Abstract
Maize (Zea mays L.) plants were grown to the nine-leaf stage. Despite a saturating N supply, the youngest mature leaves (seventh position on the stem) contained little NO3− reserve. Droughted plants (deprived of nutrient solution) showed changes in foliar enzyme activities, mRNA accumulation, photosynthesis, and carbohydrate and amino acid contents. Total leaf water potential and CO2 assimilation rates, measured 3 h into the photoperiod, decreased 3 d after the onset of drought. Starch, glucose, fructose, and amino acids, but not sucrose (Suc), accumulated in the leaves of droughted plants. Maximal extractable phosphoenolpyruvate carboxylase activities increased slightly during water deficit, whereas the sensitivity of this enzyme to the inhibitor malate decreased. Maximal extractable Suc phosphate synthase activities decreased as a result of water stress, and there was an increase in the sensitivity to the inhibitor orthophosphate. A correlation between maximal extractable foliar nitrate reductase (NR) activity and the rate of CO2 assimilation was observed. The NR activation state and maximal extractable NR activity declined rapidly in response to drought. Photosynthesis and NR activity recovered rapidly when nutrient solution was restored at this point. The decrease in maximal extractable NR activity was accompanied by a decrease in NR transcripts, whereas Suc phosphate synthase and phosphoenolpyruvate carboxylase mRNAs were much less affected. The coordination of N and C metabolism is retained during drought conditions via modulation of the activities of Suc phosphate synthase and NR commensurate with the prevailing rate of photosynthesis.
The assimilation of N in the leaves of higher plants requires both energy and C skeletons. Triose phosphate produced in the leaves as a result of photosynthetic C assimilation can be used for the synthesis of either carbohydrates or ketoacids (e.g. 2-oxoglutarate) via the anapleurotic pathway. 2-Oxoglutarate produced in the cytosol is imported into the chloroplasts, where it may serve as the acceptor for NH4+ during amino acid synthesis. To meet the needs of growth and development for both carbohydrates and amino acids, C partitioning is coordinated by a sophisticated regulatory system. Many points of reciprocal control exist between the pathways of C and N assimilation (Champigny and Foyer, 1992; Huber et al., 1992a, 1994a, 1994b, 1996b; Foyer et al., 1996). Effective regulation eliminates the competition observed in algae for available energy and C resources (Champigny and Foyer, 1992; Huppe and Turpin, 1994; Foyer et al., 1996).
During prolonged periods of drought, the decrease in water availability for transport-associated processes leads to changes in the concentrations of many metabolites, followed by disturbances in amino acid and carbohydrate metabolism. For example, there is an increase in the synthesis of compatible solutes such as special amino acids (e.g. Pro), sugars and sugar-alcohols, and Gly betaine (Yancey et al., 1982; Girousse et al., 1996). Acclimation to drought requires responses that allow essential reactions of primary metabolism to continue and enable the plant to tolerate water deficits. In the complex interplay of natural conditions, simple water deficits are unlikely to occur, since they intrinsically affect the acquisition of essential nutrients such as N and P (Talouizite and Champigny, 1988; Larsson et al., 1989, 1991; Beyrouty et al., 1994). Indeed, drought-induced N deficiency was found to limit recovery of photosynthesis in prairie grasses once water was restored (Heckathorn et al., 1997).
Studies of the effects of drought on N acquisition have frequently concerned phenomena associated with roots stressed either by the addition of PEG (Talouizite and Champigny, 1988; Larsson et al., 1989; Chazen and Neumann, 1994) or by the withdrawal of irrigation (Nonami and Boyer, 1990; Davies et al., 1994; Fambrini et al., 1994). Although there have been many studies of the physiological and molecular processes that enable the plant to tolerate drought stress and of nutrient acquisition during water deficit, relatively little information is available concerning the coordination of C and N assimilation under these circumstances (Brewitz et al., 1996).
The reduction of NO3− to NO2− catalyzed by NR is considered to be the rate-limiting step of N assimilation. NR activity is coordinated with the rate of photosynthesis and the availability of C skeletons by both transcriptional and posttranslational controls (Kaiser et al., 1993; Huber et al., 1996b). Transcription of the NR gene nia is induced by NO3− (Cheng et al., 1986) and repressed by Gln (Vincentz et al., 1993). It is also induced by sugars (Cheng et al., 1992; Vincentz et al., 1993; Krapp and Stitt, 1995). Moreover, a circadian rhythm in NR gene expression has been observed (Galangau et al., 1988; Becker et al., 1992; Scheible et al., 1997a).
In situations of water deprivation, maximal foliar extractable NR activity has been found to decrease in some cases (Plaut, 1974; Heuer et al., 1979; Talouizite and Champigny, 1988; Larsson et al., 1989) but not in others (Brewitz et al., 1996). Posttranslational regulation of NR activity is superimposed on the regulation of NR transcript accumulation (Huber et al., 1992b, 1996a). Reversible inactivation occurs when the phosphorylated NR protein interacts with an inhibitory protein in the presence of Mg2+ (Glaab and Kaiser, 1995; Mackintosh et al., 1995). Protein-phosphorylation-dependent changes in NR activity are regulated at least in part by sugars and related metabolites (Kaiser and Brendle-Brehnisch, 1991). Therefore, NR is inactivated in the light when C fixation is prevented (Kaiser and Forster, 1989). Such a situation occurs during water deprivation, which has been found to increase NR inactivation (Kaiser and Brendle-Behnisch 1991; Brewitz et al., 1996).
SPS plays a pivotal role in Suc synthesis (Kerr and Huber, 1987; Foyer and Galtier, 1996). Like NR, SPS activity is modulated by a complex series of regulatory controls that involve both allosteric regulation (Doehlert and Huber, 1983) and protein phosphorylation (Huber and Huber, 1990). Phosphorylation leads to a decrease in the affinity of the enzyme for its substrates and an increase in its susceptibility to the inhibitor Pi. Changes in the phosphorylation state of the SPS protein are implicated in the adjustment of the SPS activation state to the prevailing rates of photosynthesis and acclimation to water stress (Quick et al., 1989; Zrenner and Stitt, 1991). In contrast to metabolic regulation of SPS, little is known about the regulation of SPS gene transcription. An increase in SPS mRNA is correlated with the sink-to-source transition in leaves (Klein et al., 1993). SPS is induced when sugar levels are low (Klein et al., 1993; Hesse et al., 1995).
PEPCase catalyzes the carboxylation of PEP to oxaloacetate. This cytosolic enzyme fixes HCO3− during C4 photosynthesis and is the first enzyme of the anapleurotic pathway replenishing oxaloacetate in the tricarboxylic acid cycle (Melzer and O'Leary, 1987). Phosphorylation of PEPCase results in an increase in maximal enzyme activity and a decrease in the sensitivity of the enzyme to the inhibitor malate (Jiao and Chollet, 1991; Bakrim et al., 1993). Modulation of NR, SPS, and PEPCase activities is implicated in the coordinate adjustments of C and N assimilation to meet the needs of metabolism (Champigny and Foyer, 1992; Van Quy and Champigny, 1992). For the present study we followed the regulation of these enzymes through a period of water deficit and recovery in leaves of fully grown maize (Zea mays L.) plants to characterize molecular and metabolic mechanisms that permit coordinate control of primary C and N metabolism under these circumstances.
