Abstract
The surgical repair of degenerative mitral valve disease involves a number of technical points of importance. The use of artificial chordae for the repair of degenerative disease has increased as a part of the move from mitral valve replacement to repair of the mitral valve. The use of artificial chordae provides an alternative to the techniques pioneered by Carpentier (including the quadrangular resection, transfer of native chordae and papillary muscle shortening/plasty), which can be more technically difficult. Despite a growth in their uptake and the indications for their use, a number of challenges remain for the use of artificial chordae in mitral valve repair, particularly in the determination of the correct length to ensure optimal leaflet coaptation. Here, we analyse over 40 techniques described for artificial chordae mitral valve repair in the setting of degenerative disease.
Keywords: Mitral, Chordae, Valve repair
INTRODUCTION
Mitral valve (MV) structure and function are complex, and tightly regulated in the healthy heart. The MV consists of the anterior and posterior leaflets that sit in a fibrous annulus. The MV is supported by the subvalvular apparatus (SVA) consisting of the papillary muscles and chordae, which tether the valve leaflets to the left ventricle (LV). The SVA allows an intricate functional interaction between the MV and the LV [1]. As the MV can alter pre-load and afterload, any change in MV structure can affect the LV function. SVA changes also regulate myocardial contractility, as papillary muscle contraction modulates LV dimensions, which impacts on myocardial contractility.
Although the chordae were once thought to be simple connections between the papillary muscles and the mitral annulus [1], the critical importance of these structures is emerging. Finite element modelling studies show that variations in the length of individual chordae modulate overall chordae tension, the coaptation area of the mitral leaflets and mitral haemodynamics. Chordae that are 10% longer than normal do not make the MV incompetent, but double chordae tension [2]. If the chordae are 3% longer than normal, the chordae tension increases by one-third. This demonstrates that even minor errors in length can have dramatic consequences on the tension of the chordae, and also that the increases in chordae tension are proportional to the error in length. Using shorter-than-normal chordae produces stress concentrations at the free edge of the leaflet and impairs proper MV closure. Therefore, slight oversizing is probably the most appropriate approach clinically, and indeed intentionally oversizing the chordae during MV repair has been proposed [3].
The advent of artificial chordae has greatly enhanced the armoury of techniques available and made a great number of MV pathologies amenable to repair rather than replacement. Studies of the Society of Thoracic Surgeons Adult Cardiac Surgery Database suggest that current valve repair rates are approximately 70% [4], while reference centres are able to achieve almost 100% repair [5]. Given that MV repair is now established as superior to MV replacement [6, 7], there is a need to develop repair techniques that are simple, effective and durable. Bortolotti et al. [8] recently published an overview of the historical contributions of the development of artificial chordae and their role in MV repair for degenerative disease. The purpose and the focus of this paper are quite different from ours. In this review, we classify and appraise all the techniques that have been described for MV repair with chordae.
We provide a technically focussed analysis of over 40 techniques described for applying artificial chordae to MV repair. Each technique is placed in its evolutionary context, described with its advantages and disadvantages, and a figure is shown to illustrate the differences between the techniques. The tables describe how each technique handles the papillary muscles, leaflets and pledgets and also how their length is determined. Then we discuss technical issues related to the determination of the optimal length of artificial chordae. Finally, we examine the future use of artificial chordae in MV repair.
Artificial chordae for anatomical and physiological MV repair
The sophisticated function of the MV and subvalvular apparatus is poorly mimicked by MV replacement. An important element of this is the preservation of the SVA including the chordae [9]. Artificial chordae couple the MV and LV and restore anatomical MV structure and function. Thus, the chordae maintain the tension between the valve leaflet and the SVA. In this way, neochordae allow an anatomical repair of the MV.
Anatomical repair ideally means bi-leaflet preservation, with reattachment of chordae in their normal anatomical position, without disrupting the symmetry of the annulus and with the elimination of regurgitation. The aim of this is to maximize the valve orifice area and restore physiological valve dynamics. The use of artificial chordae is an effective, simple and reproducible means of achieving this.
This is a part of the concept termed ‘respect, do not resect’, which describes a move toward creating a surgical approach that promotes the anatomical arrangement of tissues by avoiding resection in order to maintain the dynamic links between structures [10]. Studies comparing these two strategies are discussed later, and they show that the ‘respect, do not resect’ approach improves leaflet coaptation and allows ring annuloplasty oversizing, which provides a more physiological leaflet movement. This reflects the current questions in MV repair. The important question is no longer the superior technique in terms of short-term outcomes, but rather, which provides a physiological MV with long-term durability. An important corollary of this is that there are occasions when resection is important, for example, when there is a large excess of tissue. However, with the advent of artificial chordae, the need for resection is diminishing.
MATERIALS AND METHODS
A search of Medline and internet sources (Google) was performed for all articles containing ‘MV repair’, ‘artificial chordae’, ‘neochordae’ ‘Gore-Tex’ or ‘expanded polytetrafluoroethylene’. In addition, personal collections of non-indexed supplements were used. Technical details of surgical procedures involving the use of artificial neochordae were extracted. We excluded papers describing techniques for the repair of ischaemic, congenital, genetic or any other mitral pathology apart from degenerative disease.
The history of artificial chordae materials: silk, pericardium and the advent of ePTFE Gore-Tex sutures
The use of artificial neochordae for MV repair, as David points out, dates back to the 1960s when a wide range of materials were used. Surgeons used many materials including silk and nylon [11] during its evolution. However, the use of two materials in particular had a particular role in the expansion of the use of artificial chordae: autologous pericardium and expanded polytetrafluoroethylene (ePTFE) Gore-Tex sutures.
Pericardium was used first [12] and a substantial experience was acquired in both experimental and clinical settings. Either autologous pericardium or glutaraldehyde-coated xenograft pericardium can be used for neochordae construction. The first description of expanded polytetrafluoroethylene by Herbert Vetter was in an experimental context [13]. This was adopted by pioneering surgeons like David who approached the technique with caution [11]. As experience grew, a greater number of surgeons used this material. The increase in the use of artificial chordae partly stems from the general acceptance of the ideas described in the preceding section: repair is better than replacement. ePTFE, a flexible yet strong polymer made an excellent substrate for host tissue to grow, and in a sense regenerate chordae. Although a monofilament, it is micro-porous and also non-absorbable. Early attempts to compare them suggested that pericardial chordae, although they had a longer and more certain track record, could undergo thickening and shortening over time [14]. Because of this and the growing evidence that the appearance and performance of Gore-Tex chordae were almost indistinguishable from native chordae, they became the dominant material in use. As it is the most widely used, the remainder of this article focuses on Gore-Tex neochordae.
Techniques for MV repair involving the use of artificial chordae
In this section, we review the techniques described for the use of artificial neochordae for MV repair. We first outline access to the valve. We discuss techniques aimed at repair of the single mitral leaflet and complex repairs. Each technique is described in text and figure form. As described earlier, small alterations to chordae lengths can have major effects on chordae tension and MV function. This concern has stimulated a group of techniques that have evolved out of the need for chordae of specific length—these techniques are discussed. Next, we outline the advent of repair ‘systems’ involving neochordae. We also describe the techniques where neochordae are used in Barlow's disease. Finally, we describe emerging techniques for the application of artificial chordae to MV repair.
There are a number of technical steps involved in the use of artificial chordae to repair the MV, after characterizing the defect in detail pre- and perioperatively. The repair can be considered to consist of six steps: attachment of the chordae to the papillary muscle; attachment to the leaflet; modulation of their length; insertion (or not) of an annuloplasty ring; tying knots to secure the chordae and testing the repair. Table 1 (Fig. 1A and 1B) and Table 2 (Fig. 2) detail the techniques for repairing the anterior and posterior mitral leaflets, respectively, with details of each of these operative steps. Irrespective of the details of the technique used, there are a number of important principles that apply to any use of chordae. First, the minimum number of bites through the pledget should be used when attaching the chordae to the papillary muscle as this prevents tissue damage. Secondly, it is important to avoid passing the chordae across the midline, thereby respecting the anatomical chordae-leaflet pattern. Chordae should be attached to the fibrous head of the papillary muscle, using the minimum number of pledgets, to prevent papillary muscle ischaemia. Ideally, the quality of the repair should be assessed in a full (preferably beating) heart, to mimic physiological conditions.
