Abstract
OBJECTIVES
Anaemia is a frequent complication after cardiopulmonary bypass surgery. Iron therapy has been variably employed by medical centres over the years. In our study we test o test the clinical effectiveness of intravenous and oral iron supplementation in correcting anaemia, and its impact on blood transfusion requirements, in patients undergoing cardiopulmonary bypass surgery.
METHODS
A double-blind, randomized, placebo-controlled clinical trial with three parallel groups of patients. Group I (n = 54): intravenous iron(III)-hydroxide sucrose complex, three doses of 100 mg/24 h during pre- and postoperative hospitalization and 1 pill/24 h of oral placebo in the same period and during 1 month after discharge. Group II (n = 53): oral ferrous fumarate iron 1 pill/24 h pre- and postoperatively and during 1 month after discharge, and intravenous placebo while hospitalized. Group III (n = 52): oral and intravenous placebo pre- and postoperatively, following the same protocol. Data were collected preoperatively, at theatre, at intensive care unit admission, before hospital discharge and 1 month later.
RESULTS
(1) Baseline clinical and demographic characteristics and surgical procedures were similar in the three groups; (2) no inter-group differences were found in haemoglobin and haematocrit during the postoperative period; (3) the intravenous iron group showed higher serum ferritin levels at hospital discharge (1321 ± 495 ng/ml; P < 0.001) and 1 month later (610 ± 387; P < 0.001) compared with the other groups and (4) we did not observe statistical differences in blood transfusion requirements between the three groups.
CONCLUSIONS
The use of intravenous or oral iron supplementation proved ineffective in correcting anaemia after cardiopulmonary bypass and did not reduce blood transfusion requirements. [Current Controlled Trials number: NCT01078818 (oral and intravenous iron in patients postoperative cardiovascular surgery under EC)].
Keywords: Cardiac surgery, Intravenous iron supplement, Anaemia, Transfusions, Ineffective treatments
INTRODUCTION
Anaemia is a common postoperative disorder in cardiac surgery patients after cardiopulmonary bypass (CPB) [1–3]. Blood transfusion with red blood cells, which should be avoided whenever possible, is associated with complications [4]. Many strategies have been developed to minimize the need for blood transfusion, but transfusion requirements remain high (15–85%) [4, 5].
Iron therapy has been variably employed by medical centres over the years, and this study redirects efforts to different pathways. Iron therapy is commonly used to reinforce the iron reserve to prevent anaemia after bleeding, but in cardiovascular surgery its use is controversial.
Iron supplementation is used to correct chronic anaemia in patients with renal failure and also in those with acute anaemia after surgical interventions [6, 7].
Iron plays an essential role in erythropoiesis and haemoglobin (Hb) synthesis [7]. In this regard, 150 mg of stored iron is required to increase circulating Hb levels by 1 g/dl [8].
The route of iron administration and also the type of iron preparation vary considerably. The effectiveness of oral iron supplementation is often limited by its gastrointestinal side effects (10–40%) and also by its low absorption rate (10–15%) [8–10].
Recently, some authors have suggested a role for intravenous iron in correcting anaemia after orthopaedic and oncological surgery, and in renal and obstetrics patients [10–12], but this remains controversial in cardiac surgery [13, 14].
Parenteral iron supplementation offers excellent bioavailability and is usually well tolerated [9]. Intravenous iron gluconate, iron dextrane and iron sucrose (saccharate) have been associated with infrequent side effects such as hypotension, epigastric pain, arthralgia, metallic taste, nausea, vomiting and pruritus [10, 15]. Anaphylactic reactions have been reported after the administration of intravenous iron dextrane [15]. Venofer® (iron(III)-hydroxide sucrose) is a new intravenous preparation with very few reported side effects [10, 11, 16].
The main objective of this study was to compare the safety and efficacy of two strategies of intravenous and oral iron supplementation vs placebo to correct postoperative anaemia in cardiac surgery patients undergoing CPB. A secondary objective was to compare differences in blood transfusion needs between patients receiving intravenous and oral iron vs placebo in the postoperative period.
MATERIALS AND METHODS
The study was approved by our University Hospital Ethics Committee and conducted according to the Helsinki Declaration. We report this trial in accordance with the CONSORT statement.
Patients and study design
This was a 24-month prospective, randomized, double-blind, placebo-controlled, observational clinical trial in cardiac surgery patients undergoing CPB. Written informed consent was obtained from all the patients preoperatively.
