Abstract
OBJECTIVES
Lobectomies done by video-assisted thoracic surgery (VATS) result in fewer complications and less pain and save total costs compared with the traditional approach. However, the majority of procedures are still performed via open thoracotomies, because VATS lobectomy is considered difficult to learn, requiring experience in open surgery, and causing complications in the initial phase of the learning curve. The aim of this study was to describe a training model appreciating patient safety during training and to explore the initial learning curve for a trainee rather inexperienced in open surgery.
METHODS
A trainee who had performed 14 lobectomies by thoracotomy was enrolled in a training programme at a high-volume VATS centre. The training model included courses and simulations followed by the selection of suitable patients operated on during close expert supervision. Data regarding time, a variety of quality indicators and complications were collected prospectively and compared with experts' performance.
RESULTS
Over 12 months, 29 of 214 VATS lobectomies were performed by the trainee. Twice, the supervisor had to finish the procedure due to technical difficulties. None of the operations were converted to open thoracotomy. Compared with experts, the trainee operated significantly slower [median 120 (range 74–160) vs 100 (range 42–255) min, P = 0.04]; had similar perioperative bleeding [median 100 (range 10–500) vs 50 (range 5–2500) ml, P = 0.79]; had earlier chest tube removal [median 1 (range 1–6) vs 2 (range 1–32) postoperative days, P < 0.001]; and reduced hospital stay [median 3 (range 1–10) vs 4 (range 1–41) days, P < 0.001]. Twenty-three (79%) patients had no complications, while 2 had atrial fibrillation. Pneumothorax after chest tube removal, incisional infection, prolonged pain and need for pleuracentesis were each seen once.
CONCLUSIONS
With thorough preparation of trainees and training on selected patients under close supervision, the learning curve can be overcome with good results even if the trainee has limited prior experience in open surgery.
Keywords: Thoracoscopy, Lobectomies, Education, Learning curve, Complications, Outcome
INTRODUCTION
For more than 20 years, it has been possible to perform lobectomy using video-assisted thoracic surgery (VATS) as an alternative to the traditional open thoracotomy. Unfortunately, there has only been one small, randomized study comparing the two methods [1], but the vast majority of non-randomized studies favour the minimally invasive VATS approach. Reduced pain and fewer postoperative complications have been shown [2, 3], and two studies indicate improved tolerance of chemotherapy [4, 5]. Furthermore, a systematic review of 39 studies including more than 6000 patients concluded that VATS lobectomy compared with thoracotomy lobectomy had improved overall survival rates [6]. Higher equipment costs have been a major concern when implementing VATS programmes, but recent studies all concluded that VATS lobectomy is less expensive than conventional lobectomy, because increased theatre costs were counteracted by shorter hospital stay [7–9].
Despite the many advantages of the VATS approach, the majority of lobectomies are still performed as open procedures, even though approximately 90% of lobectomies can be performed by VATS [10]. A possible explanation is the perception that VATS lobectomy is a technically challenging procedure with a shallow learning curve, where the initial phase is characterized by prolonged procedure time, frequent need for conversion to open thoracotomy and many complications. Reports on initial experiences of the procedure have not reflected the learning curve of a surgeon being trained in a standardized procedure, but the learning curve of the entire operating team learning and developing new techniques [11–13]. Earlier studies specifically exploring the necessary training requirements for VATS lobectomy have all stressed the importance of being competent in performing open major lung resections before proceeding to perform VATS lobectomies [14, 15]. However, with the spread of dedicated centres where all standard lobectomies are performed by VATS, it will be possible and perhaps even necessary to teach the standardized procedure to relatively inexperienced thoracic surgery trainees. It is unknown how this approach will impact on procedure time, blood loss, complications and need for conversions in the initial learning phase.
The aim of this study was to describe a training model appreciating patient safety during training and to explore the initial learning curve for a trainee rather inexperienced in open thoracic surgery.
MATERIALS AND METHODS
The study was conducted in a high-volume centre of thoracic surgery with a long-standing VATS programme performing more than 200 major lung resections yearly using a standardized anterior three-port technique [16]. The VATS programme is run by two dedicated VATS surgeons who developed a VATS training programme designed to teach a trainee the skills necessary for performing a VATS lobectomy in a safe way. The preparatory phase of the programme included VATS master classes at Elancourt, France, with hands-on practice on live pigs, and a VATS lobectomy course led by Dr Thomas D'Amico at Duke University Hospital, North Carolina, with training on a validated lobectomy model [17]. Observation of VATS lobectomies both on video and on the operating room was mandatory, and minor VATS procedures (wedge resections and operations for pneumothorax) were taught. Major lung resections via thoracotomies were not included in the VATS training programme.