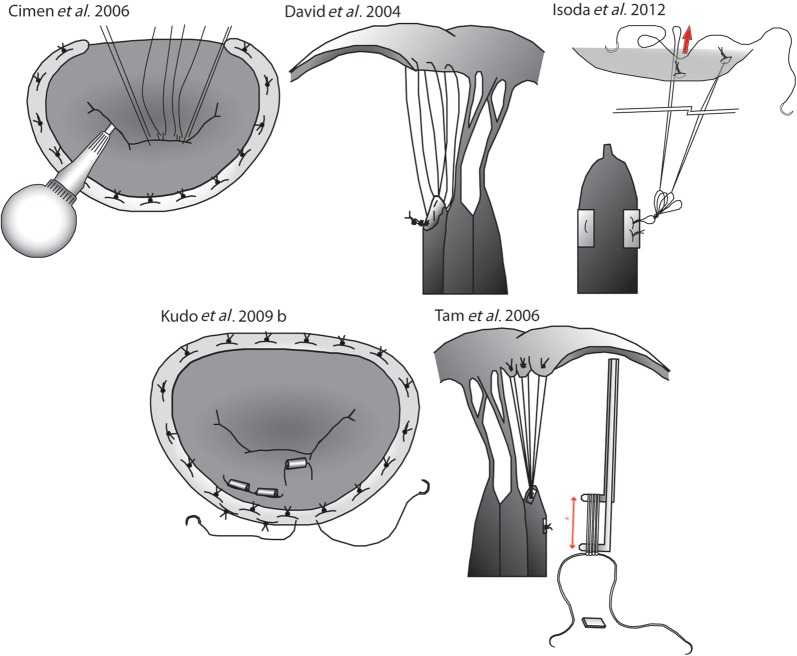
Figure 2:
Techniques for the repair of the posterior or both mitral leaflets. The figure panel shows the essential steps of the techniques detailed in Table 2 for the repair of the posterior (or both) mitral leaflet(s). Original figures adapted from the relevant publications.
Table 1:
Techniques for the repair of the anterior mitral leaflet
| Study | Method of anchor to papillary muscle | Method of anchor to leaflet | Method of adjusting length | Knot tying | Annuloplasty ring | Special features |
|---|---|---|---|---|---|---|
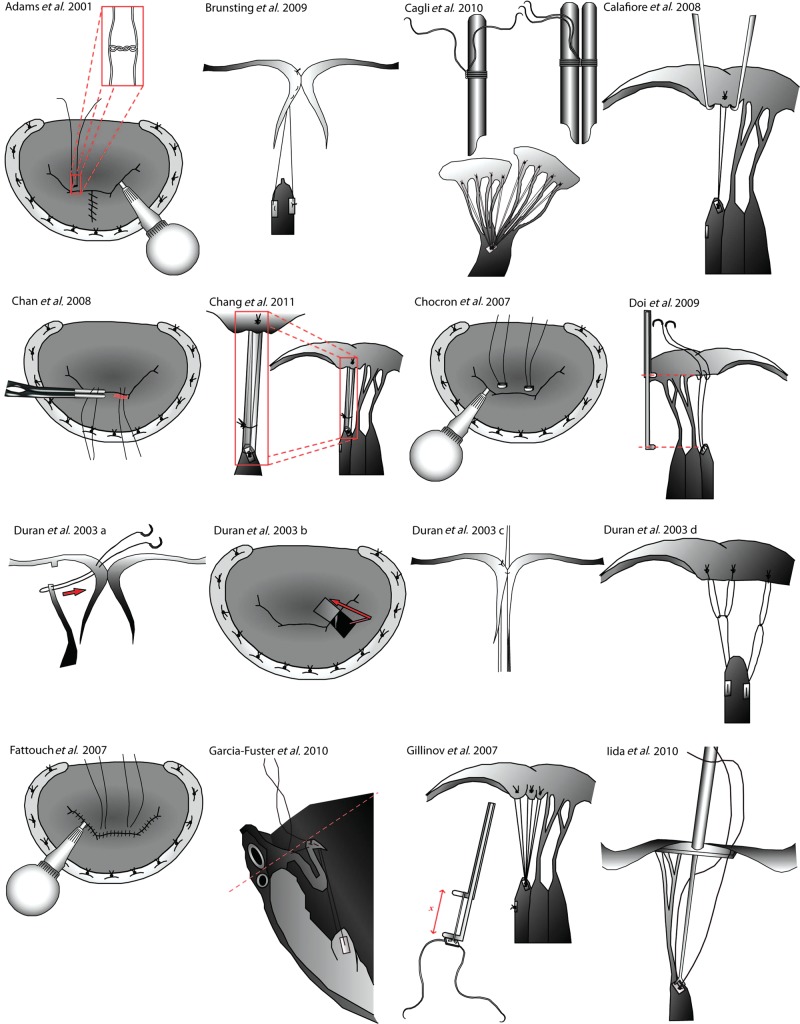
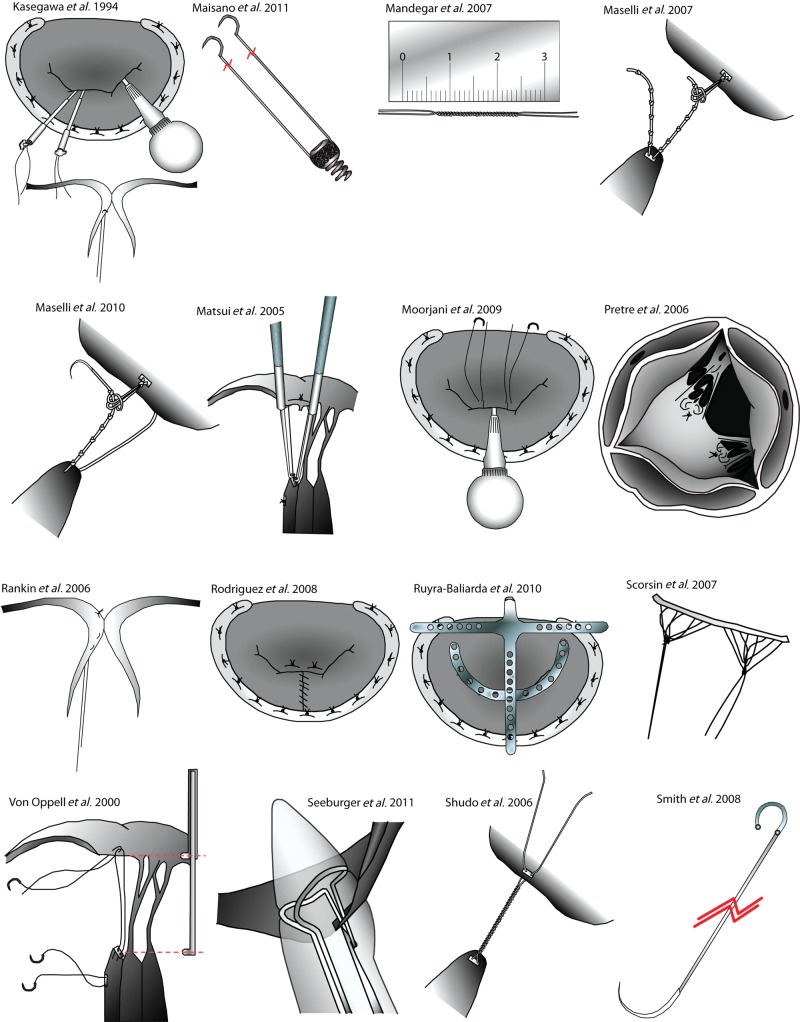
| Kasegawa et al. 1994 [18] | 1 | B1,3 | 1 | 0 | 1,2 | Tourniquets used to secure neochordae at target length during testing |
| von Oppell and Mohr 2000 [19] | 1 | A4 | 3 | 1 | 0 | Vernier callipers used to measure the PM–leaflet distance |
| Loops of neochordae are constructed to a predetermined length | ||||||
| Adams et al. 2001 [17] | 3 | B1 | 1 | 2 | 2 | Leaflet is resected prior to chordae construction |
| Duran and Pekar 2003 [28] | 1 | B2 | 4 | 1 | 1 | Temporary Alfieri stitch is used to coapt the leaflets before tying |
| Matsui et al. 2005 [21] | 1 | B3 | 3 | 0 | 2 | Specialized device measures target chordae length using surrounding normal chordae |
| Pretre et al. 2006 [16] | 0 | B2 | 1 | 0 | 1 | Requires aortotomy |
| Shudo et al. 2006 [33] | 0 | B1 | 1 | 0 | 0 | Continuous knot tying and number of knots are adjusted after knot tying |
| Rankin et al. 2006 [38] | 1 | B2 | 1 | 3 | 1 | Clip holds sutures in place over slip-knot, while competency is tested |
| The chordae is woven through the prolapsing segment, so may be particularly useful in Barlow's disease | ||||||
| Chocron 2007 [27] | 0 | B2 | 1 | 0 | 1 | Knots are tied over clips which initially hold the chordae to prevent slipping |
| Mandegar et al. 2007 [22] | 1 | A1 | 2 | 1 | 0 | A series of tight reverse knots made according to required length, which is determined by preoperative echo |
| Tied over a patch of pericardium | ||||||
| Scorsin et al. 2007 [35] | 0 | A2 | 2 | 4 | 2 | System of pre-manufactured chordae |
| Fattouch et al. 2007 [29] | 1 | B2 | 1 | 0 | 1 | Knots are tied under conditions of LV loading |
| Temporary Alfieri stitch is placed to oppose the leaflets | ||||||
| Maselli et al. 2007 [32] | 3 | B3 | 1 | 2 | 0 | Two systems of Gore-Tex sutures, one papillary and the other leaflet |
| Sutures interact and together form the neochordae | ||||||
| Smith and Stein 2008 [52] | 0 | B3 | 1 | 0 | 0 | Knots are tied leaving a little room to avoid damage to the PM |
| U-clips are used to attach the loop to the leaflet segment | ||||||
| Calafiore et al. 2008 [3] | 0 | A1 | 3 | 0 | 0 | Addition of 5 mm extra when a nerve hook is used to extend the AML that maximally avoids under-sizing the chordae |
| Rodriguez et al. 2008 [53] | 0 | A1 | 2 | 0 | 0 | No pledgets are used for the PM |
| Doi et al. 2009 [20] | 1 | A1,2 | 2,3 | 0 | 1 | Involves the construction of callipers for the measurement of the length of AML chordae |
| Brunsting et al. 2009 [43] | 1 | B2,3 | 1 | 0 | 1 | Robotic technique |
| Moorjani et al. 2009 [30] | 2 | B2 | 1 | 1 | 2 | Chordae length sets under LV loading conditions |
| Ruyra-Baliarda 2010 [36] | 0 | A1 | 1 | 0 | 1 | T-shaped no prolapse system ensures that the leaflets and annulus are at the same length |
| Cagli 2010 [34] | 1 | A2,3 | 2,3 | 3 | 1 | Hegar Dilator is used to construct the neochordae |
| Garcia-Fuster et al. 2010 [39] | 1 | B2 | 0 | 2 | 0 | Temporary stitch is used to bring the prolapsing segment to the level of the annulus, ensuring the correct chordae length is achieved |
| Maselli et al. 2010 [54] | 1 | B1 | 1 | – | – | This system involves a suture with a loop attached to the end |
| The suture contains a series of knots at a constant interval and by forming a slip noose-lace this can be adjusted | ||||||
| Maisano et al. 2011 [44] | 1 | A2,3 | 1 | 1 | 0 | A simplified device was tested in dogs to allow combination of minimally invasive, beating heart and tuneable chordae implantation |
| Chang and Kao 2011 [23] | 0 | 0 | 3 | 0 | 0 | Slit plastic tube maintains length during knot tying |