Intent-to-treat patients were randomly assigned to one of three groups: Group I patients were treated pre- and postoperatively with Intravenous iron(III)-hydroxide sucrose complex (Venofer®; Uriach Lab.). Group II patients were treated pre- and postoperatively with oral ferrous fumarate. Group III patients were treated pre- and postoperatively with oral and intravenous placebo. Randomization was performed by the Hospital Research Unit using a computer-generated random numbers list.
Dosage
Group I: patients were treated with intravenous iron (III)-hydroxide sucrose complex (Venofer®; Uriach Lab.) received three doses of 100 mg of intravenous iron/24 h during pre- and postoperative hospitalization, and 1 pill/24 h of oral placebo during the same period and during 1 month after discharge. Group II: patients were treated with ferrous fumarate iron (105 mg of iron) 1 pill/24 h orally pre- and postoperatively and during 1 month after discharge, and intravenous placebo while hospitalized, following the same protocol. Group III: patients were treated with oral and intravenous placebo pre- and postoperatively following the same protocol.
Intravenous iron was diluted in 200 ml of isotonic saline solution and injected slowly over 1 h using a syringe pump. The intravenous placebo consisted of 200 ml of isotonic saline solution and was injected in the same way.
All medication, including placebo, was prepared by the Department of Pharmacy and assigned to patients according to the random numbers list, so neither the medical staff nor the patients knew what treatment was being used. All intravenous solutions were presented in disguised form.
Inclusion criteria were: patients older than 18 years of age, elective cardiac surgery under extracorporeal circulation (EC), without previous anaemia, susceptible to treatment, without preoperative blood transfusion, able to complete all study visits per protocol and providing written informed consent. Exclusion criteria were: elective cardiac surgery patients without EC, treatment with fibrinolytic therapy 48 h before CPB surgery, history of impaired renal function (creatinine clearance <50 ml/min), previous surgery for active endocarditis, redo-surgery patients, pregnant or lactating, signs of active gastrointestinal bleeding, vitamin B12 deficit, ferropenic anaemia, clinical history of asthma or allergy, active infection, included in another clinical study, hepatic disease, history of allergy to iron, unlikely to adhere to protocol follow-up, unable to comply with the study protocol.
Criteria for red blood cell transfusion
Low output syndrome associated with Hb level <8 g/dl in coronary patients or <7 g/dl in valve surgery patients.
Data collection
Baseline analytical data, and information on demographic variables, comorbid conditions and operative risks were collected preoperatively. Hb (g/dl) and haematocrit levels (%) were collected: (1) preoperatively, (2) at operation room, (3) at ICU admission, (4) at ICU discharge, (5) at postoperative hospital discharge and (6) at 1 month after hospital discharge. Reticulocyte counts (RCs) expressed as a percentage of the total erythrocyte count and serum ferritin concentrations (ng/ml) were obtained at Day 1 of enrollment and postoperatively, approximately at Day 10 and day 30 after hospital discharge.
Statistical analysis
Sample size
The study design included an intermediate analysis with an exploratory study sample size of 20 patients per group that allowed us to determine the approximate magnitude of the effect of iron supplementation on Hb levels. Finally, we analysed 54 patients in Group I, 53 in Group II and 52 in Group III.
Data analysis
Quantitative variables are reported as means and standard deviations. Qualitative variables are expressed as frequencies and percentages.
Proportions were compared with the χ2 test or Fisher's exact test, as appropriate. The assumption of normality was tested with the Kolmogorov–Smirnov test.
The comparisons of Hb levels and RCs between groups were carried out with the ANOVA test. The comparisons of serum ferritin levels and transfusion requirements between groups were carried out with the Kruskal–Wallis test. We used median values with inter-quartile ranges (25th–5th percentiles) for red blood cell transfusion analysis.
All P-values <0.05 were considered statistically significant. Statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA) and LogXact v. 4.1 (Cytel Co., MA, USA).
