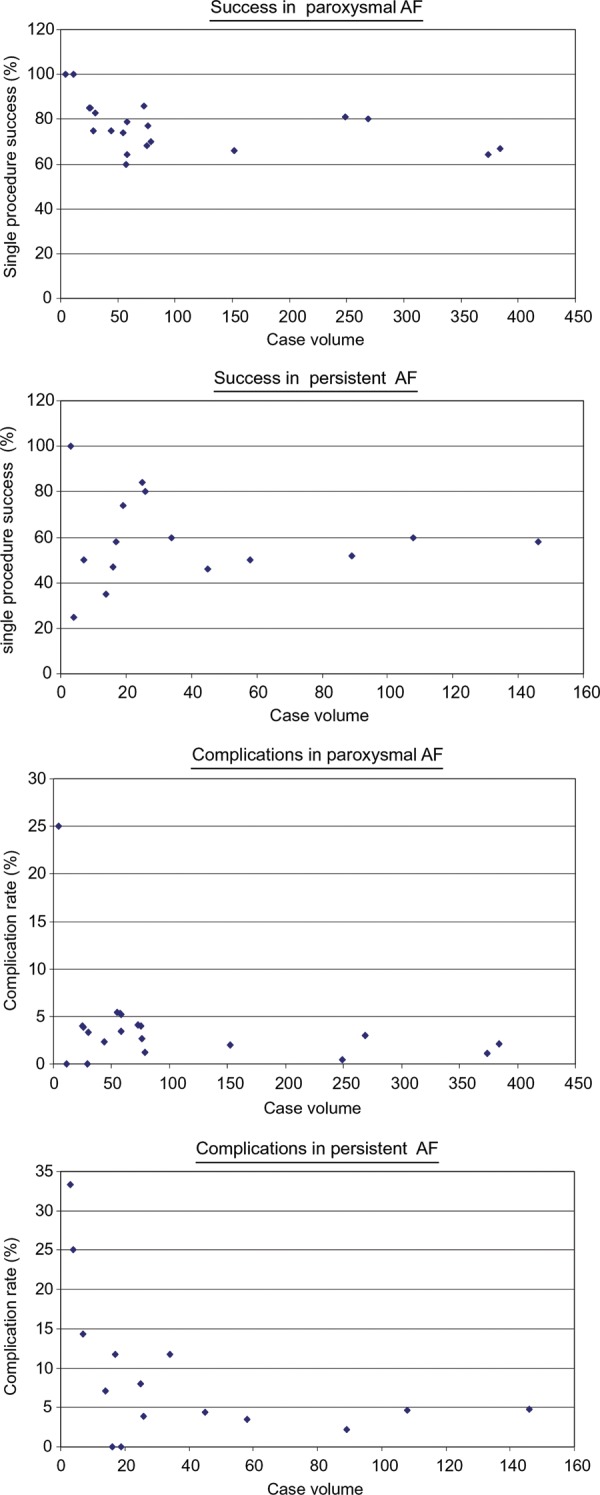
Overall success rates and total complications rates in relation to case volume.
In the 620 patients with persistent AF, 556 (90%) had a structured follow-up. Overall success was significantly lower with 70% (median 74% [IQR 60–92%]; P = 0.05) as well as the first procedure success rate of 58% (median 55% [IQR 47–81%]; P = 0.001, Table 3). Interestingly, the success rate of medication was similar with 56%, [median in persistent AF 58% (IQR 16–61%); P = 0.57]. The overall success rate was similar among higher and lower volume centres and also the two centres starting AF ablation with this technology had comparable outcome (Tables 2 and 3and Figure 1). Thus, weighed overall success rate was 79.4% (median 83% [IQR 74–94%]) in low volume and 79.1% [median 79% (IQR 68–86%)] in high volume centres (P = 0.48). First procedure success rate was 72.3% [median 75% (IQR 63–89%)] vs. 68.9% [median 67% (IQR 53–80%); P = 0.73)] but success rate without antiarrhythmic drugs was lower in low volume centres 49.7% [median 56% (IQR 26–77%)] vs. 60.8% [median 60% (IQR 58–70%), P = 0.02]. Success rates were neither dependent on the duration of experience with duty-cycled RFA (r = −0.08, P = 0.72 for final success), which ranged from 1 to 4.7 years, nor with the number of procedures with duty-cycled RFA (r = −0.17, P = 0.32). Individual predictors of success such as age and left atrial diameter could not be assessed, as not individual but average values were obtained from each centre. Still, there was a correlation between average left atrial diameter and success rate (final success r = −0.39, first procedure success r = −0.45, and success without antiarrhythmic drugs r = −0.57, all P < 0.05). The final success rate correlated highly significantly with the first procedure success rate in paroxysmal AF (r = 0.69, P = 0.001) and also with the success rate in persistent AF (r = 0.89, P < 0.0001), but was unrelated to procedure time (r = 0.08, P = 0.67), ablation time (r = −0.27, P = 0.16), and number of patients (r = −0.17, P = 0.32). Interestingly, the number of patients per centre was not significantly related to procedure time (r = −0.33, P = 0.06) and fluoroscopy time (r = −0.15, P = 0.40) in both, paroxysmal and persistent AF patients.
