Abstract
OBJECTIVES
Sternal resection is indicated for a variety of pathological conditions, mainly neoplastic or related to sternotomy complications. Resection of the sternum generally leaves a large chest-wall defect, and reconstruction is thus the most difficult part of the operation. Correct stabilization of the anterior chest wall is very important to avoid secondary complications and respiratory failure. In the last few years, different technical solutions have been used to reconstruct the sternum. We describe our technique using a sternal allograft to reconstruct the anterior chest wall after partial or complete sternal resection.
METHODS
Between June 2010 and February 2012, four patients underwent sternectomy followed by anterior chest wall reconstruction using sternal allograft. The sternal allograft was harvested from a multitissue donor following Italian legislation for tissue donation. Three patients had neoplastic involvement of the sternum, and one had a complete sternal defect as a complication of a cardiac operation.
RESULTS
We had no operative mortality. Three patients underwent partial sternal transplantation, and one underwent total sternal replacement. We had no postoperative respiratory insufficiency, infections or mechanical failure of the reconstructions. The respiratory function was preserved in all patients. The follow-up period was free from complications related to the sternal allograft implantation.
CONCLUSIONS
The technique of sternal allograft transplantation is simple, reproducible and provides excellent functional and cosmetic results. Further studies including a larger number of patients are needed to understand the biology of the allograft and the long-term results of this technique.
Keywords: Sterum, Chest wall, Sternal reconstruction, Bone allograft, Transplantation
INTRODUCTION
Sternal resection is performed for a variety of pathologies such as primary or secondary tumours, infections, trauma, radio-necrosis or mechanical dehiscence after sternotomy [1].The most challenging part of the operation is the reconstruction of the anterior chest wall in order to avoid secondary ventilatory impairment and protect the mediastinal organs [2, 3]. In the last few years, different techniques and materials have been described to reconstruct the sternum [1–6]. We propose a simple technique using sternal allograft (SA) and titanium bars to reconstruct the anterior chest wall after partial or complete sternal resection.
MATERIALS AND METHODS
Between June 2010 and February 2012, four patients underwent sternectomy followed by anterior chest wall reconstruction using SA. The preoperative characteristics of the patients are reported in Table 1. The SA was harvested from a multi-tissue donor following Italian legislation for tissue donation. The graft underwent washing immersion in sterile saline solution with antibiotics for 72 h. After packaging, the allograft was irradiated and then stored at −80°C. The day before surgery, the graft underwent defrosting at 4–6°C for 12 h, then under sterile conditions, it was immersed in a 0.9% NaCl solution with antibiotics and stored at 4–6°C until its use. The patients were positioned supine with the neck hyperextended. In the case of neoplastic disease confined between the cortical layers of the sternum or in the case of sternotomy complications, a midline skin incision was performed. In the case of neoplastic disease involving the bone and subcutaneous/cutaneous tissue, a circular skin incision, leaving at least 4 cm resection margins, was performed. The pectoralis-muscles were prepared to be used as muscle flap. The intercostal spaces were dissected extensively as far as the anterior axillary line exposing the antero-lateral rib arches. A full-thickness partial or complete resection of the sternum en-bloc with costal cartilage was performed (Fig. 1A and B). Before implantation, the SA was completely defrosted and any residual soft tissue was removed (Fig. 1C and D). The SA was then tailored using an oscillating saw to perfectly fit with the chest-wall defect (Fig. 2A) (Supplementary Video 1). After modelling the titanium bars to the best fitting shape, they were fixed to the allograft and rib stumps with locking screws (Synthes®, Solothurn, Switzerland). Usually, we use two screws on the sternum side of the bar and at least three screws for each rib side (Fig. 2B and C). It is critical to fix the screws on the bone part of the ribs as distally as possible. If the screws are wrongly fixed on the chondral segment of the ribs, mechanical failure of the implantation will develop. After partial sternectomy, the SA was fixed to the recipient sternum with an H-shaped titanium plate (Supplementary Video 2). A detailed description of the implantation technique of this titanium system has been previously published [5]. In the case of total or upper sternal replacement, the sterno-clavicular joint was reconstructed and stabilized. After making some holes in the clavicle heads and the new manubrium, hi-tension polyethylene sutures (Hi-Fi, Conmed, Linvatec) were passed between them and tied, recreating a new sterno-clavicle joint. Whenever possible, the allograft was covered with pectoralis muscle flap (Fig. 2D). The surgical results and postoperative outcomes of our series are reported in Table 1. The three patients who underwent partial sternectomy did not have impairment of respiratory function. The preoperative forced expiratory volume in 1 s (FEV1) of the patient who underwent total sternal replacement was 65% of the predicted (1.72 l). Ten days after the operation, the FEV1 improved to 75% (1.94 l). The mean follow-up time of our patients was 9.7 ± 7.3 months. Until now no mechanical failure or reconstruction related complications have been reported.