RESULTS
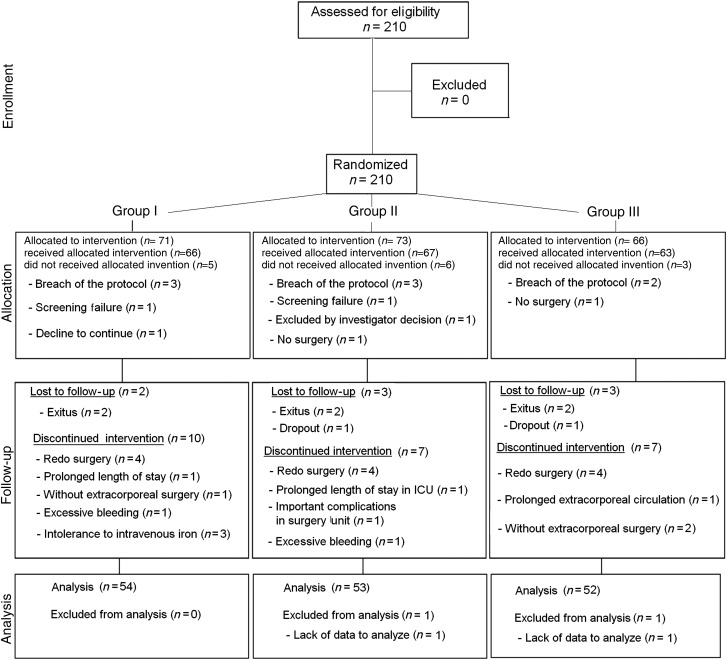
Between May 2007 and May 2009, 210 patients were assessed for eligibility. Finally, 159 consenting patients were included and completed the protocol. The final sample consisted of 116 (73%) men and 43 (27%) women, mean age 65 ± 11 years old. Figure 1 shows the consort flow chart.
Figure 1:
Consort flow chart of the study.
The three groups showed no significant differences in demographic or clinical characteristics, baseline laboratory data or operative risks (Table 1 for details).
Table 1:
Demographic and clinical characteristics of patients in each study group
| Group I (n = 54) | Group II (n = 53) | Group III (n = 52) | P-value | |
|---|---|---|---|---|
| Age (years) | 65 ± 11 | 65 ± 10 | 65 ± 12 | 0.87 |
| Sex (male/female) | 38/16 | 38/15 | 40/12 | 0.73 |
| EuroSCORE | 7.0 ± 4.8 | 6.6 ± 3.07 | 7.2 ± 4.83 | 0.99 |
| NYHA grades—no. (%) | ||||
| I | 2 (3.74) | 1(1.9) | 4 (7.7) | 0.28 |
| II | 22 (40.7) | 16 (30.8) | 26 (50) | |
| III | 29 (44.4) | 29 (55.8) | 18 (34.6) | |
| IV | 6 (11.1) | 6 (11.5) | 4 (7.7) | |
| Surgical procedure—no. (%) | 0.33 | |||
| Coronary | 19 (35.2) | 21 (39.6) | 28 (53.8) | |
| Valvular | 29 (53.7) | 25 (47.2) | 18 (34.6) | |
| Mixed | 6 (11.1) | 7 (13.2) | 6 (11.5) | |
| 24-h postoperative bleeding (ml) | 665 ± 534 | 684 ± 455 | 609 ± 310 | 0.54 |
NYHA: New York Heart Association.
No side effects were noted in the groups that received iron supplementation.
Table 2 shows repeated measures of Hb, immature reticulocyte fraction (IRF), RC and serum ferritin levels at different time intervals at 1, 10 and 30 days after discharge. Hb levels through the postoperative period were similar in the three groups. Within groups, serum ferritin levels increased at Day 10 compared with Day 1, but then decreased at discharge (Table 2).
Table 2:
Haematological parameters at different postoperative periods per study group
| Group I (n = 54) | Group II (n = 53) | Group III (n = 52) | P-value | |
|---|---|---|---|---|
| Haemoglobin (g/dl) | ||||
| Baseline | 14.0 ± 1.63 | 13.7 ± 1.46 | 14 ± 1.35 | 0.62 |
| Entry operating room | 12.7 ± 1.64 | 12.6 ± 1.70 | 12.8 ± 1.29 | 0.82 |
| Exit operating room | 10.5 ± 1.39 | 10.7 ± 1.41 | 10.5 ± 1.56 | 0.22 |
| ICU admission | 10.8 ± 1.53 | 10.6 ± 1.57 | 10.5 ± 1.50 | 0.74 |
| ICU discharge | 10.0 ± 1.23 | 10.0 ± 1.01 | 10.2 ± 1.34 | 0.59 |
| At hospital discharge | 11.1 ± 1.52 | 11.0 ± 1.28 | 11.0 ± 1.44 | 0.96 |
| One month after discharge | 12.7 ± 1.40 | 12.4 ± 1.27 | 12.3 ± 1.15 | 0.32 |
| Immature reticulocyte fraction | ||||
| Preoperative (Day 1) | 0.34 ± 0.68 | 0.35 ± 0.70 | 0.33 ± 0.07 | 0.63 |
| At discharge (around Day 10) | 0.48 ± 0.10 | 0.43 ± 0.08 | 0.44 ± 0.07 | 0.04 |
| One month after discharge | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.36 ± 0.06 | 0.81 |
| Reticulocyte count percentage | ||||
| Preoperative (Day 1) | 1.4 ± 0.60 | 1.5 ± 0.93 | 1.4 ± 0.59 | 0.87 |
| At discharge (around Day 10) | 1.8 ± 0.75 | 1.7 ± 0.71 | 1.5 ± 0.44 | 0.059 |
| One month after discharge | 1.4 ± 0.58 | 1.2 ± 0.45 | 1.2 ± 0.53 | 0.26 |
| Serum ferritin (ng/ml) | ||||
| Preoperative (Day 1) | 276 ± 186* | 229 ± 211* | 169 ± 127* | 0.02 |
| At discharge (around Day 10) | 1321 ± 495** | 541 ± 471** | 485 ± 331** | <0.001 |
| One month after discharge | 610 ± 387 | 212 ± 147 | 219 ± 222 | <0.001 |
*Comparisons (within groups) between Day 1 and Day 10: P < 0.110.