Safety
Out of 2748 patients, a total of 108 (3.9%) experienced complications, among them 31 (1.1%) neurologic events including symptomatic TIAs and stroke (Table 4). The incidence of neurologic events was similar in patients with paroxysmal and persistent AF (1.1 vs. 1.1%, P = ns) and was not related to the case load (r = 0.24, P = 0.15). The total complication rate as well as the embolus rate were unrelated to bridging anticoagulation to low molecular heparin, routine administration of heparin over the long sheath, whether a TEE was performed in every patient or not, and average procedure times (Table 5). In addition, the complication rate was not related to the number of patients treated with duty-cycled RFA as well as the duration of experience with the technology. These findings were the same when including only the 14 high-volume centres treating over 50 patients with the technology.
Table 4.
Complications
| Centre | PAF total | PAF NE | PAF phrenic palsy | PAF tamponade | PAF access vein complication | PAF PVstenosis | PAF other | CAF total | CAF NE | CAF phrenic palsy | CAF tamponade | CAF access vein complication | CAF PVstenosis | CAF other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 384 | 1 (0.3) | 1 (0.3) | 2 (0.6) | 1 (0.3) | 0 | 2 (0.6) | 146 | 2 (1.4) | 0 | 0 | 3 (2.0) | 1 (0.7) | 1 (0.7) |
| 2 | 374 | 3 (0.8) | 0 | 0 | 0 | 0 | 1 (0.3) | 89 | 0 | 0 | 1 (0.1) | 0 | 0 | 1 (0.1) |
| 3 | 269 | 4 (1.5) | 0 | 4 (1.5) | 2 (0.7) | 0 | 0 | 34 | 0 | 0 | 2 (5.9) | 2 (5.9) | 0 | 0 |
| 4 | 249 | 1 (0.4) | 0 | 0 | 6 (2.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 152 | 2 (1.3) | 1 (0.7) | 0 | 4 (2.6) | 0 | 0 | 58 | 0 | 0 | 0 | 2 (3.4) | 0 | 0 |
| 6 | 76 | 1 (1.3) | 0 | 1 (1.3) | 0 | 0 | 0 | 108 | 2 (1.8) | 0 | 2 (1.8) | 0 | 1 (0.9) | 0 |
| 7 | 58 | 1 (1.7) | 1 (1.7) | 1 (1.7) | 0 | 0 | 0 | 45 | 0 | 0 | 1 (2.2) | 1 (2.2) | 0 | 0 |
| 8 | 73 | 1 (1.4) | 0 | 2 (2.7) | 3 (4.1) | 0 | 0 | 25 | 0 | 0 | 0 | 2 (0.8) | 0 | 0 |
| 9 | 79 | 1 (1.3) | 0 | 0 | 1 (1.3) | 0 | 0 | 14 | 1 (1.3) | 0 | 0 | 1 (7.1) | 0 | 0 |
| 10 | 75 | 2 (2.7) | 0 | 1 (1.3) | 0 | 0 | 0 | 7 | 1 (1.4) | 0 | 0 | 0 | 0 | 0 |
| 11 | 44 | 0 | 0 | 1 (2.3) | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 57 | 1 (1.7) | 0 | 1 (1.7) | 1 (1.7) | 0 | 1 (1.7) | 4 | 0 | 0 | 0 | 0 | 0 | 1 (25.0) |
| 13 | 55 | 2 (3.6) | 1 (1.8) | 0 | 1 (1.8) | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 58 | 2 (0.34) | 0 | 0 | 7 (1.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 26 | 1 (3.85) | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 29 | 0 | 0 | 0 | 2 (6.9) | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 30 | 0 | 0 | 1 (3.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | 25 | 0 | 0 | 1 (4.0) | 1 (4.0) | 0 | 0 | 3 | 0 | 0 | 0 | 1 (3.3) | 0 | 0 |
| 20 | 4 | 1 (25) | 0 | 0 | 1 (25.) | 0 | 0 | 17 | 1 (5.9) | 0 | 0 | 0 | 0 | 1 (5.9) |
| Total | 2128 | 24 (1.1) | 4 (0.2) | 15 (0.7) | 30 (1.4) | 0 | 4 (2.0) | 620 | 7 (1.1) | 0 | 6 (0.2) | 12 (1.9) | 2 (0.3) | 4 (6.4) |
PAF, paroxysmal AF; CAF, chronic or persistent AF; FU, follow–up; NE, neurologic event such as transient ischaemic attack or stroke.