Table 1:
Preoperative patient profiles and operative results
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 39 | 59 | 58 | 68 |
| Gender | Female | Female | Female | Male |
| Pathology | Single metastases from ovarian cancer | Breast cancer | Breast cancer | Sternotomy complication |
| Location | Manubrium sterni | Sternal body | Manubrium sterni | Massive sternal defect |
| Comorbidity | None | Hypertension | None | Diabetes, obesity, hypertension, COPD |
| Previous operations | Bilateral oophorectomy, hysterectomy, pelvic posterior exenteration, recto-sigmoidectomy | Mammary right upper and left upper quadrantectomy | Left mastectomy | CABG (BIMA), failed thoracic refixation (Robicsek) |
| Type of resection | Partial upper sternectomy | Partial lower sternectomy | Partial upper sternectomy | Total sternal replacement |
| Associated procedures | Sterno calvicular joint reconstruction | Right mastectomy | Sterno calvicular joint reconstruction, resection of left major pectoralis muscle | Sterno calvicular joint reconstruction |
| Type of muscular flap | Bilateral advancement pectoralis major flap | Bilateral advancement pectoralis major flap | Right pectoralis major rotation flap | Bilateral advancement pectoralis major flap |
| Postoperative complications | None | Pleural effusion | None | Thoracic seroma |
| Operating time (minutes) | 243 | 167 | 183 | 134 |
| Ventilatory time (hours) | 2 | 1 | 2 | 5 |
| Hospital stay (days) | 12 | 9 | 14 | 8 |
COPD: chronic obstructive pulmonary disease; CABG: coronary artery bypass grafting; BIMA: bilateral internal mammary artery.
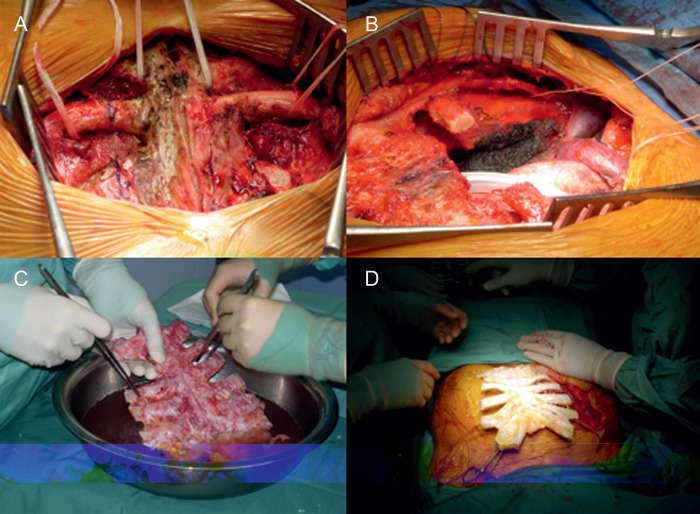
Figure 1:
(A) Operative view after complete dissection of the upper part of the sternum, (B) an example of chest-wall defect after partial upper sternectomy, (C) the SA was cleaned from the residual soft tissues and (D) the SA is ready for the implantation.