MATERIALS AND METHODS
Maize (Zea mays L.) plants were grown individually in 10-L pots in a growth chamber with a 16-h photoperiod at 23°C day/18°C night and 170 μmol photons m−2 s−1 irradiance. The plants were supplied daily with a complete nutrient solution containing 10 mm NO3− and 2 mm NH4+ (Coïc and Lesaint, 1975). When the plants had nine leaves with the seventh leaf mature, they were transferred to one of the following conditions of water availability: For 7 d, one-half of the plants received no irrigation (droughted plants), and the remaining plants received nutrient solution continuously (water-replete plants). In parallel experiments, one-half of the droughted plants was resupplied with nutrient solution after 3 d until the end of the experiment (rewatered plants). All measurements were made on the seventh (youngest mature) leaf only.
Photosynthesis Measurements and Sample Preparation
Photosynthetic CO2 assimilation and total leaf water potential were measured during the period of the experiments. The rate of photosynthetic CO2 assimilation was measured on attached leaves using an IR gas analyzer (model LCA4, Analytical Developmental Co., Hoddesdon, UK) under the conditions of irradiance prevailing in the controlled environment chambers, as described by Foyer et al. (1994). The total leaf water potential of the leaves was measured using a Scholander pressure chamber (PMS Instrument Co., Corvallis, OR). Each experiment was repeated twice, with a total of 20 plants in each case. Leaves were harvested 3 h after the beginning of the photoperiod on the days of the experiment indicated on the figures. They were weighed and metabolism was stopped by immersion in liquid N. The frozen leaf samples were ground in liquid N and divided into portions that were subsequently used for RNA extraction, enzyme assays, and determination of carbohydrate, amino acid, and NO3− contents.
Isolation of RNA and Northern-Blot Analysis
RNA was extracted from frozen leaf tissue as described by Verwoerd et al. (1989). The isolated RNA was precipitated twice, each time with 4 m LiCl for 60 min at 0°C, to remove traces of DNA and small RNA species. Electrophoretic separation of total denatured RNA samples, transfer onto blotting membranes (Zeta Probe, Bio-Rad), and hybridization with 32P-labeled cDNAs corresponding to NR (Gowrie and Campbell, 1989), PEPCase (Sheen and Bogorad, 1987), or SPS (Worrell et al., 1991) of maize were performed as previously described (Migge et al., 1996). The signals visible after autoradiography were quantified by phosphor imaging. In all cases, three replicates were performed.
Enzyme Assays
NR
NR was extracted from leaf tissue that had been reduced to a fine powder in a mortar with liquid N. The extraction buffer (50 mm Mops-KOH, pH 7.8, 5 mm NaF, 1 μm Na2MoO4, 10 μm FAD, 1 μm leupeptin, 1 μm microcystin, 0.2 g−1 fresh weight PVP, 2 mm β-mercaptoethanol, and 5 mm EDTA) was then added to the leaf tissue powder (1 mL 50 mg−1 fresh weight). The homogenate was centrifuged at 4°C for 5 min at 12,000g. NR activity was measured immediately in the supernatant. The reaction mixture consisted of 50 mm Mops-KOH buffer, pH 7.5, supplemented with 1 mm NaF, 10 mm KNO3, 0.17 mm NADH, and either 10 mm MgCl2 or 5 mm EDTA. The reaction was terminated after 8 or 16 min by the addition of an equal volume of sulfanilamide (1% [w/v] in 3 n HCl) and then naphthylethylene-diamine dihydrochloride (0.02% [w/v]) to the reaction mixture, and the A540 was measured. The activation state of NR is defined as the activity measured in the presence of 10 mm MgCl2 divided by the activity measured in the presence of 5 mm EDTA (expressed as a percentage).
SPS
Frozen leaf material was ground in a mortar with liquid N in a medium (250 mg fresh weight mL−1) containing 100 mm Tricine buffer, pH 7.5, and 200 mm KCl, 5 mm DTT, 4% (w/v) insoluble PVP, 0.33 mm PMSF, 6 μm leupeptin, 0.6 mm N-ethyl maleimide, and 1.3 mm EDTA. The homogenate was centrifuged at 12,000g for 5 min and the supernatant was desalted using a Sephadex G-25 column (PD10, Pharmacia). The proteins were eluted with 100 mm Tricine buffer, pH 7.5, containing 200 mm KCl and 5 mm DTT. SPS activity was determined under Vmax conditions or under conditions of limiting substrates in the presence of Pi (Vsel). The Vmax assay medium consisted of 50 mm Mops-NaOH buffer, pH 7.5, 15 mm MgCl2, 1 mm DTT, 10 mm Fru-6-P, 10 mm UDP-Glc, and 40 mm Glc-6-P. The Vsel assay was similar to this except that the concentrations of Fru-6-P, UDP-Glc, and Glc-6-P were 2, 6, and 6 mm, respectively, and 5 mm Pi was added to the assay medium. All reactions were incubated at 25°C for 15 min and then stopped by the addition of 7.5 m NaOH, 1/1, v/v). Unreacted Fru-6-P was destroyed by boiling for 10 min. After the assay mixture was cooled, anthrone (0.14% [w/v] in 13.8 n H2SO4) was added and the sample was incubated at 40°C for a further 20 min. The A620 was then measured.
PEPCase
Leaf tissue was ground in liquid N in a medium containing 100 mm Tris-HCl, pH 8.0, 10 mm MgCl2, 20% (v/v) glycerol, 5 mm DTT, 5 mm NaF, 1 mm PMSF, 1 μm microcystin, 20 μm leupeptin, 16 μm chymostatin, and 2% (w/v) PVP. Extracts were centrifuged at 4°C for 15 min at 15,000 rpm and desalted rapidly on Sephadex G-25. The reaction medium consisted of 50 mm Hepes-KOH, pH 7.3, 5 mm MgCl2, 1 mm NaHCO3, 5 mm NaF, 0.2 mm NADH, 10 units of malate dehydrogenase (Boehringer Mannheim), and 3 mm PEP in a final volume of 1 mL. Control cuvettes were without PEP. The reaction was followed at A340 in cuvettes maintained at 30°C. Malate sensitivity was determined by the addition of 0.8 mm malate to both the sample and control cuvettes.