Table 5.
Complication rate depending on centre-specific circumstances
| Complication rate (%) | P value | Neurologic event (%) | P value | ||
|---|---|---|---|---|---|
| Bridging anticoagulation with heparin | Yes | 3.14 | 0.54 | 1.11 | 0.95 |
| No | 2.62 | 1.15 | |||
| Heparin over long sheath | Yes | 2.38 | 0.24 | 0.95 | 0.50 |
| No | 3.37 | 1.28 | |||
| TEE in every patient | Yes | 2.75 | 0.75 | 1.38 | 0.39 |
| No | 3.03 | 0.95 |
TEE, transesophageal echocardiogram.
Weighted complication rates.
Discussion
Efficacy
The present survey on duty-cycled RFA reports a success rate for both paroxysmal and persistent AF, which is similar to conventional RFA (75 and 63%, respectively9). Interestingly, the success rate seemed unrelated to the case volume and duration of experience with the technology, which makes it interesting for centres newly developing an AF ablation program. Especially in patients with persistent AF the majority of operators reached a single procedure success rate of over 50%, which is remarkable given the procedure times of >3 h in most centres and cases. Of note most of the centres did not use all three catheters mainly because of the additional cost per catheter. The fact that success rates were independent of the number of patients is surprising at first glance. One explanation is that the technology with duty-cycled RFA applied by multielectrodes leads to a relatively uniform lesion set and standardizes the AF ablation approach. This might be especially true for the ablation of persistent AF, where a relatively uniform lesion set is applied by anatomically designed multielectrodes.2 As such, this technique could enable more physicians to treat a larger number of AF patients.
On the other hand, a substantial portion of patients (11%) needed additional conventional irrigated RFA for the cavotricuspid isthmus or the left atrium. Also three-dimensional mapping had to be used for atypical flutters. Although a linear multielectrode with duty-cycled RFA (TVAC) can map and ablate typical10 or atypical flutters,11 it has not gained widespread application because of the potential for char formation10 and inadequate energy delivery.11 In as such the duty-cycled RFA system is of limited usefulness in patients with coexisting flutters and its cost effectiveness is low if additional irrigated RFA is to be used.
Safety
Complications were more frequently in patients with persistent AF in centres with low volume. This suggests that highly complex ablations for persistent AF are preferably done at higher-volume centres. The rate of neurologic events including symptomatic TIA/stroke was 1.1%, which is in the same range as with conventional RF in a previous survey (0.94%,9) or in single centre experience (1.1%12). First of all, the fact that symptomatic neurologic events are not reported more frequently supports the users of duty-cycled RFA in the current debate on cerebral microemboli. Microlesions of uncertain sequelae might be generally accepted, as in patients undergoing on pump coronary bypass surgery.13 It remains unclear, however, as to whether the microlesions have any clinical consequences in patients without an overt symptomatic neurological event. Therefore, every measure has to be taken to reduce their occurrence. Transseptal sheath management and multiple exchanges of relatively large electrodes are potential risk factors for microemboli. This survey found a similar rate of neurological events in patients treated with one multielectrode (paroxysmal AF) compared with multiple catheters (persistent AF, Table 4). In addition, heparinization through the sheath, sheath flushing, or bridging anticoagulation had not effect on the occurrence of neurological events. Therefore, clinically symptomatic neurological events may appear not to be related to the general practice of sheath management or anticoagulation. However, the number of these complications were too small to come to definitive conclusions despite the inclusion of almost 3000 patients in this survey. In addition, performing a TEE in every patient did not lower the complication rate. The practice to perform a TEE only in patients with subtherapeutic INR seems reasonable and is certainly saving costs.