**Comparison (within groups) between Day 10 vs at discharge: P < 0.001.
A significant increase in IRF was observed in Group I compared with Groups II and III. In all groups, RC increased initially in the postoperative period and then decreased, returning to baseline values by Day 30 after discharge; there were no significant differences between the three groups at discharge (Table 2).
Regarding transfusion requirements, we found no differences between groups in the mean number of blood units transfused or the number of patients transfused (Table 3). Nevertheless, Group I patients under 75-year old needed fewer blood units per patient in the ICU (0.48 ± 1.07) compared with Group III patients of the same age group 1.15 ± 1.86 (P = 0.003).
Table 3:
Transfusion requirements (RBC units-percentage) per study group and number of patients transfused (percentage)
| Group I (n = 54) | Group II (n = 53) | Group III (n = 52) | P-value | |
|---|---|---|---|---|
| Transfusionsa (blood units per patient) | 0.84 | |||
| In the operating room | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.18 |
| In the ICU | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.56 |
| In the cardiac ward | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.38 |
| Global blood transfusions (units/patients)—no. (%) | ||||
| 0 | 34 (63) | 26 (49.1) | 26 (50.0) | 0.7 |
| 1 | 11 (20.4) | 18 (34.0) | 16 (35.6) | |
| 2 | 5 (9.3) | 4 (7.5) | 7 (13.5) | |
| 3 | 2 (3.7) | 3 (5.7) | 2 (3.8) | |
| 4 | 2 (3.7) | 1 (1.9) | 0 (0.0) | |
| 5 | 0 (0) | 1 (1.9) | 1 (1.9) | |
| Number of patients transfused—no. (%) | 20 (37) | 27 (51) | 26 (50) | 0.18 |
| In the operating room | 8 (14.8) | 6 (11.3) | 6 (11.5) | |
| In the ICU | 14 (26) | 23 (43.4) | 21 (40.4) | |
| In the cardiac ward | 3 (5.6) | 5 (9.4) | 6 (11.5) | |
RBC: red blood cells.
aBlood transfusions are expressed with the median (percentile 25th–75th).
No differences were found between the three groups in requirement for the transfusion (P = 0.70; Table 3).
Finally, no difference were found in Hb between groups at discharge analysing separately patients with (P = 0.96) and without blood transfusion (P = 0.71).
DISCUSSION
The main findings of the present study were that the administration of oral or intravenous iron did not significantly increase Hb levels, from the preoperative period to 1 month post-discharge. Nor did it reduce blood transfusion requirements.
This means that early postoperative treatment with intravenous iron does not appear to accelerate early recovery from postoperative anaemia after cardiac surgery. These findings are consistent with those of previously published studies [13, 16–18].
We found no important adverse effects or benefit in Group II (oral iron), in accordance with other studies [19]. However, Sowade et al. [20] showed that intravenous erythropoietin (EPO) in combination with oral FE2+ given 14 days before surgery increases Hb in patients undergoing cardiac surgery. This was probably due to the concomitant treatment regime administered long enough before surgery to exert a beneficial effect. However, the cost of EPO, and the difficulty of implementing this regime makes it complicated to justify in our routine practice. Intravenous iron has been successfully used to treat acute anaemia in postpartum and orthopaedic surgery [13], but not in cardiac surgery [13, 17, 21]. Intravenous iron sucrose has also been shown to safely increase Hb in patients with chronic heart failure [22].