Carbohydrate Analysis
Lyophilized leaf material was ground in a mortar with 1 m HClO4 (5–10 mg dry weight mL−1), and the extract was centrifuged at 12,000g for 5 min. The supernatant was used for the determination of soluble sugars (hexose and Suc), and the pellet was used for the estimation of the starch content. An aliquot of the supernatant fraction (500 μL) was neutralized by adding 200 μL of 0.5 m Tris-HCl, pH 7.5, and 60 μL of 5 m K2CO3. The precipitate from this reaction was removed by centrifugation at 12,000g for 5 min. Glc, Fru, and Suc in the soluble fraction were analyzed enzymatically as described by Galtier et al. (1995). The pellet reserved for starch determination was resuspended in water and incubated at 100°C for 2 h. Glc was then released by incubation at 50°C for 3 h in 20 mm sodium acetate buffer, pH 4.6, with amylase (0.66 units mL−1) and amyloglucosidase (15 units mL−1; both enzymes from Boehringer Mannheim) and assayed enzymatically as described above.
Amino Acid Analysis
Amino acids were extracted from lyophilized leaf material in 2% (w/v) 5-sulfosalicylic acid (10 mg dry weight mL−1). The extract was centrifuged at 12,000g for 5 min, and the supernatant was assayed for total amino acids by the Rosen colorimetric method with Leu as the reference. An aliquot of the supernatant was used to determine amino acid composition by ion-exchange chromatography (model LC5001 analyzer, Biotronics, Lowell, MA; Rochat and Boutin, 1989); physiological program run with lithium citrate buffers and detection at A570 and A440 after postcolumn derivatization with ninhydrin (Rochat and Boutin, 1989).
Determination of NO3−, Protein, and Chlorophyll
NO3−, protein, and chlorophyll were analyzed using the same leaf extracts as for NR activity. NO3− was determined by the method of Cataldo et al. (1975), soluble protein was determined by the method of Bradford (1976), and chlorophyll was determined by the method of Arnon (1949).
RESULTS
Total Leaf Water Potential and Photosynthesis
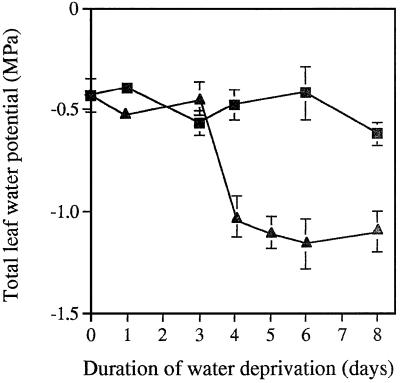
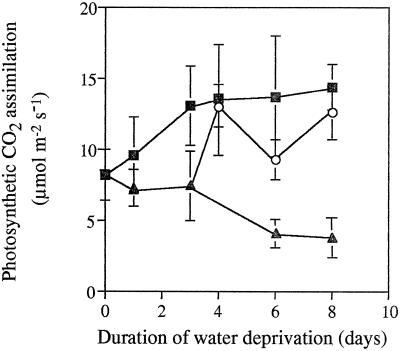
The total leaf water potential in well-watered control plants (−0.5 MPa) was constant throughout the period of the experiment (Fig. 1). In contrast, the total leaf water potential of the leaves of the droughted plants measured 3 h into the photoperiod decreased sharply after 3 d of water deprivation (Fig. 1). The rates of photosynthetic CO2 assimilation increased in well-watered plants over the first 3 d of the experiment, whereas photosynthesis was constant over this period in plants deprived of water (Fig. 2). At the point of measurement (3 h into the photoperiod) total leaf water potential was similar in leaves of all plants for the first 3 d of the experiment; therefore, it is not surprising that the measured rates of photosynthesis did not decline over this period. Nevertheless, photosynthesis in well-watered and droughted plants varied by a factor of 2 at d 3 of the experiment. Once the total leaf water potential decreased (from d 4 of water deprivation onward), there was a concomitant substantial (approximately 50%) loss of CO2 assimilation capacity in the droughted leaves (Fig. 2). Photosynthetic CO2 assimilation was comparable in all plants when water was resupplied to the droughted plants on d 3 of the experiment (Fig. 2).
Figure 1.
Total leaf water potential of maize plants continuously irrigated (▪) or subjected to 8 d of water deprivation (▴). The water potential of detached leaves was measured with a Scholander pressure chamber. Each given value represents the mean of three replicates. se is indicated for each value.
Figure 2.
The rate of net photosynthetic CO2 assimilation (μmol m−2 s−1) by leaves of maize plants continuously irrigated (▪), subjected to 8 d of water deprivation (▴), or deprived of water for 3 d and then rewatered to soil capacity (○). Each value represents the mean of three replicate experiments. se is indicated for each value.
NR Activity
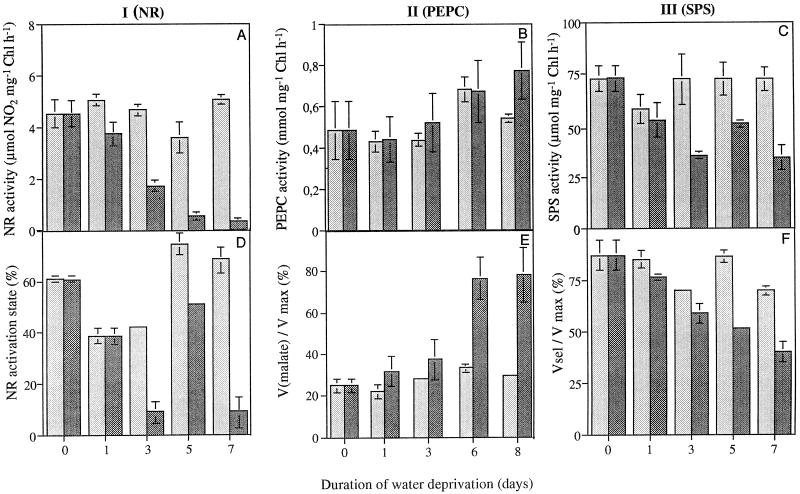
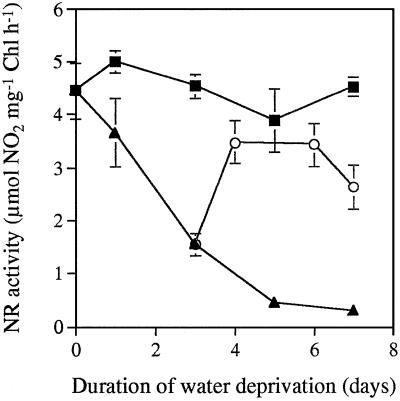
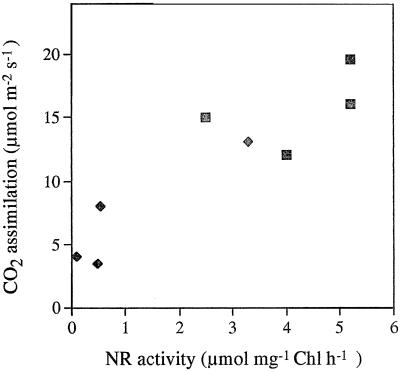
Total extractable foliar NR activity decreased as a result of water stress (Fig. 3A). Less than 10% of the original maximal NR activity remained after 7 d of water deprivation. NR activity extracted and assayed in the presence of Mg2+ (Fig. 3D) and compared with the total activity extracted and assayed in the presence of EDTA (Fig. 3A) gives an indication of the activation state of the enzyme (Kaiser et al., 1993; Huber et al., 1996b). NR was about 60% activated in water-replete leaves harvested 3 h into the photoperiod (Fig. 3D). The NR activation state decreased in droughted leaves compared with well-watered controls. The values of NR activity were very low in the presence of the inhibitor Mg2+, causing considerable variation in calculated values for NR activation state (Fig. 3D). Maximal extractable NR activity recovered rapidly when water was restored at d 3 of the experiment (Fig. 4). There was a clear relationship between total extractable NR activity and the rate of photosynthetic CO2 assimilation such that the inhibition of photosynthesis caused by water stress correlated with a marked decrease in total NR activity (r2 = 0.832; Fig. 5).