| Iida et al. 2010 [49] | 0 | 1 or 3 | 3 | 0 | 0 | A system pulls chordae to the correct tension at the site of the opposing leaflet to ensure correct length of the chordae |
| Points of tension are the leaflet and the papillary muscle | ||||||
| Matsui et al. 2011 [24] | 1 | 3 | 3 | 0 | 2 | Chordae are passed through tubes of predetermined length which are then cut after tying |
| Seeburger et al. 2012 [41] | 1 | 3 | 1 | 0 | 0 | Neochord DS 1000 device |
| Beating heart, minimally-invasive technique with custom device | ||||||
| Animal study | ||||||
| Chan et al. 2008 [25] | 1 | 1 | 0 | 2 | 0 | Clips are used to maintain the length of the chordae at the desired location, tied and cut |
PM: papillary muscle; AML: anterior mitral valve leaflet.
The table lists techniques described for the repair of the anterior mitral leaflet. Each technique is accompanied by an illustration shown in Fig. 1A–B, except Matsui et al. 2011 which is similar to Chang et al. 2011. Techniques are listed in chronological order in table and alphabetical order in Fig. 1A–B.
Method of anchor to papillary muscle: 0: not defined; 1: pledgetted support; 2: figure-of-eight conventional suture; 3: neither 0, 1, 2, e.g. fixation device. Method of anchor to leaflet: 0: not defined; A: chordae tied permanently before or without length adjustment; B: chordae are left untied or are only partially tied until competence testing; 1: at the site of anatomical chordae attachment; 2: at the site of maximal prolapse; 3: use of special device used. Method of adjusting length: 0: not defined; 1: adjusted after testing; 2: pre-determined by echo; 3: pre-determined by direct measurement (see special features if a custom device is used); 4: other. Knot tying: 0: not defined; 1: reverse knotting so that final length is not affected by tying; 2: surgeon's knot (reef knot with an extra throw); 3: slip-knots; 4: continuous suture. Annuloplasty ring: 0: not defined; 1: before chordae placement; 2: after chordae placement.
Figure 1A:
Techniques for the repair of the anterior mitral leaflet. The figure panel shows the essential steps of the techniques detailed in Table 1 for the repair of the anterior mitral leaflet. Original figures adapted from the relevant publications.
Table 2:
Techniques for the repair of the posterior (or both) mitral leaflets
| Study | Method of anchor to papillary muscle | Method of anchor to leaflet | Method of adjusting length | Knot tying | Annuloplasty ring | Special features |
|---|---|---|---|---|---|---|
| David 2004 [11] | 3 | B1 | 1 | 0 | 1 | The length of each chordae loop varies with the stress pattern |
| Cimen et al. 2006 [31] | 1 | A1 | 1 | 2 | 0 | Knots are tied under LV filling conditions |
| Tam et al. 2006 [26] | 1 | A2 | 3 | 0 | 1 | Callipers are used to make loops of predetermined length, which are attached to leaflet and PM |
| Not adjustable | ||||||
| Kudo et al. 2009 [37] | 1 | A4 | 3 | 1 | 0 | After standard loop technique, a reduction 4-0 polyester suture is used to reduce prolapse of the leaflet in Barlow's disease |
| Isoda et al. 2012 [51] | 3 | 3 | 1 | 0 | 0 | This involves an anchor loop fixed to the papillary muscle |
| A series of loops to the approximate length required is made and tied to the papillary anchor point | ||||||
| The loop is fixed to the leaflet using additional small anchoring loops | ||||||
| If the length need to be adjusted, additional anchor sutures are placed to shorten the loop |
Figure 1B:
Techniques for the repair of the anterior mitral leaflet. The figure panel shows the essential steps of further techniques detailed in Table 1 for the repair of the anterior mitral leaflet. Original figures adapted from the relevant publications.
These principles are designed to replicate the natural arrangement and stress patterns of the mitral–SVA, and also to prevent failure of the repair. The anatomical relation between the papillary muscle and the MV leaflet must be maintained This means preserving the relationship between chordae from the anterior papillary muscle to the lateral half of the anterior and posterior leaflets, and between the posterior papillary muscle and the medial half of the anterior and posterior leaflets. The chordae should not cross over these anatomical boundaries.
