Abstract
OBJECTIVES
The purpose of this study was to compare the clinical outcomes of induction chemoradiotherapy and chemotherapy and to identify the prognostic factors for non-small-cell lung cancer patients with mediastinal lymph node metastasis who were treated with induction therapy.
METHODS
Between August 1995 and December 2010, 50 non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis were scheduled to receive induction therapy followed by surgery. Irinotecan plus cisplatin was used for induction chemotherapy from June 1995 to April 1999, and docetaxel plus cisplatin with concurrent radiation at a dose of 40–46 Gy has been used for induction chemoradiotherapy since May 1999.
RESULTS
Thirty-five patients were treated with induction chemoradiotherapy and 15 were treated with induction chemotherapy. For the entire population, the 3-year and 5-year overall survival rates were 64.1 and 53.9%, respectively, and the 1-year and 2-year disease-free survival rates were 70.0 and 53.1%, respectively. Among the clinicopathological factors, the chemoradiotherapy group exhibited longer overall survival and disease-free survival than the chemotherapy group (overall survival, P = 0.0020; disease-free survival, P = 0.015). Pathological downstaging was also significantly associated with favorable overall survival (P = 0.0042) and disease-free survival (P = 0.021). A multivariate analysis showed that chemoradiotherapy (P = 0.0099) and pathological downstaging (P = 0.039) were independent prognostic factors.
CONCLUSIONS
Our results indicated that induction chemoradiotherapy was superior to induction chemotherapy with regard to the outcome of non-small-cell lung cancer patients with mediastinal lymph node metastasis.
Keywords: Lung cancer, Induction therapy, Chemoradiotherapy, Chemotherapy
INTRODUCTION
Surgical resection is the first therapeutic option for the control of local disease in patients with non-small-cell lung cancer (NSCLC). Locally advanced disease status is associated with a possibility of micrometastasis to distant sites, which is often the cause of disease recurrence, typically resulting in a poor outcome. In this situation, surgery does not contribute to a disease cure. Mediastinal lymph node metastasis of NSCLC without clinical distant metastasis is one of the categories of locally advanced disease for which the prognosis remains unsatisfactory. NSCLC patients with mediastinal lymph node metastasis form a heterogeneous population, ranging from unresectable N stage with a tumor mass that was either not discrete or unmeasurable to resectable N stage with a single node with a short-axis diameter of 1 cm on a transverse computer tomography (CT) scan image [1]. Thus, the clinical manifestations and treatment option for N2 disease also exhibit substantial heterogeneity. Indeed, numerous clinical trials including various combinations of chemotherapy with or without radiotherapy followed by surgery or definitive chemoradiotherapy have been adapted to establish an appropriate strategy for patients with mediastinal lymph node metastasis [2–8]. The two recent studies failed to demonstrate a benefit from the addition of surgery in the entire population [2–8]. However, in the subset analysis of the intergroup trial 0139 for patients who underwent a lobectomy vs a matched subset undergoing chemoradiotherapy, the surgical group showed a significantly more favorable survival rate. This result strongly suggests the possible advantage of surgical resection after induction chemoradiotherapy for a select population of patients with N2 disease [2–8]. Regarding the comparison between induction chemoradiotherapy and chemotherapy, no prospective randomized studies have been reported. As retrospective study, only Higgins and colleagues reported the outcome: induction chemoradiotherapy was associated with a higher rate of mediastinal downstaging but not an improvement in overall survival (OS) [9].
We have been using cisplatin-based induction therapy for the treatment of NSCLC patients with locally advanced diseases such as N2/3 and T3/4 diseases since 1995 [10–13]. In this study, we compared the clinical outcome of induction chemoradiotherapy and chemotherapy and investigated prognostic factors for NSCLC patients with mediastinal lymph node metastasis who were treated with induction therapy.
MATERIAL AND METHODS
Patient selection and evaluation
Between July 1995 and December 2010, a total of 86 NSCLC patients with clinical N2/3 disease were scheduled for treatment with induction therapy followed by surgery at Okayama University Hospital. Mediastinal lymph node metastasis was pathologically confirmed in 50 patients prior to induction therapy and they were the subjects of this study. Thirty-six patients who were not examined for mediastinal nodal metastasis were excluded from this study to avoid including false positive N2/3 cases. Among 50 patients, the outcome of 37 patients was reported in our previous reports and they were prospectively treated with induction chemotherapy and chemoradiotherapy [10, 11]. Briefly, previously untreated NSCLC patients with pathologically confirmed mediastinal nodal metastasis were eligible for enrollment in those studies. Patient information was shared at a meeting among pulmonary oncologists, radiation oncologists and general thoracic surgeons to determine whether induction therapy was indicated for the treatment of individual patients with locally advanced NSCLC. When mediastinal lymph node metastasis was suspected based on the findings of a chest CT or an 18-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET)/CT scan, a cervical mediastinoscopy or endobronchial ultrasound-guided transbronchial biopsy (EBUS) was performed to evaluate stations 2, 4 and 7. An anterior mediastinoscopy was also performed when metastasis was suspected at stations 5 or 6.