The preparatory phase was followed by a clinical practice phase, where suitable patients were selected for the trainee based on tumour localization and the absence of major co-morbidity. A supervised ‘whole-training’-approach was used where the trainee performed the entire procedure including systematic lymph node dissection except when perioperative complications forced the supervisor to take over. All procedures were performed using three ports and a standard anterior approach described earlier [16].
Data regarding time, a variety of quality indicators and complications were collected prospectively in an institutional database. Perioperative data (removed lobe(s), procedure time, blood loss and possible complications) and baseline patient data (age, gender, lung function, preoperative stage group [18] and co-morbidity) were entered in the database immediately after the completion of each operation. The single chest tube was removed when there was no air leakage, and the patient was discharged when he or she was fully mobilized. Days with chest tube, length of hospital stay and possible postoperative complications were registered upon discharge, and the database was double-checked when the patient returned to the out-patient clinic approximately 2 weeks later. The registration of all major VATS procedures at the centre allowed for comparisons between the trainee and the expert consultants.
Statistical analysis
Characteristics of the patients operated on by the trainee and the experts were compared using the independent samples t-test (for age and lung function), Pearson's chi-square test (for gender) and Fisher's exact test (for preoperative stage group and removed lobe(s)). Independent samples t-tests were used to compare the procedures performed by the trainee and the experts. The following parameters were compared: procedure time, perioperative bleeding, days with chest tube and days of admittance. To explore a possible development in procedure time and perioperative bleeding as the trainee progressed along the learning curve, they were plotted against procedure number and the correlations were calculated using Spearman's rho. Per- and postoperative complications were merely reported as the limited number of procedures did not allow a meaningful statistical comparison with the register data.
Statistical analysis was performed using a statistical software package (PASW, version 18.0; SPSS Inc.; Chicago, IL, USA). Differences were considered to be statistically significant when the P-value was <0.05.
Ethics
All procedures were performed as part of normal clinical practice and no ethical approval was needed.
RESULTS
In the preparatory phase, the trainee practiced on pigs and simulators, performed more than 100 minor VATS procedures and observed more than 100 VATS lobectomies. Traditional open lung surgery was not a part of the training programme, and the trainee had performed only 14 major lung resections through open thoracotomies before performing the first VATS lobectomy: six right upper lobes, three middle lobes, one right lower lobe, two left upper lobes, two left lower lobes and two pneumonectomies. No additional thoracotomies were performed during the performing phase of the training programme.
Over 12 months, the trainee performed 29 VATS lobectomies and the two expert consultants performed 185 VATS lobectomies. Table 1 shows the patient characteristics of these patients (age, gender, lung function, preoperative stage group, pathological diagnosis and the removed lobe(s)), and Table 2 shows the surgical outcomes (procedure time, perioperative bleeding, days with chest tube and days of admittance).
Table 1:
Patient characteristics of the 214 patients who had a lobectomy performed by VATS either by a trainee (n = 29) or by an expert (n = 185)
| Operated by trainee (n = 29) | Operated by experts (n = 185) | |
|---|---|---|
| Age [years; median (range)] | 67 (44–82) | 66 (19–90) |
| Gender [n (%)] | ||
| Female | 22 (76) | 97 (52) |
| Male | 7 (24) | 88 (48) |
| FEV1 [l; mean (SD)] | 2.3 (0.72) | 2.3 (0.72) |
| FEV1 in percentage of predicted value [%; mean (SD)] | 90.3 (16.6) | 82.1 (17.7) |
| Stage group [n (%)] | ||
| IA | 12 (48) | 60 (38) |
| IB | 10 (40) | 74 (46) |
| IIA | 2 (8) | 15 (9) |
| IIB | 1 (4) | 7 (4) |
| IIIA | 0 | 4 (3) |
| Pathology [n (%)] | ||
| Adenocarcinoma | 18 (62) | 99 (54) |
| Squamous cell carcinoma | 6 (21) | 40 (22) |
| Other lung cancera | 1 (3) | 21 (11) |
| Lung metastasisb | 3 (10) | 15 (8) |
| Benignc | 1 (3) | 10 (5) |
| Removed lobe(s) [n (%)] | ||
| RUL | 7 (24) | 73 (40) |
| ML | 3 (10) | 13 (7) |
| RLL | 8 (28) | 25 (14) |
| LUL | 3 (10) | 46 (25) |
| LLL | 7 (24) | 24 (13) |
| Upper bi-lobectomy | 0 | 3 (2) |
| Lower bi-lobectomy | 1 (3) | 1 (1) |
The table shows the age, gender, FEV1, FEV1 in percentage of predicted value, preoperative stage group, pathology and the removed lobe(s).