What access can be used?
Artificial chordae can be placed in a number of access settings, including minimally-invasive/robotic and open surgery, which is the most common [15]. Access to the valve is usually achieved by atriotomy, but Pretre et al. [16] have described a method for repairing the anterior leaflet using combined atriotomy and aortotomy. The authors initially used this approach to salvage failed repairs due to excessive anterior leaflet prolapse. Access to the anterior leaflet via the aortic valve gave excellent results, so this was used electively in a number of patients with good results in the short to medium term, with no valve-related complications and either no or trivial mitral regurgitation, although this remains used only rarely.
Techniques for the repair of single MV leaflet pathology
The most straightforward application of artificial chordae is in the case of isolated prolapse of a single MV leaflet due to degenerative disease. In its simplest form, by passing a double needle Gore-Tex suture through the leaflet and papillary muscle, two artificial chordae are constructed [17]. The advantages of this technique are that it is simple and creates fixed-length neochordae that allow the preservation of the SVA. However, the disadvantage of this technique is that it creates neochordae with a fixed length. An early approach to solving the problem of needing to readjust the chordae length after testing was the tourniquet technique described by Kasegawa et al. [18]. This involves placing tourniquets on the neochordae, holding them at a specific length during competence testing. If competence testing indicates satisfactory length, the chordae are tied down. The advantage of this technique is that the chordae length is adjusted to an anatomically correct length. The disadvantage is that the required length is obtained by a process of trial and error.
Multiple-loop technique (David technique)
The construction of neochordae using multiple loops provides a system of interconnected and self-adjustable chordae that work with the functional anatomy of the MV [11]. Such a system is able to modulate the distribution of tension along and between valve cusps. This is of special importance when attempting to repair severe prolapse or when both cusps are diseased. A number of techniques for the use of loops in neochordae construction have been described.
In its simplest form, a double-arm 5CV or 6CV Gore-Tex suture is passed through the papillary muscle, and the suture is tied to leave two arms of unequal length. The longer arm is passed through the cusp in question at the free margin. It is then passed back through the papillary muscle once more, then again through the free margin of the prolapsing cusp and back through the papillary muscle. Multiple bites are taken, until six or more neochordae are constructed, which anchor a prolapsing cusp segment. These neochordae should be closely aggregated to limit the tension maintained on any single chordae. This technique benefits from its dynamism: the length of each individual loop varies as a function of its tension, so that tensions are normalized to within a range. This acts as a negative feedback system, reducing the tension on chordae by increasing in length, while still preventing the leaflet from prolapsing. Its main disadvantage is that the length of the loop is not fixed and so may result in suboptimal leaflet coaptation at some points during the cardiac cycle.
Fixed length loop techniques using callipers
To generate loops of fixed lengths, von Oppell and Mohr [19] described the loop technique. This is a foundational technique on which many others are based. First, the required loop length is calculated using a reference point on a nearby segment of normal valve to the respective papillary muscle. Callipers are set to this length and used to construct the loop, which is tied off over a pledget. The arms of the ePTFE sutures are then pulled through the pledget to ensure that further knots do not alter the length of the loop. The chordae is secured to the papillary muscle between two pledgets by passing the needle through the pledget, then the fibrous portion of the muscle and through a second pledget. The loop is then sutured to the atrial aspect of the prolapsing cusp using a second Gore-Tex suture, with knots tied on the ventricular side of the cusp. Doi et al. [20] describe a technique where the suture is passed through the rough zone of the leaflet (from atrial to ventricular), and then through the free edge of the leaflet. This leaves a loop, into which the calliper is introduced (having been set at the required length). The loop is then tied over the calliper. Matsui et al. [21] describe a calliper with a round distal tip (for the papillary muscle) and a proximal hook (for the leaflet), which is also inserted within a preconstrucetd loop, and used to tie over. These techniques are advantageous because they allow accurate length determination, but they require the use of a (often customized) calliper.
Fixed length loop techniques without a calliper
Mandegar et al. [22] have proposed a method for accurate length determination without the use of a calliper. The predetermined length is constructed using a series of tight reverse knots; this accurately produces a specific length as the number of reverse knots corresponds to a certain length. An alternative method for tying loops at a predetermined length involves temporarily fixing them at a specific length using a slit plastic tube. A knot can then be tied without changing the length of the chordae [23]. Mutsui et al. [24] describe an additional technique for fixed-length chordae using a tube. After tying the chordae to the papillary muscle using a pledget, the arms of the suture are each passed through plastic tubes cut to the required length. The sutures are tied down over the tube. After tying, the tubes are cut-off the chordae. The advantages of this technique are that the apparatus is all subvalvular, so there is no clutter of the operative field, and secondly, that they do not require an assistant to hold a calliper in place. Chan et al. [25] first determine the normal length of chordae by using normal chordae as a reference. Having secured the chordae to the papillary muscle with a pledget, and passed them through the free edge of the leaflet, they mark the correct length with a marker pen. A covered clip holds the chordae at the correct length, allowing them to be tied without movement.
While these techniques provide lengths that are specific and based on anatomically correct chordae, a possible disadvantage is that because the measurements are made in an arrested, open heart, the final length could fail to replicate the required length in the full, beating heart. A second potential disadvantage is damage to the papillary muscle due to the multiple passes through the pledgets may also alter the tension on the papillary muscles. This has led to the development of techniques that aim at avoiding damage to the head of the papillary muscle and techniques that aim to obtain anatomical-length chordae.
Techniques limiting the number of passes through the papillary muscle
Tam et al. [26] have proposed a modification of the loop technique that involves only a single pass through the papillary muscle and pledget. A calliper is used to measure the correct chordae length as before. This calliper is maintained at the desired length and is used to construct multiple loops that are tied, and so are connected by a single suture, instead of tying each loop separately. A similar method has been described using clips rather than tourniquets by Chocron [27]. These techniques have the advantage of preserving the papillary muscle integrity by reducing the number of passes through the structure to the minimum.
Techniques for anatomical length chordae
The techniques described above may be criticized because the opened, empty heart is not an anatomical setting in which to perform length measurements. Other methods have been designed to make the mitral–SVA complex more anatomical during the repair process.
A simple method has been described by Duran and Pekar [28]. They propose the placement of a temporary Alfieri stitch, which brings the leaflets in contact. This replicates their structure under physiological conditions to some extent and the chordae are tied in this position. Alternatively, the length of the chordae can be calculated during preoperative echocardiography; this strategy can be used in conjunction with the techniques described above for the generation of specific lengths (see later section on the decision process of length determination).
An alternative approach is to tie the chordae down under conditions of LV loading (filled with saline), while also making the leaflets coapt with a temporary Alfieri stitch. This symmetrical repair technique proposed by Fattouch et al. [29] replicates the shape and stress patterns of a loaded, if still heart. The same approach is used taken by Moorjani et al. [30] and Cimen et al. [31]. The major disadvantage of these techniques is that once a specific length has been achieved, corrections cannot be made.
Techniques for tuneable length chordae
Although a number of methods have been described for making neochordae of a specific length, many suffer from the fact that ePTFE sutures are very slippery, and the final length may vary at the time of knot tying.
A method that avoids this has been described by Maselli et al. [32]. The ‘tuneable’ loop technique involves the creation of an extended length of ePTFE with multiple knots at defined length intervals. This allows for tuning of the loop length in situ. This system consists of two ePTFE components, one fixed on the leaflet and another on the papillary muscle. The papillary component is secured to the fibrous portion of the papillary muscle using two pledgets and contains an ePTFE chordae with knots at a defined distance from one another (approximately 2 mm). The leaflet component is attached to the free edge of the cusp, forming a free loop on the atrial aspect. This loop is positioned between two knots on the papillary chordae component. The length of the papillary component is approximately half the circumference of the loop. This creates a single artificial chordae, and multiple neochordae should be constructed to ensure proper coaptation of valve leaflets. After testing the repair by filling the LV with saline and examining for regurgitation, the length of any chordae can be readjusted by loosening the loop and replacing it at a new position. This can be done without damaging the neochordae anchors at the leaflet or papillary component. This has the advantage of being modifiable after testing, but has the disadvantage of the potential for chordae slippage during tying.