Figure 3.
The maximal extractable activities of NR (I), PEPCase (II), or SPS (III) of leaves of maize plants continuously irrigated (shaded bars) or exposed to 1 week of water deprivation (black bars). Total extractable NR activity (μmol mg−1 chlorophyll [Chl] h−1) was assayed in the presence of 5 mm EDTA (A) or in the presence of 10 mm Mg2+, allowing calculation of the activation state (D). Total extractable PEPCase activities (mmol mg−1 Chl h−1) were measured in the absence of malate (B). In E, each given value represents the ratio of total extractable PEPCase activity and the respective PEPCase activity assayed in the presence of 3 mm malate. Maximal extractable SPS activities (μmol mg−1 Chl h−1) are given in C, and F shows the ratio of maximal catalytic SPS activity to that measured under conditions of limiting substrates in the presence of Pi. Each value represents the mean of three replicate experiments. se is indicated for each value.
Figure 4.
Maximal extractable NR activities in leaves from droughted plants (▴), water-replete controls (▪), and plants rewatered 3 d after the onset of water deprivation (○). Chl, Chlorophyll. Results are means ± se.
Figure 5.
The relationship between the CO2 assimilation rate in air in the growth conditions and maximal extractable NR activity in the same leaves. Samples were taken from individual water-replete plants (▪) or plants deprived of water for 7 d (♦). Each value represents the mean of three replicate experiments. Chl, Chlorophyll.
PEPCase and SPS Activities
The maximal catalytic activity of PEPCase significantly increased in water-stressed maize leaves compared with well-watered controls (Fig. 3B). At the same time, the sensitivity to the inhibitor malate was decreased in droughted plants compared with the water-replete controls (Fig. 3E). Total extractable SPS activity decreased in droughted leaves compared with the well-watered controls (Fig. 3C), and there was a concomitant increase in the sensitivity of the enzyme to the inhibitor Pi (Fig. 3F).
Transcript Levels
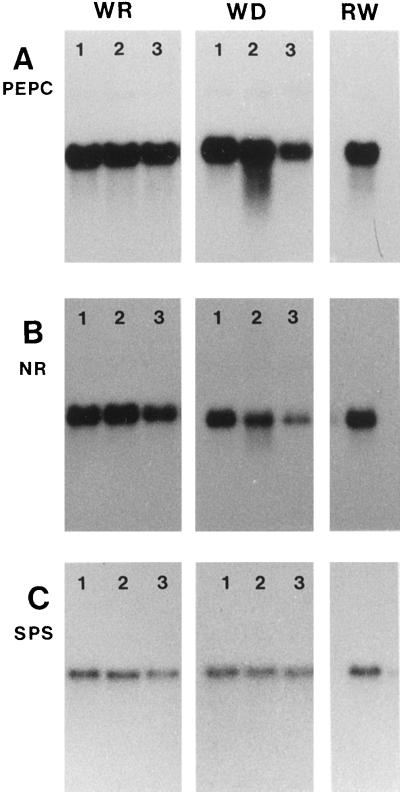
NR transcript abundance rapidly decreased following the imposition of water stress (Fig. 6). After 7 d of water deprivation, NR mRNA was about 80% lower than in water-replete plants (Fig. 6B); however, the NR mRNA pool was restored within 24 h after droughted plants were rehydrated after 3 d (Fig. 6B). In contrast to the severe drought-induced decrease in NR mRNA, SPS and PEPCase transcripts were much less affected (Fig. 6, A and C). PEPCase transcripts decreased by about 30% after 7 d of water stress (Fig. 6A), whereas the effect on the SPS mRNA pool was even less severe (10–20%; Fig. 6C). In both cases the levels of transcript were rapidly restored to control values following rehydration at d 3 of the experiment.
Figure 6.
The NR (A), PEPCase (B), or SPS (C) transcript levels in leaves of water-replete controls (WR) or water-deprived plants (WD) at 3 d (1), 5 d (2), and 7 d (3) after withholding irrigation or 24 h after irrigation to soil capacity of maize plants that had been deprived of water for 7 d (RW). Denatured total RNA (10 μg) samples were fractionated on a formaldehyde-agarose gel (1.5% agarose) and transferred onto a membrane. The RNA blots were probed with the partial 2.2-kb cDNA clone pZmnrl encoding NR of maize (Gowrie and Campbell, 1989), a partial 1.0-kb cDNA clone corresponding to PEPCase of maize (Sheen and Bogorad, 1987), or a full-length 3.4-kb cDNA clone encoding SPS of maize (Worrell et al., 1991).
Foliar Carbohydrate Contents
The Suc contents of leaves from water-stressed and well-watered maize plants were similar throughout the 7 d of the experiment (Table I). In contrast, foliar Fru and Glc contents increased by 3.7- and 6-fold, respectively, in the leaves of plants deprived of water for 7 d (Table I) compared with the water-replete plants (Table I). There was about twice the amount of starch in leaves of maize plants deprived of water for 7 d than in well-watered controls (Table I). When water was restored to droughted plants at d 3 of the experiments, the foliar carbohydrate contents rapidly returned to values comparable to those measured in water-replete controls (data not shown).
Table I.
The accumulation of soluble starch, sugars, and NO3− in maize leaves
| Time of Sampling | Starch | Suc | Glc | Fru | NO3− |
|---|---|---|---|---|---|
| mmol GEamg−1 Chl | μmol mg−1 Chl | μg mg−1 Chl | |||
| d 0 | 0.11 ± 0.03 | 0.344 ± 0.033 | 0.269 ± 0.15 | 0.182 ± 0.037 | <30 |
| Well-watered plants on d 7 of the experiment | 0.08 ± 0.02 | 0.285 ± 0.066 | 0.134 ± 0.03 | 0.076 ± 0.013 | <30 |
| Plants deprived of water for 7 d | 0.15 ± 0.02 | 0.294 ± 0.039 | 0.527 ± 0.023 | 0.275 ± 0.006 | Not detectable |
GE, Glc equivalents; Chl, chlorophyll.