RUL: right upper lobe; ML: middle lobe; RLL: right lower lobe; LUL: left upper lobe; LLL: left lower lobe; FEV1: forced expiratory volume in 1 s.
aLarge cell carcinoma, small cell carcinoma, bronchioloalveolar carcinoma and carcinoid tumour.
bMetastases from colon cancer, malignant melanoma, sarcoma, mamma cancer and kidney cancer.
cBronchial ectasia, hamartoma, middle lobe syndrome, fibrosis and pneumonia.
Table 2:
Characteristics of 29 VATS lobectomies performed by a trainee and 185 performed by two experts during the same 12 months
| Trainee | Experts | |
|---|---|---|
| Procedure data | ||
| Time | median 120 min (74–160 min) | median 100 min (42–255 min) |
| Perioperative bleeding | median 100 ml (10–500 ml) | median 50 ml (5–2500 ml) |
| Postoperative data | ||
| Days with chest tube | median 1 day (1–6 days) | median 2 days (1–32 days) |
| Days of admittance | median 3 days (1–10 days) | median 4 days (1–41 days) |
The patients operated on by the trainee and the experts were of equal age, mean 66.6 years (standard deviation [SD] = 9.6 years) and mean 64.7 years (SD 11.2 years), respectively, P = 0.39. However, the trainee operated on a significantly larger proportion of women than the experts, 76 and 52%, respectively, P = 0.026. The preoperative stage group of the patients operated on by the trainee and the experts was not significantly different, P = 0.93, but their lung function was better, mean forced expiratory volume in 1 s in percentage of predicted value 90 vs 82%, P = 0.021, and the trainee removed a larger proportion of lower lobes and middle lobes than the experts, left lower lobe 24 vs 13%, right lower lobe 28 vs 14% and middle lobe 10 vs 7%, P = 0.039.
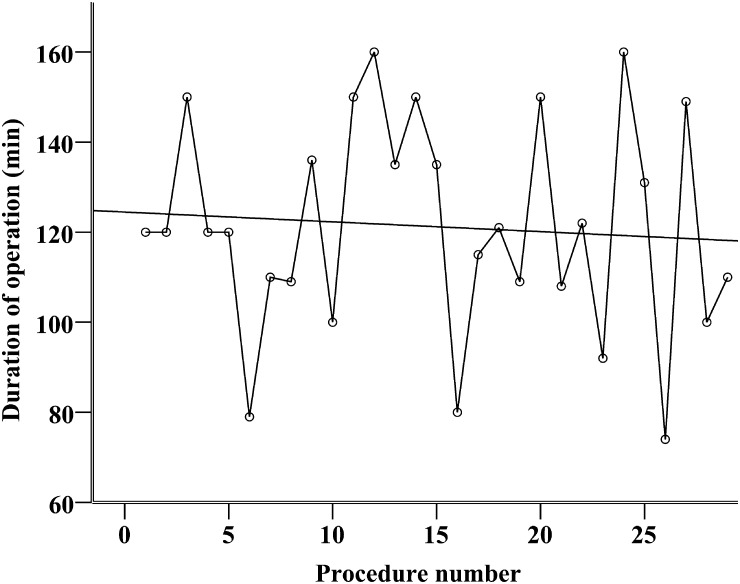
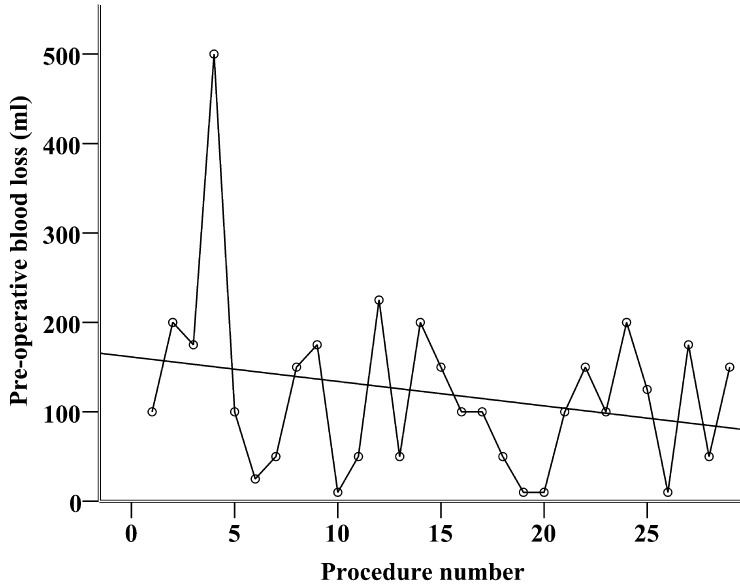
The trainee operated significantly slower than the experts [median 120 (range 74–160 min) vs 100 min (range 42–255 min), P = 0.04], but perioperative bleeding was not significantly different [median 100 (range 10–500 ml) vs 50 ml (range 5–2500 ml), P = 0.79]. The patients operated on by the trainee had the chest tube removed earlier: median 1 (range 1–6) vs 2 (range 1–32) postoperative days, P < 0.001, and spent fewer days in the hospital: median 3 (range 1–10) vs 4 (range 1–41) days, P < 0.001. As shown in Figs. 1 and 2, there was no development in procedure time or perioperative bleeding during the first 29 operations performed by the trainee. Spearman's rho was −0.09 and −0.16, respectively, and these correlations were not statistically significant, P = 0.64 and 0.42, respectively. The perioperative performance of the trainee was characterized by huge variance from case to case and failed to reach a plateau phase.