An alternative method that prevents knot-slipping is that proposed by Shudo et al. [33], the continuous knot-tying method. This involves securing the chordae to the papillary muscle first and tying multiple tight knots to a normal leaflet edge. This gives a good idea of the number of knots required for the prolapsing segment. Final knots are tied after competence testing. The advantage of this technique is that it is modifiable and does not require additional apparatus. However, it requires some experience to generate knots in the chordae of a uniform length and can be time consuming. It is not known whether the presence of series of multiple knots in the subvalvular space could result in a higher risk of haemolysis due to the passage of cells between knots.
Artificial chordae repair systems
Artificial chordae repair systems have been made in an attempt to provide reproducibility and enable surgeons with less repair experience to adopt the use of artificial chordae in MV lesions which have otherwise been destined for replacement. Some of these are innovative ways of using readily available equipment, such as Cagli's [34] description of the use of Hegar Dilators to construct neochordae. Others are focussed on the chordae themselves, for example Scorsin et al.'s [35] artificial chordae strip system, which involves the pre-manufacture of loops of chordae, avoiding the need for intraoperative loop construction, and offering significant timesaving benefit. This system allows the distribution of stresses along the whole free margin of the mitral leaflet. Its disadvantage is that it is an added cost, and is not widely available.
More complex repair systems are designed to provide maximal control over chordae placement. Ruyra-Baliarda [36] devised a T-shaped no-prolapse system, which involves suturing the leafets onto an oversized T-bar sitting above the annulus. This ensures that the chordae are placed at a length that brings the leaflets to the annular level. The advantage of this technique is that it does not involve the judgement of selecting a length and does not require an assistant to hold the apparatus in place. However, it involves the use of custom-made equipment. Clinical evaluation, animal studies and commercialization of such systems may bring about their wider adoption.
Artificial chordae for Barlow's disease
When the leaflet prolapse is severe with large bellowing cusps, quadrangular or triangular resections have been the most common strategies. This is because the use of the loop technique can leave an excessive portion of the leaflet, as in Barlow's disease, which is a known risk factor for systolic anterior motion (SAM). While the use of quadrangular/triangular resection and sliding plasty techniques for MV repair can be useful, they are not universally applicable. An alternative is a modification of the loop technique that can provide control of the excessive leaflet area [37]. When the posterior leaflet height measures 1.5 cm or more, an additional reduction of 4-0 polyester braid suture is pulled through the leaflet via a spaghetti support, until the height of the leaflet on the posterior side is 1.5 cm or less. This prevents excessive leaflet area impinging on the LV outflow tract. This technique involves readily available materials and is simple, but could change the shape of the leaflet, and produces excessive tension on the leaflet edge. Rankin et al. [38] also describes a method for repairing Barlow's disease using artificial chordae. This involves a clip that holds the chordae at the level of the leaflet during competency testing. The chordae is then repeatedly woven though the prolapsing segment. This reduces the leaflet to the correct level and prevents the oversized Barlow leaflets from distending. Rankin et al.'s technique has the benefit of taking multiple bites so that the tension is spread over a wider area, reducing the risk of tearing of the valve leaflet.
Artificial chordae are useful for the repair of Barlow's disease using the multiple interdependent loop technique [11] described earlier. This prevents bellowing of the leaflet, but also allows them to give a little at points of maximal strain to avoid damage to the leaflet which may occur if the chordae is of a fixed length. However, achieving the balance between enough tension to prevent excessive leaflet motion and too much tension on the leaflet requires significant experience.
Another technique that has been described for Barlow's disease (but can also be used for other lesions) is the ‘folding leaflet’ technique [39]. This involves chordae that pass over the free edge of the leaflet and are tied on the atrial side, creating a ‘hockey-stick’ folding zone that enhances leaflet coaptation. A holding stitch approximates the leaflet to the annulus, thus ensuring that the chordal length measures to the annular level. These holding stitches (held under tension by the assistant) ensure that the first knot is tied at the annular level. This technique has the advantage of being simple and promotes the maintenance of functional anatomy of the valve. Its disadvantage is that although if performed correctly it should provide chordae of the correct length, if the holding stitches are not under tension, the repair might fail.
Emerging techniques
There is a trend to the application of minimally-invasive techniques in cardiac surgery in general, and this is also true in MV repair [19]. The combination of minimally-invasive and robotic mitral surgery with artificial chordae is a major emerging theme, as discussed earlier. The development of simplified techniques designed specifically for this is needed to accelerate the rate of progress. An exciting advance has been the early clinical and laboratory testing of a system to apply artificial chordae to prolapsing MV components in a closed-chest, off-pump setting [40]. This system involves advancing a specialized device through the apex, tethering the end of the chordae to the prolapsing leaflet segment and then tying the remaining end to the LV apex. A clinical device (NeoChord DS 1000 Device) is emerging that achieves off-pump transapical implantation of artificial chordae [41]. This consists of a grasping mechanism, which captures the leaflet free edge, a fibre-optic device monitor to demonstrate successful grasping of the leaflet and a needle system that atraumatically pierces the leaflet and implants the chordae. Access is via 5 cm mini-thoracotomy. The device is passed into the left atrium under echocardiographic guidance. The chordae are then secured to the LV apex. Further clinical experience is required to evaluate this device. An important consideration is that it relies solely on echocardiographic determination of the proper chordae length and the selection of the prolapsing segment. Questions also remain as to whether the implantation of the chordae in the apical position will allow for anatomical repairs, and some data suggest that apical fixations result in inferior MV structure and function compared with papillary muscle fixation [42].
Another example of emerging chordae techniques is the use of robotic approaches, as described by Brunstig et al. [43]. This robotic on-pump technique under cardioplegic arrest involves first passing chordae through the papillary muscle, followed by a pledget. A full annuloplasty ring is then inserted, and then the neochordae are retrieved and woven into the free margin of the flail segment in three full thickness bites. The chordae is tied against the robotic forceps that apply a permanent clip retaining adequate chordae length. Valve competence is tested with saline, and the chordae are adjusted to measure competence.
A third area of emerging interest is the development of specific devices for the easy implantation of chordae of the correct length. Such a system has recently been described in an experimental setting [44]. A chordae adjustment system is temporarily tied to the head of the papillary muscle. The system contains two neochordae. Without estimating length based on echocardiographic measurements, the distal end of the neochordae was sutured to the flail segment of the mitral leaflet. The adjustment device is removed. The chordae length is then adjusted off-pump using echocardiography, leaving a finely tuned mitral chordae. The atriotomy is then closed with a purse-string suture.
Evaluation of the impact of artificial chordae on MV function
There is relatively little clinical data about the effect of artificial chordae insertion on leaflet coaptation areas, but Perier et al. [10] show that in selected patients who underwent chordae insertion, the mean coaptation area is 15 ± 2 mm. Implantation of artificial chordae during MV replacement surgery improves survival and reduces the rates of ventricular rupture [45]. Further studies show that implantation of artificial chordae may improve outcomes and left ventricular function [9]. However, the benefit of artificial chordae insertion is dependent on their proper anatomical placement with respect to the leaflet, the papillary muscles, the location of the valve defect and especially on the correct determination of their length.
Seeburger et al. [46] showed that the use of the loop technique significantly reduces post-operative mitral regurgitation, increased mitral orifice area and reduced the mean pressure gradient compared with leaflet resection. Falk et al. [47] showed that artificial chordae implantation significantly increases the length of the MV coaptation line.
These series are heterogeneous in the pathologies they include, the techniques for MV repair they apply and the nature of the study. There is consensus that MV repair using artificial chordae is both safe and effective. However, there is also some debate as to whether results using artificial chordae are superior to resection or not [46]. However, in such cases, the versatility of artificial loop chordae for the use in the minimally-invasive approach may make it the favoured option.