Foliar Amino Acid and NO3− Contents
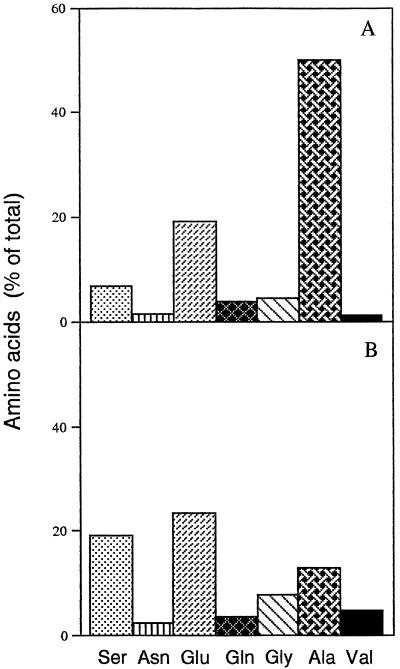
The amino acid contents of maize leaves increased about 2-fold, from approximately 4 μmol mg−1 chlorophyll at the beginning of the experiment to about 9 μmol mg−1 chlorophyll after 7 d of water stress. Gln, Glu, Asn, Gly, and Ser accounted for a large part of the total amino acid pool in water-replete maize plants (Fig. 7). The foliar contents of Gln, Glu, and Asn were not greatly changed in leaves of plants deprived of water, but there was a marked accumulation of Ala (Fig. 7). Small increases in other amino acids were also observed (data not shown). The increase in the total amino acid pool of leaves of water-stressed plants was therefore due to the accumulation of Ala. The maize plants studied in these experiments were large but contained little stored NO3− in their leaves despite being supplied with saturating N throughout the period of growth and development (Khamis and Lamaze, 1990; Khamis et al., 1990). In water-stressed maize leaves, foliar NO3− levels decreased below the level of detection (Table I).
Figure 7.
The contribution of major amino acids to the total amino acid pool in droughted maize leaves (A) and in water-replete controls (B). Measurements were made 7 d after the onset of water deprivation.
DISCUSSION
Drought induces complex changes in C and N metabolism resulting from water deficits and from modifications in the availability of nutrients (Talouizite and Champigny, 1988; Larsson et al., 1991; Beyrouty et al., 1994). The photosynthetic apparatus is known to be relatively resistant to water stress (Cornic et al., 1989; Quick et al., 1989; Chaves and Pereira, 1992). This appears to be true in maize leaves, in which photosynthesis quickly recovered when plants were rehydrated (Fig. 2). Carbohydrate-mediated repression of transcription of photosynthetic genes, such as those coding for the Rubisco large and small subunits, is a possible mechanism of control when carbohydrate accumulates in leaves (Krapp et al., 1993; Sheen, 1994; Graham, 1996; Koch, 1996). This may occur in water-stressed maize leaves following several days of water stress and provides at least a partial explanation for the reduction in photosynthetic capacity. Depletion of essential nutrients, particularly NO3−, can also cause changes in gene expression and enzyme activity in stress situations (Scheible et al., 1997a, 1997b). Although measurements at single time points such as those presented here provide only a limited snapshot of metabolism, they are nevertheless indicative of the overall shift in C and N metabolism that occurs as a result of drought.
Carbohydrate-mediated repression of PEPCase gene transcription has been observed in isolated mesophyll protoplasts of maize (Sheen, 1989). The decrease in the PEPCase transcript pool that occurred with carbohydrate accumulation in water-stressed maize leaves (Fig. 5) in the current study is consistent with this observation. In contrast, the maximal extractable foliar PEPCase activities were increased in plants subjected to water deficit compared with water-replete controls (Fig. 3). In marked contrast to the increased turnover of the PEPCase transcript pool, the PEPCase protein and PEPCase activity were stabilized during drought, as has been observed previously (Saccardy et al., 1996). The measured drought-induced decrease in the sensitivity of PEPCase activity to the inhibitor malate (which is a measure of the phosphorylation state of the enzyme; Jiao and Chollet, 1991; MacKintosh et al., 1995) indicates an additional shift in metabolism to increased PEPCase activity (Fig. 3).
Although photosynthetic rates were decreased during drought (Fig. 2), the residual photosynthetic capacity of the maize leaves still accounted for the observed increases of carbohydrate accumulation during water stress when export of Suc from the leaf was restricted (Table I). The loss of photosynthetic CO2 assimilation caused by decreased leaf water potentials was accompanied by a decrease in maximal extractable SPS activity and an increase in the phosphorylation state of the enzyme. To study changes in total extractable SPS activity, we used an assay that contained saturating concentrations of substrates (the Vmax assay) and found that SPS activity was decreased following water stress (Fig. 3). We explored the activation state of SPS by exploiting the changes in the kinetic properties of the enzyme, such as the sensitivity to the inhibitor Pi, caused by protein phosphorylation (Siegl et al., 1990).
SPS activities were measured in the presence of limiting substrate levels and with Pi (Vsel). Water stress led to a decrease in Vmax (Fig. 3E) and Vsel (Fig. 3F) compared with water-replete controls. Thus, in contrast to SPS in the leaves of C3 plants (Quick et al., 1989), maize leaf SPS showed no metabolic compensation for decreased maximal extractable SPS activities by changes in the kinetic properties that would favor an increase in activity in substrate-limited conditions. In maize leaves drought-induced modulation of SPS activity regulates the carbohydrate pool in relation to the decreased rates of net photosynthesis and N assimilation. The decrease in total extractable SPS activity and the increase in SPS phosphorylation state would serve to decrease the flux of C to Suc in a situation of declining photosynthetic capacity and export.
Observations on the compartmentation of Suc synthesis, seen exclusively in the mesophyll cells of maize leaves (Furbank et al., 1985; Lunn et al., 1997) and the ATP-mediated SPS activation in other C4 plants (Lunn et al., 1997) suggest that Suc biosynthesis is subject to different mechanisms of regulation in C3 and C4 plants. This may explain the observed inactivation of maize SPS observed here during water deficit, which is in marked contrast to the activation of SPS observed in spinach under similar circumstances (Quick et al., 1989).
In maize there was a clear water-deficit-induced decrease in both maximal extractable SPS activity and SPS activation state (as indicated by the Vsel activity; Fig. 3). This would favor decreased Suc biosynthesis in water-stressed maize leaves, which showed significant hexose accumulation (Table I). Water deficit favored starch breakdown in C3 species (Fox and Geiger, 1985) but caused accumulation of starch in maize (Table I). There was also a substantial increase in free Glc in droughted maize leaves, which may indicate that considerable starch turnover is favored under conditions of water deficit.