Figure 1:
Scatter dot showing the duration (in minutes) of the first 29 VATS lobectomies performed by a trainee. Interpolation line shows a non-significant development (Spearman's rho = −0.09, P = 0.64).
Figure 2:
Scatter dot showing the perioperative blood loss (in ml) of the first 29 VATS lobectomies performed by a trainee. Interpolation line shows a non-significant development (Spearman's rho = −0.16, P = 0.42).
None of the operations performed by the trainee were converted to open thoracotomy, but twice the supervisor had to finish the procedure due to technical difficulties (tumour adherent to middle lobe bronchus and damage to pulmonary vein branch, respectively). Twenty-three (79%) patients had no postoperative complications, while 2 had atrial fibrillation. Pneumothorax after chest tube removal, incisional infection, prolonged pain and need for pleuracentesis were each seen once.
DISCUSSION
VATS lobectomy is a complex procedure with a shallow learning curve, but the trainee in this study managed to achieve good results during the initial part of his training. The trainee needed significantly longer time to finish the procedure than the experts, which is consisting with earlier findings [15], and not surprising as rapid task completion is a recognized feature of expert performance [19]. However, the median procedure time was only 20 min longer and that must be considered acceptable even in today's busy operating theatres. The other quality indicators achieved by the trainee were either equal to (perioperative blood loss) or better (days with chest tube and length of stay) than the experts'. Studies describing initial experiences with major VATS resections in single centres have reported conversion rates between 5 and 20% [12–14], but in our small series of 29 cases, it was not necessary to convert to open thoracotomy at all. Unplanned conversion prolongs the operating time and hospital stay, but does not prejudice short-term or long-term surgical outcomes when compared with scheduled open thoracotomy [20]. Conversion is not a failure, and fear of conversion should not prevent eligible procedures to be performed by VATS. The trainee's patients had no intra- or perioperative mortality, but 21% had minor complications. Two expert centres in the United States have reported complication rates of 15.3 and 23.8%, respectively [21, 22], and even though comparison with these large series should be done with caution, we believe that the frequency and type of complications in the 29 patients in this study were definitely satisfactory.