We did not find adverse effects in the group receiving intravenous iron supplementation, or increased infection rate, indicating that it is a safe therapy for use in the postoperative setting. This finding confirms those of other studies [23].
Initially, intravenous iron increases ferritin, but this increase is transitory, as previously reported. The subsequent significant decrease is explained by the iron consumption necessary for erythropoiesis. In patients without iron supplementation, ferritin was lower than in those with supplementation. This is explained by the well-known state of functional iron deficiency induced by accelerated erythropoiesis associated with acute anaemia [7, 10].
The significant elevation in IRF observed in patients receiving intravenous iron compared with those who received placebo and oral supplementation reflects the erythropoiesis process [20].
As reported by other authors, we observed a rapid increase in the RCs as from the first day after the injection of intravenous iron, which peaked on Day 5; parenteral iron may act by treating functional iron deficiency and/or by increasing endogenous EPO synthesis [18, 24, 25].
We did not find differences between groups in the number of transfused patients or the number of blood units required per patient, as reported elsewhere [22]. As a result of these findings, iron supplementation no longer forms part of the postoperative treatment plan in our CPB patients.
Study limitations
As a limitation, the interval between iron administration and surgery was only 5–6 days, which may influence the magnitude of the effect of iron supplementation. But frequently, that waiting time is the bigger one to delay the main cardiosurgical procedures. In addition, assessment at 30 days may be premature from the point of view of long-term patient recovery, but our particular concern is early recovery from anaemia and reduced transfusion requirements. A 30-day period of iron (intravenous or specially oral treatment) supplementation could conceivably show more positive results. A new design may include a long preoperative period of receiving iron treatment.
There were more patients undergoing valve procedures than coronary procedures in the two treatment groups, where theoretically there could be more damage to red cells.
With respect to the power of this study, the sample size and the Hb levels at 1 month after discharge (showed in Table 2), to reach a power of 80% with a 95% confidence level (one-tailed), 247 patients per group would be needed to demonstrate a difference between Groups I and II; 128 patients per group to demonstrate a difference between Groups I and III and 2043 patients per group to demonstrate a difference between Groups II and III.
CONCLUSION
This study showed that postoperative intravenous iron administration offers excellent bioavailability as shown by significantly increased IRF and serum ferritin. However, it failed to show any beneficial effects on the correction of anaemia after cardiac surgery.
We found no reduction in blood transfusion requirements between the three groups.
The findings of the present study are consistent with those of other authors who found no evidence for the effectiveness of iron supplementation to correct anaemia after cardiac surgery. Although the findings of this study were negative, it presents important data that may help in the search for new and effective strategies to avoid anaemia and transfusions after open heart surgery.
Randomized studies with longer iron treatment time and/or larger doses before surgery, given by different pathways, are required to confirm the results of this study.
Conflict of interest: none declared.
REFERENCES
- 1.Butler J, Rocker G, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55:552–9. doi: 10.1016/0003-4975(93)91048-r. [DOI] [PubMed] [Google Scholar]
- 2.Huyzen RJ, van Oeveren W, Wei F, Stellingwerf P, Boonstra PW, Gu YJ. In vitro effect of hemodilution on activated clotting time and high-dose thrombin time during cardiopulmonary bypass. Ann Thorac Surg. 1996;62:533–7. [PubMed] [Google Scholar]
- 3.Boyle E, Verrier E, Spiess B. Endothelial cell injury in cardiovascular surgery: the procoagulant response. Ann Thorac Surg. 1996;62:549–1557. doi: 10.1016/0003-4975(96)00836-3. [DOI] [PubMed] [Google Scholar]
- 4.Leal Noval S, Rincon-Ferrari MD, Garcia Curiel A, Herruzo Avilés A, Camacho Laraña P, Garnacho Montero J, et al. Transfusión of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461–8. doi: 10.1378/chest.119.5.1461. [DOI] [PubMed] [Google Scholar]