Limitations
The major limitation of a self-reported survey is the lack of independent data acquisition and monitoring. However, despite the same limitations, the survey on conventional RFA of AF has been conducted twice because of the importance of the results.8,9 Selected study populations do not reflect overall outcome of a procedure and it is, therefore, important to collect results from real-world settings. Another limitation is that 13 invited centres did not respond the survey. It cannot be excluded that they could have worse results. Only a prospective monitored data registry for predefined patient set with a structured follow-up can give robust data on the safety of duty-cycled RFA.
Conclusion
Duty-cycled RFA has a self-reported success and complication rate similar to conventional RFA. A prospective registry with controlled data monitoring should be conducted to assess outcome.
Conflict of interest: C.S.:Obtained unrestricted research grants from Medtronic, Biotronik, St Jude Medical and Bard Electrophysiology. In addition he is Advisor to Biotronik, Founder and stockholder of Acutus Medical Inc.
Electrophysiology. Advisor to Biotronik. Founder and stockholder of Acutus Medical Inc.
A.S.: Investigator in Clinical Trials with St Jude Medical, Biotronik, Medtronic. Research grant from St Jude Medical.
M.W.: Consultant to Medtronic.
S.S.: Consultant to St Jude Medical.
S.B.: Consulting grants from Medtronic, Boston Scientific, Biosense Webster, Sorin Group, Co-founder and stockholder of B2M Inc.
Ng G.A.: Research grant from Medtronic, St Jude Medical and Sorin. Consultant: Medtronic and St Jude Medical.
S.S.F.: advisor to Medtronic
S.M.: advisor to Medtronic
L.V.A.B.: Consultant to Medtronic, Boston Scientific, Cameron Health
All other authors have no conflict of interest to declare.
Supplementary material
Funding
The conduction of this research was funded by a research grant from Medtronic Inc., Minneapolis, MN.
Supplementary Material
References
- 1.Boersma LV, Wijffels MC, Oral H, Wever EF, Morady F. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm. 2008;5:1635–42. doi: 10.1016/j.hrthm.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Scharf C, Boersma L, Davies W, Kanagaratnam P, Peters NS, Paul V, et al. Ablation of persistent atrial fibrillation using multielectrode catheters and duty-cycled radiofrequency energy. J Am Coll Cardiol. 2009;54:1450–6. doi: 10.1016/j.jacc.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Tivig C, Dang L, Brunner-La Rocca HP, Oezcan S, Duru F, Scharf C. Duty-cycled unipolar/bipolar versus conventional radiofrequency ablation in paroxysmal and persistent atrial fibrillation. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.12.010. epub ahead of press. [DOI] [PubMed] [Google Scholar]
- 4.Gaita F, Leclercq JF, Schumacher B, Scaglione M, Toso E, Halimi F, et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon. J Cardiovasc Electrophysiol. 2011;22:961–8. doi: 10.1111/j.1540-8167.2011.02050.x. [DOI] [PubMed] [Google Scholar]
- 5.Herrera Siklody C, Deneke T, Hocini M, Lehrmann H, Shin DI, Miyazaki S, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol. 2011;58:681–8. doi: 10.1016/j.jacc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Deneke T, Shin DI, Balta O, Bunz K, Fassbender F, Mugge A, et al. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm. 2011;8:1705–11. doi: 10.1016/j.hrthm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 8.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 9.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 10.Boll S, Dang L, Scharf C. Linear ablation with duty-cycled radiofrequency energy at the cavotricuspid isthmus. Pacing Clin Electrophysiol. 2010;33:444–50. doi: 10.1111/j.1540-8159.2009.02658.x. [DOI] [PubMed] [Google Scholar]
- 11.Naegeli B, Dang L, Boll S, Tivig C, Scharf C. Initial results of linear duty-cycled radiofrequency for atypical flutter and atrial tachycardia. Pacing Clin Electrophysiol. 2011;34:1128–37. doi: 10.1111/j.1540-8159.2011.03118.x. [DOI] [PubMed] [Google Scholar]
- 12.Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran S, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006;114:759–65. doi: 10.1161/CIRCULATIONAHA.106.641225. [DOI] [PubMed] [Google Scholar]
- 13.Kruis RW, Vlasveld FA, Van Dijk D. The (un)importance of cerebral microemboli. Semin Cardiothorac Vasc Anesth. 2010;14:111–8. doi: 10.1177/1089253210370903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