Several factors could explain the positive results above. The trainee was inexperienced in open thoracotomy but had performed many basic VATS procedures and had seen a lot of VATS lobectomies before entering the VATS lobectomy training programme. Furthermore, the trainee had the opportunity to practice on live pigs and in black-box simulators. The effect of simulation-based training in VATS lobectomy has not been explored, but construct validity has been established for the cadaver-model used at Duke University by showing the ability to discriminate between operators of different skill levels [17]. The evidence for positive effects of simulation-based training in general is good, and based on our experience, we would recommend this training as one way of ensuring basic thoracoscopy competency before undertaking VATS lobectomies.
Other deciding factors for the good results are the presence of the expert supervisors and the standardized operative approach. The trainee in our study did not have to learn from his own mistakes, search for appropriate instruments or develop new techniques during his learning phase. In a dedicated, high-volume VATS centre, the whole team surrounding the surgeon is accustomed to the minimally invasive approach, and when a trainee encounters difficulties the supervising expert is able to help overcome these and avoid conversion to open thoracotomy. An analysis of a national American database found that a high hospital VATS/total lobectomy ratio was associated with fewer total complications and shorter length of stay, and we support the conclusion that experienced VATS centres may be recommended [23].
The high volume of VATS lobectomies also allowed for a selection of suitable patients and procedures. Removal of the middle lobe and the lower lobes is technically easier, and the trainee in our study had to complete 10 of these operations before advancing to upper lobe lobectomies. This approach helped to successfully overcome the first and steepest part of the learning curve. As the trainee improved, he was exposed to more difficult procedures. This could explain why there was no significant improvement in procedure time and blood loss during the first 29 procedures (Figs. 1 and 2). The figures also show highly variable performance even after 20 procedures, which indicate that more procedures are needed to reach the plateau of the learning curve. According to the classic three-stage learning model presented by Fitts and Posner, the highly variable performance is a trademark of the first, ‘cognitive’, stage, whereas experienced people in the third, ‘autonomous’, stage perform consistently well [19]. VATS lobectomy is a complicated procedure requiring prolonged deliberate practice to master, and it is not surprising that the learning curves showed that the trainee had not reached expert performance level after 29 procedures.
During the entire training period, patients with severe co-morbidity, inferior lung function and increased risk of prolonged postoperative recovery were operated on by the experts. This selection explains why the mean number of days with chest tube and days of admittance were higher than in patients operated by the trainee.
In line with the European Working Time Directive, a 48-h work week for doctors in training was enforced in August 2009, and in the United States, the current 80-h work week has made experienced surgeons wonder, whether the new duty hours restrictions provide sufficient time to reach the level of proficiency necessary to support the safe, independent practice of medicine. There is no doubt that shorter work weeks increase the need for proactive, focused training and specialization. Currently, many educational programmes have difficulty in providing appropriate training in VATS lobectomy, and in a recent questionnaire among early career thoracic surgeons in the United States, only 58% of the responders considered themselves skilled in VATS lobectomies [24]. This correlates well with an earlier finding that only 55% of thoracic surgery residents felt that their residency provided appropriate training for VATS lobectomy, whereas 92% felt that this was true for open lobectomy [24]. This will probably change in the future when a larger proportion of major lung resections will be performed by VATS, making it more difficult for the trainees to obtain experience in open thoracotomy. Our data showed that experience in open surgery is not a prerequisite for learning VATS lobectomies, and VATS training can be introduced early in the education of thoracic surgeons. In our supervised setting, this was done with no mortality or major morbidity. However, when trainees start to operate unsupervised, they must be able to perform a quick conversion to open thoracotomy and control the situation in case of a catastrophic intraoperative complication. Fortunately, these only occur in about 1% of the cases [25], but as the consequences can be fatal, focus needs to be maintained on preventing and dealing with them. Future studies on large series should address this issue and how training programmes can be tailored to meeting these challenges.
The prospective design and complete follow-up in the out-patient clinic ensured good data quality and minimized underreporting of complications, which added strength to the study. The major limitation was the small number of patients operated on by a single trainee. Caution has to be used before generalizing these experiences to other centres with different volumes, techniques, etc.
Conclusions
With thorough preparation of trainees and training on selected patients under close supervision, the learning curve for VATS lobectomies can be overcome with good results even if the trainee has limited prior experience in open surgery.
Conflict of interest: René Petersen and Henrik Hansen are consultants for Covidien VATS MasterClass in Elancourt, France.