Remaining problems with artificial chordae-optimal length of chordae
Either short or long neochordae are detrimental to this structural and functional interaction. Short neochordae exert high tension on the valve leaflet and papillary muscle. This may cause rupture, or impair proper leaflet motion. Long neochordae may fail to repair the prolapse and increase the risk of SAM. Calafiore [48] proposes to calculate the distance between the edge of the prolapsing leaflet and the mitral annulus using preoperative echocardiography. Intraoperatively, the elongated chordae is measured and that length is subtracted from the preoperative leaflet-annulus value. This value is the target length of the neochordae. Mandegar et al. [22] propose measuring the distance between the posterior papillary muscle head to the mitral annulus plane at the coaptation of the leaflets. Alternatively, many of the techniques described above use callipers to directly calculate the correct chordae length.
There are a number of conceptual stages to preparing neochordae of the correct length. First, the correct target length must be determined. Secondly, the nature of the disease must be assessed (for example, billowing leaflets should have artificial neochordae attached further away from the free margin, as this will compensate for the distorted leaflet morphology). Thirdly, neochordae should be attached and tested. These processes are interdependent, and a number of investigators have integrated them, for example by determining the correct length by using testing protocols such as saline injections into the LV.
There are two broad strategies to generating neochordae of the correct size. First, the length of neochordae may be predetermined. In these techniques, either pre- or perioperative assessment of length is performed by transoesophageal echocardiogram (TOE) or direct measurement by use of a normal landmark (e.g. neighbouring non-prolapsing segments). However, it is important to take into consideration that the LV dimensions and geometry are very different in the empty non-beating heart, and measurements made in this setting poorly reflect the situation in vivo. This may be avoided by either making measurements before CPB using TOE or by filling the open LV with saline. A second strategy is to leave the length undetermined and adjust neochordae length by testing the valve function. Lengths are adjusted until any regurgitation is eliminated. The most widely used approaches for predetermination and adjustments after testing are described in Tables 3 and 4, respectively.
Table 3:
Techniques for the creation of artificial chordae of a specific length
| Study | Length determination method | Key features | Comment/figure |
|---|---|---|---|
| Gillinov and Banbury 2007 [55] | Normal adjacent chordae or papillary-annular distance. Length measured using callipers | Chordae measured on a set or callipers locked to the measured distance | Includes reference lengths |
| The knot is buried. Usually up to four chordae loops are made | |||
| Mandegar et al. 2007 [22] | Preoperative TOE: posterior papillary muscle head to the mitral annulus plane at the coaptation of the leaflets | A 4-0 Gore-Tex suture is fixed to fibrous head of papillary muscle using a pledget and a loose knot. The length of the suture is measured by using a variable number of reverse knots (if knots are tight, there will be two reverse knots per millimetre of braided 4-0 Gore-Tex). Knots tied over a portion of pericardium on the atrial side. This neochordae is used to measure all subsequent neochordae | The mean number of neochordae for each patients was 3.4 |
| Shudo et al. 2006 [33] | Normal adjacent chordae | A Gore-Tex suture is tied in the fibrous head of papillary muscle. Multiple knots are made until the length of the neochordae approximates that of adjacent chordae. Both arms of the suture are then brought through the leaflet head | This technique avoids the problems of knot-sliding, as the leaflet is fixed between the knots on the ventricular side and the knots on the atrial side |
| Doi et al. 2009 [20] | Transthoracic and TOE, as well as direct calliper measurement of a normal posterior leaflet | After securing the Gore-Tex suture to the papillary head, the arms of the Gore-Tex suture are taken through the leaflet and tied around the calliper directly in situ. This ensures that the posterior and anterior leaflets are of the same height, ensuring they coapt properly | The use of a range of measurement tools ensures an accurate definition of the required chordae length |
| Tam et al. 2006 [26] | Direct calliper measurement | A calliper is used to measure a nonprolapsing segment. This fixed length is used to make loops of the required length, ex situ | It is important to secure the loop using non-sliding knots, while still on the calliper, otherwise the length may change |
Table 4:
Techniques for the creation of artificial chordae of adjustable length
| Study | Key features | Comment/figure |
|---|---|---|
| Fattouch et al. 2007 [29] | Secure 5-0 Gore-Tex suture to papillary head before annuloplasty | The placement of neochordae when the LV is filled ensures that the length is appropriate to normal dimensions and geometry |
| Attach to prolapsing segment | Can be used for complex lesions. In such cases, the orifice is almost completely closed and tested. Care must be taken to avoid damage to the neochordae when performing edge-to-edge repair | |
| Perform temporary edge-to-edge repair (Alfieri stitch) | ||
| Annuloplasty performed | ||
| LV injection with saline, adjustments made if MR noted and then Gore-Tex sutures tied securely | ||
| Pretre et al. 2006 [16] | Neochordae are constructed to be similar to adjacent normal chordae. Artificial chordae are temporarily locked and then inspected, while the LV is filled from an aortotomy. Aortotomy is performed after posterior leaflet repair. Neochordae are adjusted and then tied definitively from the aortotomy | Only 25 min extra CPB time required for aortotomy and repositioning. Initially used to salvage failed repair from the atriotomy |
| Chocron 2007 [27] | Neochordae are placed through the papillary muscle and the free edge of the MV. Annuloplasty is then performed. Measurement of correct length made from papillary muscle to annuloplasty ring. Clips are then used to prevent sliding of valve during the competency test. LV filled via aorta. Clips adjusted and MV re-tested until MR is eliminated. Knots tied over the clips, which can be removed or left in place | Clips avoid knot sliding |
| Kasegawa et al. 1994 [18] | A small tourniquet is used to maintain chordae length during LV filling. Fine adjustments are then made and the final length is knotted in place | Good results obtained and reduced the time necessary to eliminate MR |
| Calafiore et al. 2008 [3] | This technique is used to obtain correct chordae lengths for the AML and to inhibit excessive movement of the PML | Obtained good results |
| The AML is pulled with nerve hooks to its maximum extent and the chordae is attached 5 mm higher than the border of the AML. For the PML, the same method was used without the added 5 mm. In both cases, after an initial chordae is tied, nerve hooks are used to fine tune the required length | Calafiore et al. had previously proposed the TOE approach but now use this technique | |
| Moorjani et al. 2009 [30] | After securing the suture to the papillary muscle, both ends of the Gore-Tex suture are passed to the atrial side. One limb is then placed back through the leaflet to the ventricular aspect. Annuloplasty rings are inserted and then the repair is tested by LV filling. The height of the sutures is easily adjusted. When MR has been eliminated, the limb of the suture is passed back through the leaflet and tied | Passing the suture through the leaflet, a third time prior to knot tying prevents the knot from altering the height of the neochordae |
| Rankin et al. 2006 [38] | An anchor suture is placed on the papillary muscle. A Gore-Tex suture is tied to this anchor and left in the ventricular cavity. After annuloplasty, the sutures are retrieved and tied by use of a slip-knot on the atrial aspect of the area of maximal prolapse of the leaflet. A clip prevents unintentional slipping. The length is tested by filling the LV with saline, adjustments are made by replacing the clip and then eight knots are tied at the correct position | |
| Maselli et al. 2007 [32] | Knots are tied at fixed intervals in a neochordae loop attached to the papillary muscle. These can be temporarily fixed by connecting them to a loop fixed to the papillary component (see description in earlier section) | Easily adjustable and does not involve damage to the leaflets or loops |
| Isoda et al. 2012 [51] | Two anchor system. Anchoring loop is placed at the papillary muscle. An additional anchoring loop is placed at the leaflet and the main chordae loop is tied between them. If adjustment of chordae length is required, an additional anchoring loop can be placed towards the annulus of the valve leaflet | This involves an anchor loop fixed to the papillary muscle |
| A series of loops to the approximate length required is made and tied to the papillary anchor point | ||
| The loop is fixed to the leaflet using additional small anchoring loops | ||
| If the length need to be adjusted, additional anchor sutures are placed to shorten the loop |
A new device has been designed specifically for length determination. It consists of a central bar that is secured at the level of the papillary muscle by a T-section (at the distal end) and secured at the level of the valve leaflets by an additional T-section (at the proximal level) inside the artificial chordae loop. This allows the tying of the artificial chordae at the level of the normal leaflet. By using the opposing, normal leaflet as a point of support for the device, the correct length of the chordae is ensured [49].