The patient inclusion criteria were an age of 75 years or younger, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 [14], and adequate organ functional reserves, as described previously [10, 11]. Written informed consent was obtained from all the patients. The study protocol was approved by the Institutional Review Board/Ethical Committee of Okayama University. The disease stage was evaluated using chest radiography, enhanced chest and abdominal CT scans, including the adrenal glands, enhanced brain magnetic resonance imaging (MRI), a radionuclide bone scan or an FDG PET/CT scan and bronchoscopy. The International Association of the Study of Lung Cancer TNM staging system for NSCLC, seventh edition, was used to determine the disease staging and nodal location [15].
Treatment plans
The details of the treatment regimens were described in our previous study [10, 11]. Irinotecan plus cisplatin was used for induction chemotherapy from June 1995 to April 1999, and docetaxel plus cisplatin with concurrent radiation at a dose of 40 Gy (1999–2000)–46 Gy (2000–10) has been used for induction chemoradiotherapy since May 1999. Following the induction therapy, patient response was evaluated based on a chest radiograph and CT scans. Patients without progressive disease were, in principle, scheduled to undergo surgery within 6 weeks of the completion of the induction therapy, as described previously. Briefly, the surgical procedure was determined based on the disease extent before induction treatment. While a posterolateral thoracotomy was used as the basic approach, a median sternotomy was applied for patients with contralateral mediastinal lymph node metastasis or when great vessels, such as the main pulmonary artery, needed to be secured for a safe resection. A lobectomy with mediastinal lymph nodal dissection was basically the resection of first choice; however, a bilobectomy or pneumonectomy was performed in cases requiring these procedures because of disease extension [12]. A sleeve resection was preferred to avoid a pneumonectomy, if appropriate. A complete ipsilateral superior mediastinal and subcarinal lymphadenectomy was performed in all cases. For patients with primary lower lobe lesions, stations 8 and 9 lymph nodes were also resected. Patients with primary left pulmonary lesions also underwent the resection of stations 5 and 6 lymph nodes. The bronchial stump was covered with pericardial fat tissue or pedicled intercostal muscle. When a sleeve resection was performed, the greater omentum was, in principle, used to wrap the anastomosis. Post-operative adjuvant treatment was left to the physician's discretion.
Survival and statistical analysis
After completion of scheduled therapy, chest and abdominal CT and enhanced brain MRI were repeated every 3 months for at least 2 years. During 3–5 years after completion, chest and abdominal CT and enhanced brain MRI were repeated every 6 months. A radionuclide bone scan or PET-CT was performed if necessary. After 5 years, chest X-ray was repeated every year and further image analyses were performed if necessary. The OS and the disease-free survival (DFS) were calculated from the date of initiation of induction therapy until the date of death or the last follow-up for OS and until confirmed disease recurrence or death for DFS. The survival curve was calculated by the Kaplan–Meier method and the difference between groups was compared with the log-rank test. A multivariate analysis was performed using the Cox proportional hazard model. Fisher's exact tests were applied to examine differences in categorical factors across groups. All data were analyzed usingJMP® 9.0.0 Program for Windows (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided and probability values <0.05 were defined as being statistically significant.
RESULTS
Patient characteristics
Fifty NSCLC patients with pathological mediastinal lymph node metastasis were the subjects of this study. Thirty-five patients were treated with induction chemoradiotherapy and 15 were treated with induction chemotherapy. The patient characteristics were as shown in Table 1. The median patient age was 59 years (range: 31–74 years). There were 37 men and 13 women in the series. The histological subtype was adenocarcinoma in 26 patients, squamous cell carcinoma in 18, large cell carcinoma in 4 and adenosquamous carcinoma in 2. Forty-five patients had stage IIIA disease, and 5 had stage IIIB disease. Forty-six patients underwent a mediastinoscopy, and 4 underwent an endobronchial EBUS. Patients who had bulky or extra-nodal N3 diseases were not enrolled. There was no significant difference in clinicopathological factors between the chemoradiotherapy group and chemotherapy group (Table 1).