A consistent relationship between photosynthesis and maximal catalytic NR activity was observed in maize leaves. The maximum catalytic NR activity was strongly decreased in water-stressed maize leaves (Fig. 3). This is consistent with previous observations of water-stress-induced losses in maximal extractable foliar NR activity in other species (Plaut, 1974; Heuer et al., 1979; Talouizite and Champigny, 1988; Larsson et al., 1989; Wellburn et al., 1996). NR is known to be posttranslationally regulated by reversible phosphorylation/dephosphorylation changes in maize leaves (Huber et al., 1994a). Since deprivation of CO2 causes inactivation of NR via this mechanism (Kaiser and Brendle-Behnisch, 1991), it is not surprising that drought has also been found to decrease the NR activation state in tomato (Brewitz et al., 1996), as it did in the present study with maize (Fig. 3D).
During water stress the decrease in maximal NR activity was accompanied by a sharp decline in NR transcript levels (Fig. 6). This appears to be relatively specific for NR transcripts, because other mRNA pools (e.g. those corresponding to SPS or PEPCase) were much less affected by drought (Fig. 6). NR gene transcription in leaves is induced by NO3− and carbohydrates (Cheng et al., 1992; Vincentz et al., 1993) and inhibited by Gln (Vincentz et al., 1993). In the current study, Suc and Gln did not significantly increase in water-stressed maize leaves (Table I and Fig. 7, respectively). The decrease in the quantity of NR message observed following the onset of water deprivation (Fig. 6) may have been caused by the decrease in foliar NO3−. Even when the maize plants were watered daily with nutrient solution, leaves contained little stored NO3−. In droughted maize leaves foliar NO3− was below the level of detection (Table I).
NO3− not only modulates NR transcription but is also an important determinant of the stability of NR transcripts (Galangau et al., 1988). A severe or prolonged NO3− deficit may not only result in a reduction of NR gene transcription but also in reduced stability of both the NR transcripts and NR protein (Galangau et al., 1988; Ferrario et al., 1995). The rapid recovery of NR transcripts (Fig. 6) and NR activity (Fig. 4) following the onset of rewatering would tend to support this view. NR protein degradation appears to be regulated, since NR activity and NR protein decrease toward the end of the photoperiod (Galangau et al., 1988). A modified form of NR with a truncated N terminus did not show dark inactivation, and the corresponding decline of NR protein levels was abolished (Nussaume et al., 1995). Regardless of the mechanisms associated with NR protein turnover, the drought-induced decrease in maximal catalytic NR activity together with decreases in the NR activation state would be sufficient to prevent primary N assimilation in water-stressed maize leaves.
Plants have developed physiological and biochemical strategies to tolerate water deficits. In optimal and stress conditions, the appropriate provision of carbohydrates and amino acids in the required amounts and stoichiometries must be achieved by efficient communication and regulation. Our data show that in maize leaves subjected to water deficit there is clearly a concerted down-regulation of NR activity and photosynthesis (Fig. 5). A correlation between maximal extractable NR activity and ambient photosynthesis was observed (Fig. 5). During drought, Suc production in maize leaves is limited by a down-regulation of the activity of SPS. The decreases in the capacities of NO3− assimilation and Suc synthesis can be considered to be coordinated to adjust the production of amino acids and sugars to reduced demand under these conditions.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Wilbur Campbell (Michigan Technological University, Houghton) for providing the cDNA clone encoding maize NR and to Dr. Lawrence Bogorad (Harvard University, Cambridge, MA) for sending us a cDNA clone corresponding to maize PEPCase. T.W.B. wishes to thank Professor Alfred Pühler (Universtität Bielefeld, Germany) for the opportunity to work at the Lehrstuhl für Genetik.
Abbreviations:
- NR
nitrate reductase
- PEPCase
phosphoenolpyruvate carboxylase
- SPS
Suc phosphate synthase
Footnotes
This work was supported by European Economic Community Biotechnology (contract no. BIO2 CT93 0400), by the Project of Technical Priority, Network D–Nitrogen Utilization and Efficiency, and by a research grant from the Deutsche Forschungsgemeinschaft (Be 1108/5-1 Be 1108/5-33).
LITERATURE CITED
- Arnon DI. Plant Physiol. 1949;34:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakrim N, Peril J-L, Helen E, Rocker J-PK, Arrio-Dupont M, Vidal J, Gadal P, Chollet R. Regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase: a cardinal event influencing the photosynthesis rate in Sorghum and maize. Plant Physiol. 1993;101:891–897. doi: 10.1104/pp.101.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TW, Foyer CH, Caboche M. Light regulated expression of the nitrate-reductase and nitrite-reductase genes in tomato and in the phytochrome-deficient aurea mutant of tomato. Planta. 1992;188:39–47. doi: 10.1007/BF00198937. [DOI] [PubMed] [Google Scholar]
- Beyrouty CA, Grigg BC, Norman RJ, Wells BR. Nutrient uptake by rice in response to water management. J Plant Nutr. 1994;17:39–55. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewitz E, Larsson C-M, Larsson M. Responses of nitrate assimilation and N translocation in tomato (Lycopersicon esculentum Mill) to reduced ambient air humidity. J Exp Bot. 1996;47:855–861. [Google Scholar]
- Cataldo DA, Haroon M, Schrader LE, Yougs VL. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commum Soil Sci and Plant Analysis. 1975;6:71–80. [Google Scholar]
- Champigny M-L, Foyer CH. Nitrate activation of cytosolic protein kinases diverts photosynthetic carbon from sucrose to amino acid biosynthesis. Plant Physiol. 1992;100:7–12. doi: 10.1104/pp.100.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves M, Pereira J. Water stress, CO2 and climate change. J Exp Bot. 1992;43:1131–1139. [Google Scholar]
- Chazen O, Neumann PM. Hydraulic signals from the roots and rapid cell-wall hardening in growing maize (Zea maize L.) leaves are primary responses to polyethylene glycol-induced water deficits. Plant Physiol. 1994;104:1385–1392. doi: 10.1104/pp.104.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Acedo GN, Christinsin M, Conkling MA. Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA. 1992;89:1861–1864. doi: 10.1073/pnas.89.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Dewdney J, Kleinhofs A, Goodman HM. Cloning and nitrate induction of nitrate reductase mRNA. Proc Natl Acad Sci USA. 1986;83:6825–6826. doi: 10.1073/pnas.83.18.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coïc Y, Lesaint C. Alsace. 1975;23:1–22. [Google Scholar]