Durability of artificial chordae techniques
Artificial chordae of various types have been used since the 1960s (see historical review [8]). A recent review of the main outcome studies shows that artificial chordae show excellent long-term durability, regardless of the setting in which they are used, and for both adult and paediatric settings [8]. Perhaps some of the best evidence of artificial chordae durability comes from a recent study, by one of the early adopters of Gore-Tex artificial chordae. David et al. [50] recently reported a series of over 600 patients who underwent artificial chordae replacement over the course of 25 years. At 18 years, freedom from MV reoperation was 90.2 ± 2.4%, indicating excellent durability.
One challenge is that many of the techniques described in this paper have been applied to a limited number of patients, and there is insufficient evidence to compare them directly. This field is rapidly evolving with the advent of new techniques and the move towards minimally-invasive placement. Therefore, direct comparison of different techniques will require a more data than are currently available.
CONCLUSIONS
Classical Carpentier techniques make MV repair feasible in many cases and their value should not be downplayed. Artificial neochordae are durable, versatile structures that facilitate MV repair in the great majority of cases. However, their use is technically challenging and there is a learning curve. The challenges involved in their use have stimulated the development of technical solutions to maintain their longevity, apply them to different anatomical situations and to ensure their correct length. Artificial neochordae constitute the strategy of choice for a range of MV lesions and their indications are likely to expand. There is a move to use them in robotic or minimally-invasive settings and as a part of specially designed mitral repair systems. The advent of such systems will shorten the learning curve, may improve outcomes and should help to increase the number of repairs rather than replacements.
FUNDING
This work was supported by the NIHR Biomedical Research Centre Funding Scheme.
Conflict of interest: none declared.
REFERENCES
- 1.Yun KL, Niczyporuk MA, Sarris GE, Fann JI, Miller DC. Importance of mitral subvalvular apparatus in terms of cardiac energetics and systolic mechanics in the ejecting canine heart. J Clin Invest. 1991;87:247–54. doi: 10.1172/JCI114978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reimink MS, Kunzelman KS, Cochran RP. The effect of chordal replacement suture length on function and stresses in repaired mitral valves: a finite element study. J Heart Valve Dis. 1996;5:365–75. [PubMed] [Google Scholar]
- 3.Calafiore AM, Scandura S, Iaco AL, Contini M, Di Mauro M, Bivona A. A simple method to obtain the correct length of the artificial chordae in complex chordal replacement. J Card Surg. 2008;23:204–6. doi: 10.1111/j.1540-8191.2008.00570.x. [DOI] [PubMed] [Google Scholar]
- 4.Gammie JS, Zhao Y, Peterson ED, O'Brien SM, Rankin JS, Griffith BP. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2010;90:1401–8. doi: 10.1016/j.athoracsur.2010.05.055. 1410 e1401 discussion 1408–1410. [DOI] [PubMed] [Google Scholar]
- 5.Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg. 2012;144:308–12. doi: 10.1016/j.jtcvs.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Gillinov AM, Cosgrove DM, Blackstone EH, Diaz R, Arnold JH, Lytle BW. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg. 1998;116:734–43. doi: 10.1016/S0022-5223(98)00450-4. [DOI] [PubMed] [Google Scholar]
- 7.Yacoub M, Halim M, Radley-Smith R, McKay R, Nijveld A, Towers M. Surgical treatment of mitral regurgitation caused by floppy valves: repair versus replacement. Circulation. 1981;64:II210–16. [PubMed] [Google Scholar]
- 8.Bortolotti U, Milano AD, Frater RW. Mitral valve repair with artificial chordae: a review of its history, technical details, long-term results, and pathology. Ann Thorac Surg. 2012;93:684–91. doi: 10.1016/j.athoracsur.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Athanasiou T, Chow A, Rao C, Aziz O, Siannis F, Ali A. Preservation of the mitral valve apparatus: evidence synthesis and critical reappraisal of surgical techniques. Eur J Cardiothorac Surg. 2008;33:391–401. doi: 10.1016/j.ejcts.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Perier P, Hohenberger W, Lakew F, Batz G, Urbanski P, Zacher M. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: midterm results of the ‘respect rather than resect’ approach. Ann Thorac Surg. 2008;86:718–25. doi: 10.1016/j.athoracsur.2008.05.015. discussion 718–25. [DOI] [PubMed] [Google Scholar]
- 11.David TE. Artificial chordae. Semin Thorac Cardiovasc Surg. 2004;16:161–8. doi: 10.1053/j.semtcvs.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Frater RW, Berghuis J, Brown AL, Jr, Ellis FH., Jr The experimental and clinical use of autogenous pericardium for the replacement and extension of mitral and tricuspid value cusps and chordae. J Cardiovasc Surg (Torino) 1965;6:214–28. [PubMed] [Google Scholar]
- 13.Vetter HO, Factor SM, Frater RW. The use of glycerol-treated homologous pericardium as a substitute for cusps and chordae tendineae of the mitral valve in sheep. Thorac Cardiovasc Surg. 1987;35:11–5. doi: 10.1055/s-2007-1020189. [DOI] [PubMed] [Google Scholar]
- 14.Frater RW, Vetter HO, Zussa C, Dahm M. Chordal replacement in mitral valve repair. Circulation. 1990;82:IV125–30. [PubMed] [Google Scholar]
- 15.Nifong LW, Chitwood WR, Pappas PS, Smith CR, Argenziano M, Starnes VA. Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg. 2005;129:1395–404. doi: 10.1016/j.jtcvs.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Pretre R, Khatchatourov G, Kadner A, Genoni M. Application and adjustment of artificial chordae to the mitral valve using an approach through the aortic valve. Ann Thorac Surg. 2006;82:761–2. doi: 10.1016/j.athoracsur.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 17.Adams DH, Kadner A, Chen RH. Artificial mitral valve chordae replacement made simple. Ann Thorac Surg. 2001;71:1377–8. doi: 10.1016/s0003-4975(00)02184-6. discussion 1378–1379. [DOI] [PubMed] [Google Scholar]
- 18.Kasegawa H, Kamata S, Hirata S, Kobayashi N, Mannouji E, Ida T. Simple method for determining proper length of artificial chordae in mitral valve repair. Ann Thorac Surg. 1994;57:237–8. doi: 10.1016/0003-4975(94)90413-8. discussion 238–239. [DOI] [PubMed] [Google Scholar]
- 19.von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg. 2000;70:2166–8. doi: 10.1016/s0003-4975(00)02047-6. [DOI] [PubMed] [Google Scholar]
- 20.Doi A, Iida H, Sunazawa T. Intracardiac calipers for artificial chordae replacement in mitral valve repair. Ann Thorac Surg. 2009;87:326–8. doi: 10.1016/j.athoracsur.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Matsui Y, Fukada Y, Naito Y, Sasaki S, Yasuda K. A new device for ensuring the correct length of artificial chordae in mitral valvuloplasty. Ann Thorac Surg. 2005;79:1064–5. doi: 10.1016/j.athoracsur.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Mandegar MH, Yousefnia MA, Roshanali F. Preoperative determination of artificial chordae length. Ann Thorac Surg. 2007;84:680–2. doi: 10.1016/j.athoracsur.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 23.Chang JP, Kao CL. Slit stent technique for ensuring the correct length of artificial chordae in mitral repair. J Card Surg. 2011;26:259–60. doi: 10.1111/j.1540-8191.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsui Y, Kubota S, Sugiki H, Wakasa S, Ooka T, Tachibana T. Measured tube technique for ensuring the correct length of slippery artificial chordae in mitral valvuloplasty. Ann Thorac Surg. 2011;92:1132–4. doi: 10.1016/j.athoracsur.2011.03.111. [DOI] [PubMed] [Google Scholar]