REFERENCES
- 1.Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy–video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg. 1995;109:997–1001. doi: 10.1016/S0022-5223(95)70326-8. [DOI] [PubMed] [Google Scholar]
- 2.Whitson BA, Andrade RS, Boettcher A, Bardales R, Kratzke RA, Dahlberg PS, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–70. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Handy JR, Jr, Asaph JW, Douville EC, Ott GY, Grunkemeier GL, Wu Y. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg. 2010;37:451–5. doi: 10.1016/j.ejcts.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RP, Pham D, Burfeind WR, Hanish SI, Toloza EM, Harpole DH, Jr, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007;83:1245–9. doi: 10.1016/j.athoracsur.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Nicastri DG, Wisnivesky JP, Litle VR, Yun J, Chin C, Dembitzer FR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008;135:642–7. doi: 10.1016/j.jtcvs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–16. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: can we afford it? Eur J Cardiothorac Surg. 2009;35:423–8. doi: 10.1016/j.ejcts.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Burfeind WR, Jr, Jaik NP, Villamizar N, Toloza EM, Harpole DH, Jr, D'Amico TA. A cost-minimisation analysis of lobectomy: thoracoscopic versus posterolateral thoracotomy. Eur J Cardiothorac Surg. 2010;37:827–32. doi: 10.1016/j.ejcts.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Swanson SJ, Meyers BF, Gunnarsson CL, Moore M, Howington JA, Maddaus MA, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg. 2012;93:1027–32. doi: 10.1016/j.athoracsur.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 10.McKenna RJ, Jr, Houck WV. New approaches to the minimally invasive treatment of lung cancer. Curr Opin Pulm Med. 2005;11:282–6. doi: 10.1097/01.mcp.0000166589.08880.44. [DOI] [PubMed] [Google Scholar]
- 11.Swanson SJ, Herndon JE, D'Amico TA, Demmy TL, McKenna RJ, Jr, Green MR, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802–a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–7. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez D, de la Torre M, Paradela M, Fernandez R, Delgado M, Garcia J, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg. 2011;40:e21–8. doi: 10.1016/j.ejcts.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 13.Amer K, Khan AZ, Vohra HA. Video-assisted thoracic surgery of major pulmonary resections for lung cancer: the Southampton experience. Eur J Cardiothorac Surg. 2011;39:173–9. doi: 10.1016/j.ejcts.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Belgers EH, Siebenga J, Bosch AM, van Haren EH, Bollen EC. Complete video-assisted thoracoscopic surgery lobectomy and its learning curve. A single center study introducing the technique in The Netherlands. Interact CardioVasc Thorac Surg. 2010;10:176–80. doi: 10.1510/icvts.2009.212878. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg. 2010;37:516–20. doi: 10.1016/j.ejcts.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc. 2011;25:1263–9. doi: 10.1007/s00464-010-1355-9. [DOI] [PubMed] [Google Scholar]
- 17.Tong BC, Gustafson MR, Balderson SS, D'Amico TA, Meyerson SL. Validation of a thoracoscopic lobectomy simulator. Eur J Cardiothorac Surg. 2012;42:364–9. doi: 10.1093/ejcts/ezs012. [DOI] [PubMed] [Google Scholar]
- 18.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–71. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 19.Magill RA. The Stages of Learning. Motor Learning and Control. 8th edn. New York: McGraw-Hill; 2007. pp. 263–89. [Google Scholar]
- 20.Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes? Ann Thorac Surg. 2008;86:235–9. doi: 10.1016/j.athoracsur.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 21.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–5. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 22.Onaitis MW, Petersen RP, Balderson SS, Toloza E, Burfeind WR, Harpole DH, Jr, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg. 2006;244:420–5. doi: 10.1097/01.sla.0000234892.79056.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HS, Detterbeck FC, Boffa DJ, Kim AW. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg. 2012;93:372–9. doi: 10.1016/j.athoracsur.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Boffa DJ, Gangadharan S, Kent M, Kerendi F, Onaitis M, Verrier E, et al. Self-perceived video-assisted thoracic surgery lobectomy proficiency by recent graduates of North American thoracic residencies. Interact CardioVasc Thorac Surg. 2012;14:797–800. doi: 10.1093/icvts/ivr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores RM, Ihekweazu U, Dycoco J, Rizk NP, Rusch VW, Bains MS, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg. 2011;142:1412–7. doi: 10.1016/j.jtcvs.2011.09.028. [DOI] [PubMed] [Google Scholar]