- 5.Reddy SM, Talwar S, Velayoudam D, Parag Charde P, Mallick V, Jha RJ, et al. Multi-modality blood conservation strategy in open-heart surgery: an audit. Interact CardioVasc Thorac Surg. 2009;9:480–3. doi: 10.1510/icvts.2009.203034. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M. Erythropoieis, erythropoietin and iron metabolism in elective surgery: preoperative strategies for avoiding allogenic blood exposure. Am J Surg. 1995;170:S37–43. doi: 10.1016/s0002-9610(99)80057-9. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg DS, Laina A, Peer G, Kaplan E, Levi BA, Frank N, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients nor receiving dialysis. Am J Kidney Dis. 1996;27:234–8. doi: 10.1016/s0272-6386(96)90546-6. [DOI] [PubMed] [Google Scholar]
- 8.Rohling RG, Zimmerman AP, Breymann C. Intravenous versus oral iron supplementation for preoperative stimulation of haemoglobin synthesis using recombinant human erythropoietin. J Hematother Stem Cell Res. 2000;9:497–500. doi: 10.1089/152581600419161. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer RM, Schaefer L. Iron monitoring and supplementation: how do we achieve the best results? Nephrol Dial Transplant. 1998;13:9–12. doi: 10.1093/ndt/13.suppl_2.9. [DOI] [PubMed] [Google Scholar]
- 10.Macdougall IC. Strategies for iron supplementation: oral versus intravenous. Kidney Int Suppl. 1999;69:S61–6. doi: 10.1046/j.1523-1755.1999.055suppl.69061.x. [DOI] [PubMed] [Google Scholar]
- 11.Beris P. Perisurgical intravenous iron therapy. Transfusion alternatives. Transfus Med. 1999;4:35–8. [Google Scholar]
- 12.Berniere J, Dehullu JP. Le fer intraveineux dans le traitement des anèmies postoperatoires dans la chirurgie du rachis de l'enfant et de l'adolescent. Rev Chir Orthoped. 1998;84:319–22. [PubMed] [Google Scholar]
- 13.Kulier A, Levin J, Moser R, Rumpold-Seitlinger G, Tudor IC, Snyder Ramos SA, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–9. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 14.Beris P, Muñoz M, Garcia-Erce JA, Thomas D, Maniatis A, Van der Linden P. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A. Pathogenesis of anaphylactoid reactions to intravenous iron. Am J Kidney Dis. 2000;35:360–3. doi: 10.1016/s0272-6386(00)70352-0. [DOI] [PubMed] [Google Scholar]
- 16.Beris P, Muñoz M, García Erce JA, Thomas D, Maniatis A, Linden V. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 17.Madi-Jebara SN, Sleilaty GS, Achouh PE, Yazigi AG, Haddad FA, Hayek GM, et al. Postoperative intravenous iron used alone or in combination with low-dose erythropoietin is not effective for correction of anemia after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:59–63. doi: 10.1053/j.jvca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Karkouti K, McCluskey SA, Ghannam M, Salpeter MJ, Quirt I, Yau TM. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Can J Anaesth. 2006;53:11–9. doi: 10.1007/BF03021522. [DOI] [PubMed] [Google Scholar]
- 19.Crosby L, Palarski VA, Cotington E, Cmolik B. Iron supplementation for acute blood loss anemia after coronary artery bypass surgery: a randomized, placebo-controlled study. Heart Lung. 1994;23:493–9. [PubMed] [Google Scholar]
- 20.Sowade O, Messinger D, Franke W, Sowade B, Scigalla P, Warnke H. The estimation of efficacy of oral iron supplementation during treatment with epoetin beta (recombinant human erythropoietin) in patients undergoing cardiac surgery. Eur J Haematol. 1998;60:252–9. doi: 10.1111/j.1600-0609.1998.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 21.Muñoz M, Leal-Noval R. Intravenous iron in cardiac surgery. Semin Hematol. 2006;43(Suppl. 6):46–9. [Google Scholar]
- 22.Bolger AP, Bartlett FR, Penstons HS, O'Leary J, Pollock N, Kaprielian R, et al. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:225–7. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Torres S, Kuo YH, Morris K, Neibart R, Holtz JB, Davis JM. Intravenous iron following cardiac surgery does not increase the infection rate. Surg Infect. 2006;7:61–6. doi: 10.1089/sur.2006.7.361. [DOI] [PubMed] [Google Scholar]
- 24.Ahsan N. Intravenous infusion of total dose iron is superior to oral iron in treatment of anemia in peritoneal dialysis patients: a single center comparative study. J Am Soc Nephrol. 1998;9:664–8. doi: 10.1681/ASN.V94664. [DOI] [PubMed] [Google Scholar]
- 25.Huli S, Durandy Y. Post-haemodilution anaemia in paediatric cardiac surgery: benefit of intravenous iron therapy. Ann Fr Anesth Reanim. 2005;24:1262–5. doi: 10.1016/j.annfar.2005.05.023. [DOI] [PubMed] [Google Scholar]