- 25.Chan DT, Chiu CS, Cheng LC, Au TW. Artificial chordae: a simple clip and tie technique. J Thorac Cardiovasc Surg. 2008;136:1597–9. doi: 10.1016/j.jtcvs.2007.12.080. [DOI] [PubMed] [Google Scholar]
- 26.Tam R, Joshi P, Konstantinov IE. A simple method of preparing artificial chordae for mitral valve repair. J Thorac Cardiovasc Surg. 2006;132:1486–7. doi: 10.1016/j.jtcvs.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Chocron S. Removable clips for mitral valve repair. J Thorac Cardiovasc Surg. 2007;133:1682–3. doi: 10.1016/j.jtcvs.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Duran CM, Pekar F. Techniques for ensuring the correct length of new mitral chords. J Heart Valve Dis. 2003;12:156–61. [PubMed] [Google Scholar]
- 29.Fattouch K, Bianco G, Sbraga F, Sampognaro R, Ruvolo G. Simple, safe and easy technique to ensure the correct length of artificial chordae in mitral valve repair. Ann Thorac Surg. 2007;83:1902–3. doi: 10.1016/j.athoracsur.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Moorjani N, Viola N, Janusauskas V, Livesey S. Adjusting the length of artificial polytetrafluoroethylene chordae in mitral valve repair by a single loop technique. J Thorac Cardiovasc Surg. 2009;138:1441–2. doi: 10.1016/j.jtcvs.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 31.Cimen S, Ketenci B, Ozay B, Demirtas M. Neo-chordae length adjustment in mitral valve repair. Eur J Cardiothorac Surg. 2006;29:843–4. doi: 10.1016/j.ejcts.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 32.Maselli D, De Paulis R, Weltert L, Salica A, Scaffa R, Bellisario A. A new method for artificial chordae length ‘tuning’ in mitral valve repair: preliminary experience. J Thorac Cardiovasc Surg. 2007;134:454–9. doi: 10.1016/j.jtcvs.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Shudo Y, Taniguchi K, Takahashi T, Matsue H. Simple and easy method for chordal reconstruction during mitral valve repair. Ann Thorac Surg. 2006;82:348–9. doi: 10.1016/j.athoracsur.2005.05.087. [DOI] [PubMed] [Google Scholar]
- 34.Cagli K. A simple method of making artificial chordal loops for mitral valve repair. Ann Thorac Surg. 2010;89:e12–4. doi: 10.1016/j.athoracsur.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 35.Scorsin M, Al-Attar N, Lessana A. A novel technique of utilizing artificial chordae for repair of mitral valve prolapse. J Thorac Cardiovasc Surg. 2007;134:1072–3. doi: 10.1016/j.jtcvs.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Ruyra-Baliarda X. Preliminary experience with the no prolapse system. A new device for ensuring the proper length of artificial chordae in mitral valve repair. Interact CardioVasc Thorac Surg. 2010;10:165–7. doi: 10.1510/icvts.2009.207159. [DOI] [PubMed] [Google Scholar]
- 37.Kudo M, Yozu R, Kokaji K, Kimura N. A simple method of prevention for systolic anterior motion in mitral valve repair by loop technique method. Ann Thorac Surg. 2009;87:324–5. doi: 10.1016/j.athoracsur.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 38.Rankin JS, Orozco RE, Rodgers TL, Alfery DD, Glower DD. Adjustable artificial chordal replacement for repair of mitral valve prolapse. Ann Thorac Surg. 2006;81:1526–8. doi: 10.1016/j.athoracsur.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Fuster R, Gil O, Vazquez A, Garcia A, Martinez-Leon J. The folding leaflet: a simple method for neochordal repair. Ann Thorac Surg. 2010;89:1682–4. doi: 10.1016/j.athoracsur.2009.06.082. [DOI] [PubMed] [Google Scholar]
- 40.Bajona P, Katz WE, Daly RC, Zehr KJ, Speziali G. Beating-heart, off-pump mitral valve repair by implantation of artificial chordae tendineae: an acute in vivo animal study. J Thorac Cardiovasc Surg. 2009;137:188–93. doi: 10.1016/j.jtcvs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Seeburger J, Leontjev S, Neumuth M, Noack T, Hobartner M, Misfeld M. Trans-apical beating-heart implantation of neo-chordae to mitral valve leaflets: results of an acute animal study. Eur J Cardiothorac Surg. 2012;41:173–6. doi: 10.1016/j.ejcts.2011.03.058. discussion 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber A, Hurni S, Vandenberghe S, Wahl A, Aymard T, Vogel R. Ideal site for ventricular anchoring of artificial chordae in mitral regurgitation. J Thorac Cardiovasc Surg. 2012;143:S78–81. doi: 10.1016/j.jtcvs.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 43.Brunsting L, Rankin S, Braly K, Binford R. Robotic artificial chordae replacement for repair of mitral valve prolapse. Innov Technol Tech Cardiothorac Vasc Surg. 2009;4:229–232. doi: 10.1097/IMI.0b013e3181b0aa5d. [DOI] [PubMed] [Google Scholar]
- 44.Maisano F, Cioni M, Seeburger J, Falk V, Mohr FW, Mack MJ. Beating-heart implantation of adjustable length mitral valve chordae: acute and chronic experience in an animal model. Eur J Cardiothorac Surg. 2011;40:840–7. doi: 10.1016/j.ejcts.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Miller DW, Jr, Johnson DD, Ivey TD. Does preservation of the posterior chordae tendineae enhance survival during mitral valve replacement? Ann Thorac Surg. 1979;28:22–7. doi: 10.1016/s0003-4975(10)63386-3. [DOI] [PubMed] [Google Scholar]
- 46.Seeburger J, Falk V, Borger MA, Passage J, Walther T, Doll N. Chordae replacement versus resection for repair of isolated posterior mitral leaflet prolapse: a egalite. Ann Thorac Surg. 2009;87:1715–20. doi: 10.1016/j.athoracsur.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Falk V, Seeburger J, Czesla M, Borger MA, Willige J, Kuntze T. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg. 2008;136:1205. doi: 10.1016/j.jtcvs.2008.07.028. discussion 1205–6. [DOI] [PubMed] [Google Scholar]
- 48.Calafiore AM. Choice of artificial chordae length according to echocardiographic criteria. Ann Thorac Surg. 2006;81:375–7. doi: 10.1016/j.athoracsur.2004.08.071. [DOI] [PubMed] [Google Scholar]
- 49.Iida H, Sunazawa T, Doi A, Ishida K, Irabu S. A device for ensuring the neochordae replacement in mitral valve repair. Ann Thorac Surg. 2010;90:2071–2. doi: 10.1016/j.athoracsur.2009.12.085. [DOI] [PubMed] [Google Scholar]
- 50.David TE, Armstrong S, Ivanov J. Chordal replacement with polytetrafluoroethylene sutures for mitral valve repair: a 25-year experience. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Isoda S, Osako M, Kimura T, Mashiko Y, Yamanaka N, Nakamura S, et al. The "loop with anchor" technique to repair mitral valve prolapse. Ann Thorac Cardiovasc Surg. 2012;18:170–3. doi: 10.5761/atcs.nm.11.01705. [DOI] [PubMed] [Google Scholar]
- 52.Smith JM, Stein H. Endoscopic placement of multiple artificial chordae with robotic assistance and nitinol clip fixation. J Thorac Cardiovasc Surg. 2008;135:610–14. doi: 10.1016/j.jtcvs.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez E, Nifong LW, Chu MW, Wood W, Vos PW, Chitwood WR. Robotic mitral valve repair for anterior leaflet and bileaflet prolapse. Ann Thorac Surg. 2008;85:438–44. doi: 10.1016/j.athoracsur.2007.04.122. discussion 444. [DOI] [PubMed] [Google Scholar]
- 54.Maselli D, Ficarra E, Weltert L, Barberi F, Scaffa R, Bellisario A. A method to avoid knot-tying in artificial chordae implantation for mitral valve repair. J Heart Valve Dis. 2010;19:249–53. [PubMed] [Google Scholar]
- 55.Gillinov AM, Banbury MK. Pre-measured artificial chordae for mitral valve repair. Ann Thorac Surg. 2007;84:2127–9. doi: 10.1016/j.athoracsur.2007.04.046. [DOI] [PubMed] [Google Scholar]