Table 1:
Patient characteristics
| Characteristics | Total | ChRT | ChT | P-value |
|---|---|---|---|---|
| Median and range of age (years) | 59.8 (31–74) | 59 (31–74) | 59 (34–74) | |
| Sex | ||||
| Male | 37 | 24 | 13 | 0.29 |
| Female | 13 | 11 | 2 | |
| Histological subtypes | ||||
| Adenocarcinoma | 26 | 18 | 8 | 0.9 |
| Squamous cell carcinoma | 18 | 14 | 4 | |
| Adenosquamous carcinoma | 2 | 1 | 1 | |
| Large cell carcinoma | 4 | 2 | 2 | |
| Stage | ||||
| IIIA | 45 | 30 | 15 | 0.3 |
| T1N2M0 | 9 | 6 | 3 | |
| T2N2M0 | 29 | 19 | 10 | |
| T3N2M0 | 7 | 5 | 2 | |
| IIIB | 5 | 5 | 0 | |
| T2N3M0 | 2 | 2 | 0 | |
| T4N2M0 | 3 | 3 | 0 | |
| Pulmonary resectiona | ||||
| Lobectomy | 33 | 25 | 8 | 0.079 |
| Sleeve lobectomy | 5 | 4 | 1 | |
| Bilobectomy | 7 | 4 | 3 | |
| Right pneumonectomy | 2 | 0 | 2 | |
| Left pneumonectomy | 2 | 1 | 1 | |
ChRT: chemoradiotherapy; ChT: chemotherapy.
aPulmonary resection (pneumonectomy vs others).
Induction therapy
In the chemotherapy group, 11 patients (73.3%) completed induction chemotherapy without dose modification. Four patients (27.7%) required dose modification, consisting of 2 patients with completion of the scheduled therapy with dose modification and two with a modified scheduled therapy in which each patient skipped once or twice. In 35 chemoradiotherapy patients, 13 (37.1%) completed the planned full-dose induction chemotherapy with radiation at a dose of 46 Gy (12 patients) and 40 Gy (1 patient). Eleven patients (31.4%) completed the planned dose modified induction chemotherapy with radiation at a dose of 46 Gy (9 patients), 42 Gy (1 patient) and 32 Gy (1 patient). Eleven patients (31.4%) received the modified induction chemotherapy with single omission (9 patients; 7 with a 46 Gy radiation) or double omission (2 patients with a 46 Gy radiation) of drug administration.
Surgery, pathological response and postoperative adjuvant therapy
The median time from the end of induction therapy until surgery was 35 days (range: 25–59 days). Surgical resection was performed in 49 patients. The surgical procedures in the chemoradiotherapy group included a lobectomy in 25 patients, a sleeve lobectomy in 4, a bilobectomy in 4 and a left pneumonectomy in 1. Those in the chemotherapy group included a lobectomy in 8 patients, a sleeve lobectomy in 1, a bilobectomy in 3, a right pneumonectomy in 2 and a left pneumonectomy in 1. One patient in the chemoradiotherapy group did not undergo surgery because of severe congestive heart failure. Regarding resectability, 1 of the 34 patients in the chemoradiotherapy group exhibited incomplete tumor resection with pleural dissemination. Four of the 15 patients in the chemotherapy group exhibited incomplete tumor resection with mediastinal lymph node invasion to the paratracheal region and superior vena cava [10]. The rate of incomplete resection was significantly higher in the chemotherapy group than in the chemoradiotherapy group (P = 0.026).
The pathological responsiveness of the resected specimens was estimated. In the chemoradiotherapy group, pathological downstaging and pathological complete response were confirmed in 16 (45.7%) and 7 (20.6%) of the 34 patients, respectively. In the chemotherapy group, 2 (13.3%) and 1 (6.7%) of the 15 patients exhibited pathological downstaging and pathological complete response, respectively. The rate of pathological downstaging was significantly higher in the chemoradiotherapy group than in the chemotherapy group (P = 0.021). In the chemoradiotherapy group, 2 patients with N2 disease at the start of induction chemoradiotherapy exhibited N3 disease upon pathological examination of the resected specimens. Of the 49 patients who underwent surgical resection, 14 of the 34 chemoradiotherapy patients and 13 of the 15 chemotherapy patients received post-operative adjuvant therapy.