- Cornic G, Le Gouallec JL, Briantais JM, Hodges M. Effect of dehydration and high light on photosynthesis of two C3 plants (Phaseolus vulgaris L.) and (Elatostema repens [Lour], Hall f) Planta. 1989;177:84–90. doi: 10.1007/BF00392157. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Tardieu F, Trejo CL. How do chemical signals work in plants that grow in drying soil? Plant Physiol. 1994;104:309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert DC, Huber SC. Regulation of spinach leaf sucrose-phosphate synthase by glucose 6-phosphate, inorganic phosphate and pH. Plant Physiol. 1983;73:989–994. doi: 10.1104/pp.73.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrini M, Pugliesi C, Vernieri P, Pardossi A, Baroncelli S. Characterization of a wilty sunflower (Helianthus annuus L.) mutant. II. Water relations, stomatal conductance, abscisic acid content in leaves and xylem sap of plants subjected to water deficiency. J Exp Bot. 1994;45:1809–1815. [Google Scholar]
- Ferrario-Méry S, Valadier M-H, Foyer CH. Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol. 1998;117:293–302. doi: 10.1104/pp.117.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario S, Valadier M-H, Morot-Gaudry J-F, Foyer CH. Effects of constitutive expression of nitrate reductase in transgenic Nicotiana plumbaginifolia L. in response to varying nitrogen supply. Planta. 1995;196:288–294. [Google Scholar]
- Fox TC, Geiger DR. Osmotic response of sugar beet leaves at CO2 compensation point. Plant Physiol. 1985;80:239–241. doi: 10.1104/pp.80.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Champigny ML, Valadier MH, Ferrario S. Partitioning of photosynthetic carbon: the role of nitrate activation of protein kinases. In: Shewry P, Halford N, Hooley R, editors. Protein Phosphorylation in Plants. Proceedings of the Phytochemical Society of Europe. Oxford, UK: Clarendon Press; 1996. pp. 35–51. [Google Scholar]
- Foyer CH, Galtier N (1996) Source-sink interaction and communication in leaves. In E Zamski, AA Schafer, eds, Photoassimilate Distribution in Plants and Crops: Source Sink Relationships. Marcel Dekker, Inc., pp 311–340
- Foyer CH, Valadier MH, Ferrario S. Co-regulation of nitrogen and carbon assimilation in leaves. In: Smirnoff N, editor. Environment and Plant Metabolism, Flexibility and Acclimation. Guildford, UK: Bios Scientific Publishers; 1994. pp. 17–33. [Google Scholar]
- Furbank RT, Stitt M, Foyer CH. Intercellular compartmentation of sucrose synthesis in leaves of Zea mays. Planta. 1985;163:172–178. doi: 10.1007/BF00396079. [DOI] [PubMed] [Google Scholar]
- Galangau F, Daniel-Vedel F, Moureaux T, Dorbe MF, Leydecker MT, Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988;88:383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N, Foyer CH, Murchie E, Alred R, Quick P, Voelker TA, Thepenier C, Lasceve G, Betsche T. Effects of light and atmosphere CO2 enrichment on photosynthetic carbon partitioning and carbon/nitrogen ratios in tomato (Lycopersicon esculentum L.) plants over-expressing sucrose phosphate synthase. J Exp Bot. 1995;46:1335–1344. [Google Scholar]
- Girousse C, Bournitrateville R, Bonnemain J-L. Water deficit-induced changes in concentration in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol. 1996;111:109–113. doi: 10.1104/pp.111.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaab J, Kaiser WM. Inactivation of nitrate reductase is a two-step mechanism involving NR-protein phosphorylation and subsequent ‘binding’ of an inhibitor protein. Planta. 1995;195:514–518. [Google Scholar]
- Gowrie G, Campbell WH. cDNA clones for corn leaf NADH: nitrate reductase and chloroplast NAD(P)+ glyceraldehyde-3-phosphate dehydrogenase. Plant Physiol. 1989;90:792–798. doi: 10.1104/pp.90.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham IA. Carbohydrate control of gene expression in higher plants. Res Microbiol. 1996;147:435–594. doi: 10.1016/0923-2508(96)84014-9. [DOI] [PubMed] [Google Scholar]
- Heckathorn SA, De Lucia EH, Zielinski RE. The contribution of drought-related decreases in foliar nitrogen concentration to decreases in photosynthetic capacity during and after drought in prairie grasses. Physiol Plant. 1997;101:173–182. [Google Scholar]
- Hesse H, Sonnewald U, Willmitzer L. Cloning and expression analysis of sucrose phosphate synthase from sugar beet (Beta vulgaris L.) Mol Gen Genet. 1995;195:514–518. doi: 10.1007/BF00293155. [DOI] [PubMed] [Google Scholar]
- Heuer B, Plaut Z, Federman E. Nitrate and nitrite reduction in wheat leaves as affected by different types of water stress. Physiol Plant. 1979;46:318–323. [Google Scholar]
- Huber JL, Huber SC, Campbell WH, Redinbaugh MG. Comparative studies of the light modulation of nitrate reductase and sucrose-phosphate synthase activities in spinach leaves. Plant Physiol. 1992a;100:706–712. doi: 10.1104/pp.100.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JL, Huber SC, Campbell WH, Redinbaugh MG. Reversible light/dark modulation of spinach leaf nitrate reductase activity involves protein phosphorylation. Arch Biochem Biophys. 1992b;296:58–65. doi: 10.1016/0003-9861(92)90544-7. [DOI] [PubMed] [Google Scholar]
- Huber JL, Redinbaugh MG, Huber SC, Campbell WH. Regulation of maize leaf nitrate reductase activity involves both gene expression and protein phosphorylation. Plant Physiol. 1994a;106:1667–1674. doi: 10.1104/pp.106.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Bachmann M, Huber JL. Post-translational regulation of nitrate reductase activity: a role for Ca2+ and 14–3-3 proteins. Trend Plant Sci. 1996a;1:432–438. [Google Scholar]
- Huber SC, Huber JL. Regulation of spinach leaf sucrose-phosphate synthase by multisite phosphorylation. Curr Top Plant Biochem Physiol. 1990;9:329–343. [Google Scholar]
- Huber SC, Huber JL, McMichael RW. Control of plant enzyme activity by reversible protein phosphorylation. Int Rev Cytol. 1994b;149:47–98. [Google Scholar]
- Huber SC, McMichael RW, Bachmann M, Huber JL, Shannon JC, Kang KK, Paul M (1996b) Regulation of leaf sucrose-phosphate synthase and nitrate reductase by reversible protein phosphorylation. In PR Shewry, NG Halford, R Hooley, eds, Protein Phosphorylation in Plants. Proceedings of the Phytochemical Society of Europe. Oxford Science Publications, Clarendon Press, Oxford, UK, pp 19–34
- Huppe HC, Turpin DH. Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:577–607. [Google Scholar]