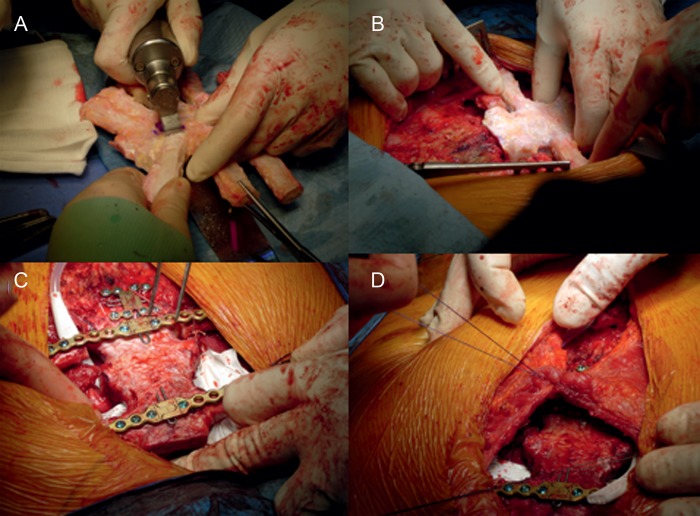
Figure 2:
(A) Tailoring of the SA using the oscillating saw, (B) the positioning of the SA to fill the chest defect after tailoring, (C) the SA fixed in place using titanium bars, plate and screws and (D) the advancement major pectoralis muscle flap was used to cover the SA.
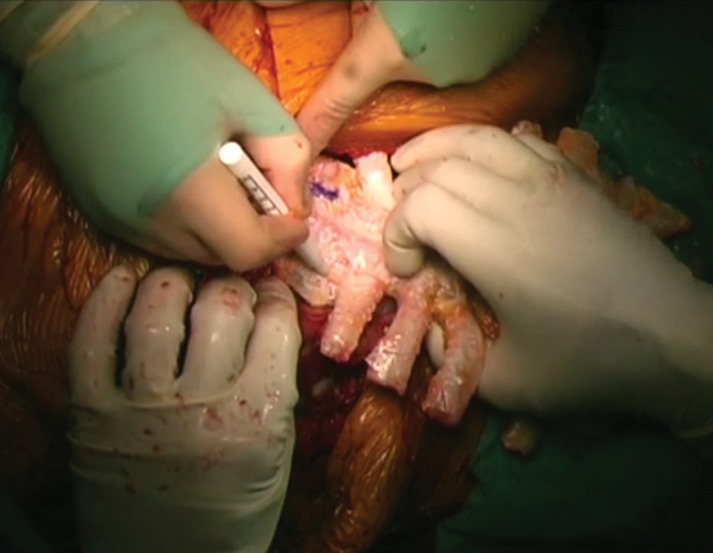
Supplementary Video 1: In a separate table, a second surgical team prepares the sternal allograft for the implantation. The allograft was completely defrosted and any residual soft tissues were removed. The SA was then tailored using an oscillating saw to perfectly fit with the chest-wall defect.
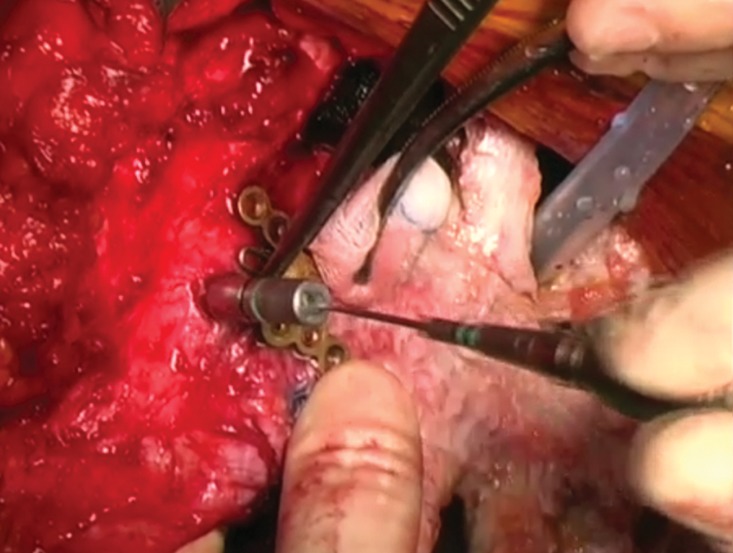
Supplementary Video 2: After modelling the titanium bars to the best fitting shape following the template, they were fixed to the allograft and rib stumps with locking screws. A drill was used to making the holes for the screws. In case of partial sternectomy, the allograft was fixed to the recipient sternum with an H-shaped or star-shaped titanium plate. The allograft was then covered with a major advancement pectoralis muscle flap, that it was prepared at the beginning of the surgical procedure.