- Jiao J-A, Chollet R. Post-translational regulation of phosphoenolpyruvate carboxylase in C4 and crassulacean acid metabolism plants. Plant Physiol. 1991;95:981–985. doi: 10.1104/pp.95.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Brendle-Behnisch E. Rapid modulation of spinach leaf nitrate reductase by photosynthesis. I. Modulation in vivo by CO2 availability. Plant Physiol. 1991;96:363–367. doi: 10.1104/pp.96.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Forster J. Low CO2 prevents nitrate reduction in leaves. Plant Physiol. 1989;91:970–974. doi: 10.1104/pp.91.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Spill D, Glaab J. Rapid modulation of nitrate reductase in leaves and roots: indirect evidence for the involvement of protein phosphorylation/dephosphorylation. Physiol Plant. 1993;89:557–562. [Google Scholar]
- Kerr PS, Huber SC. Co-ordinate control of sucrose formation in soybean leaves by sucrose phosphate synthase and fructose-2,6-bisphosphate. Planta. 1987;170:197–204. doi: 10.1007/BF00397888. [DOI] [PubMed] [Google Scholar]
- Khamis S, Lamaze T. Maximal biomass production can occur in corn (Zea mays) in the absence of NO3− accumulation in either leaves or roots. Physiol Plant. 1990;78:388–394. [Google Scholar]
- Khamis S, Lamaze T, Lemoine Y, Foyer CH. Adaptation of the photosynthetic apparatus in maize leaves as a result of nitrogen limitation. Plant Physiol. 1990;94:1436–1443. doi: 10.1104/pp.94.3.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Crafts-Brandner S, Salvucci M. Cloning and development expression of the sucrose phosphate synthase gene from spinach. Planta. 1993;190:498–510. doi: 10.1007/BF00224789. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–510. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Krapp A, Hofmann B, Schafer C, Stitt M. Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the “sink-regulation” of photosynthesis? Plant J. 1993;3:817–828. [Google Scholar]
- Krapp A, Stitt M. An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta. 1995;195:313–323. [Google Scholar]
- Larsson C-M, Larsson M, Purves JV, Clarkson DT. Translocation and cycling through roots of recently absorbed nitrogen and sulphur in Triticum aestivum during vegetative and generative growth. Physiol Plant. 1991;82:345–352. [Google Scholar]
- Larsson M, Larsson C-M, Whitford PN, Clarkson DT. Influence of osmotic stress on nitrate reductase activity in wheat (Triticum aestivum L.) and the role of abscisic acid. J Exp Bot. 1989;40:1265–1271. [Google Scholar]
- Lunn JE, Furbank RT, Hatch MD. Adenosine 5′-triphosphate-mediated activation of sucrose-phosphate synthase in bundle sheath cells of C4 plants. Planta. 1997;202:249–256. [Google Scholar]
- MacKintosh C, Douglas P, Lillo C. Identification of a protein that inhibits the phosphorylation form of nitrate reductase from spinach (Spinacia oleracea) leaves. Plant Physiol. 1995;107:451–457. doi: 10.1104/pp.107.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer E, O'Leary M. Anapleurotic fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiol. 1987;84:58–60. doi: 10.1104/pp.84.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migge A, Meya G, Carrayol E, Hirel B, Becker TW. Regulation of the subunit composition of tomato plastidic glutamine synthetase by light and the nitrogen source. Planta. 1996;200:213–220. [Google Scholar]
- Nonami H, Boyer JS. Primary events regulating stem growth at low water potentials. Plant Physiol. 1990;94:1601–1609. doi: 10.1104/pp.93.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussaume L, Vincentz M, Meyer C, Boutin JP, Caboche M. Post-transcriptional regulation of nitrate reductase by light is abolished by an N-terminal deletion. Plant Cell. 1995;7:611–621. doi: 10.1105/tpc.7.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut Z. Nitrate reductase activity of wheat seedlings during exposure to and recovery from water stress and salinity. Physiol Plant. 1974;30:212–217. [Google Scholar]
- Quick WP, Siegl G, Neuhaus HE, Feil R, Stitt M. Short-term water stress leads to a stimulation of sucrose synthesis by activating sucrose phosphate synthase. Planta. 1989;177:536–546. doi: 10.1007/BF00392622. [DOI] [PubMed] [Google Scholar]
- Rochat C, Boutin J-P. Carbohydrates and nitrogenous compounds changes in the hull and in the seed during the pod development of pea. Plant Physiol Biochem. 1989;27:881–887. [Google Scholar]
- Saccardy K, Cornic G, Brulfert J, Reyss A. Effect of drought stress on net CO2 uptake by Zea leaves. Planta. 1996;199:589–595. [Google Scholar]
- Scheible W-R, Gonzàlez-Fontes A, Lauerer M, Müller-Rober B, Caboche M, Stitt M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 1997a;9:783–98. doi: 10.1105/tpc.9.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible W-R, Lauerer M, Schulz E-D, Caboche M, Stitt M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997b;11:671–91. [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1989;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J (1994) Feedback control of gene expression. Photosynth Res 39: 427–438 [DOI] [PubMed]
- Sheen J, Bogorad L. Regulation of levels of nuclear transcripts for C4 photosynthesis in bundle sheath and mesophyll cells of maize leaves. Plant Mol Biol. 1987;4:227–238. doi: 10.1007/BF00015031. [DOI] [PubMed] [Google Scholar]
- Siegl G, Mackintosh C, Stitt M. Sucrose-phosphate synthase is dephosphorylated by protein photophatase 2A in spinach leaves. Evidence from the effects of okadaic acid and microcystin. FEBS Letts. 1990;270:198–202. doi: 10.1016/0014-5793(90)81267-r. [DOI] [PubMed] [Google Scholar]
- Talouizite A, Champigny ML. Response of wheat seedlings to short-term drought with particular respect to nitrate utilization. Plant Cell Environ. 1988;11:149–155. [Google Scholar]
- Van Quy L, Champigny ML. Nitrate enhances the kinase activity for phosphorylation of phosphoenolpyruvate carboxylase and sucrose phosphate synthase proteins in wheat leaves. Plant Physiol. 1992;99:344–347. doi: 10.1104/pp.99.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BM, Hoekma A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz M, Moureaux T, Leydecker MT, Vaucheret H, Caboche M. Regulation of nitrate and nitrite reductase expression in Nicotiana plumbaginifolia leaves by nitrogen and carbon metabolites. Plant J. 1993;3:315–324. doi: 10.1111/j.1365-313x.1993.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Wellburn FAM, Lau K-K, Milling PMK, Wellburn AR. Drought and air pollution affect nitrogen cycling and free radical scavenging in Pinus halepensis (Mill) J Exp Bot. 1996;47:1361–1367. [Google Scholar]
- Worrell AC, Bruneau JM, Summerfelt K, Boersig M, Voelker TA. Expression of maize sucrose phosphate synthase in tomato alters leaf carbohydrate partitioning. Plant Cell. 1991;3:1121–1130. doi: 10.1105/tpc.3.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero CN. Living with water stress: evolution of osmolyte system. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Zrenner R, Stitt M. Comparison of the effect of rapidly and gradually developing water stress on carbohydrate metabolism in spinach leaves. Plant Cell Envriron. 1991;14:939–946. [Google Scholar]