COMMENT
Many surgical techniques have been described over the years, involving different materials for sternal replacement and anterior chest wall reconstruction, but none of them is considered the gold-standard procedure [2, 7]. The most used were methylmethacrilate, polythetrafluoroethylene, marlex-mesh, metallic-plates, bone-homograft and musculocutaneous flaps. The clinical application of these materials has both potential advantages and drawbacks [3, 4, 7, 8]. Synthetic materials have some disadvantages such as excessive rigidity, the risk of erosion of the adjacent structures, the risk of infection, insufficient strength, rupture, migration, immunological reaction and impossibility of incorporation into the host tissue [4, 8]. Bone-autografts have optimal biomechanical characteristics and are fully compatible. Bone grafts also act as a scaffold for osteoprogenitor cells and bone formation [4, 7, 8]. The main disadvantage is that in the case of a large defect, the use of autologous bone would involve complex harvesting and additional aesthetic and functional insult in other body areas. Bone-allografts have the same advantage as bone-autografts in terms of infection risk, compatibility and host tissue incorporation, but they do not require additional incision or tissue removal for harvesting, and they are also readily available from tissue-banks [4, 8]. At the same time, SAs have bone-induction, activated by bone-morphogenic-proteins and act as a three-dimensional scaffold for new angiogenesis and bone formation [4]. Recently, some authors [4, 7, 8] have used bone-allografts to replace the sternum. Until now, no complications related to the bone-allograft have been reported during the follow-up period, confirming the optimal biocompatibility and mechanical characteristics of this material. In all cases the allograft was fixed to the thoracic wall using titanium devices. Titanium is extremely biocompatible, malleable, has a low density (lightweight), a high thermal and mechanical resistance and is magnetic-resonance compatible with minimal diffraction and artefacts during computed tomography. We had no postoperative respiratory insufficiency, infections or mechanical failure of the reconstructions.
In conclusion, the technique of SA transplantation is simple and reproducible and provides excellent functional and cosmetic results. Further studies including a larger number of patients are needed to understand the biology of the allograft and the long-term results of this technique.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
REFERENCES
- 1.Arnold PG, Pailorelo PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804–10. doi: 10.1097/00006534-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Incarbone M, Nava M, Lequaglie C, Ravasi G, Pastorino U. Sternal resection for primary or secondary tumors. J Thorac Cardiovasc Surg. 1997;114:93–9. doi: 10.1016/S0022-5223(97)70121-1. [DOI] [PubMed] [Google Scholar]
- 3.Weyant MJ, Bains MSD, Venkatraman E, Downey RJ, Park BJ, Flores RM, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg. 2006;81:279–85. doi: 10.1016/j.athoracsur.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Marulli G, Hamad AM, Cogliati E, Breda C, Zuin A, Rea F. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J Thorac Cardiovasc Surg. 2010;139:e69–70. doi: 10.1016/j.jtcvs.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Voss B, Bauernschmitt R, Will A, Krane M, Kross R, Brockmann G, et al. Sternal reconstruction with titanium plates in complicated sternal dehiscence. Eur J Cardiothorac Surg. 2008;34:139–45. doi: 10.1016/j.ejcts.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Chapelier AR, Missana MC, Couturaud B, Fadel E, Fabre D, Mussot S, et al. Sternal resection and reconstruction for primary malignant tumors. Ann Thorac Surg. 2004;77:1001–7. doi: 10.1016/j.athoracsur.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Rocco G. Overview on current and future materials for chest wall reconstruction. Thorac Surg Clin. 2010;20:559–62. doi: 10.1016/j.thorsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Stella F, Dell'Amore A, Dolci G, Cassanelli N, Caroli G, Zamagni C, et al. Allogenic sternal transplant after sternectomy for metastasis of ovarian carcinoma. Ann Thorac Surg. 2012;93:e71–2. doi: 10.1016/j.athoracsur.2011.10.004. [DOI] [PubMed] [Google Scholar]