Post-operative adjuvant therapy was performed in 14 patients in the chemoradiotherapy group and 13 patients in the chemotherapy group. The content of adjuvant therapy of chemoradiotherapy group consisted of docetaxel plus cisplatin in 9 patients, irinotecan plus cisplatin in 3 patients, gemcitabine plus cisplatin in 1 patient and gemcitabine plus carboplatin in 1 patient. That of chemotherapy group consisted of irinotecan plus cisplatin in 8 patients, radiation therapy to a total dose of 50 Gy in 5 patients.
Pattern of relapse
At the time of the final data analysis in February 2012, the median follow-up period for the surviving patients was 5.9 years, ranging from 1.2 to 12.6 years. Twenty-eight patients (56.0%) were alive. Disease relapse had occurred in 26 patients, consisting of only distant relapse in 14 (chemoradiotherapy group, 10 patients; chemotherapy group, 4 patients), only loco-regional relapse in 7 (chemoradiotherapy group, 2 patients; chemotherapy group, 5 patients) and both distant and loco-regional relapse in 5 (chemoradiotherapy group, 2 patients; chemotherapy group, 3 patients) at the time of the initial diagnosis of relapse. The rate of local relapse was significantly higher in the chemotherapy group than in the chemoradiotherapy group (4 [11.4%] of 35 cases vs 7 [46.7%] of 15 cases; P = 0.010). Total distant relapse occurred in 12 (34.3%) of the 35 patients in the chemoradiotherapy group and 7 (46.7%) of the 15 patients in the chemotherapy group, with no significant difference observed between the groups.
Survival
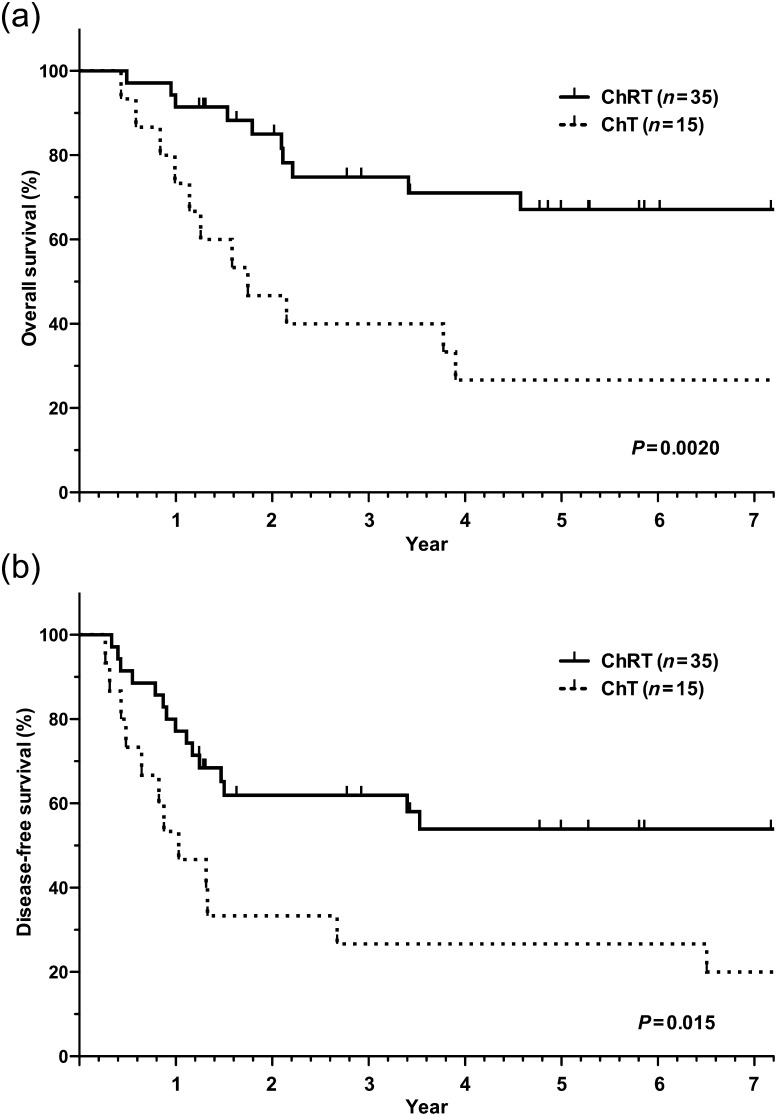
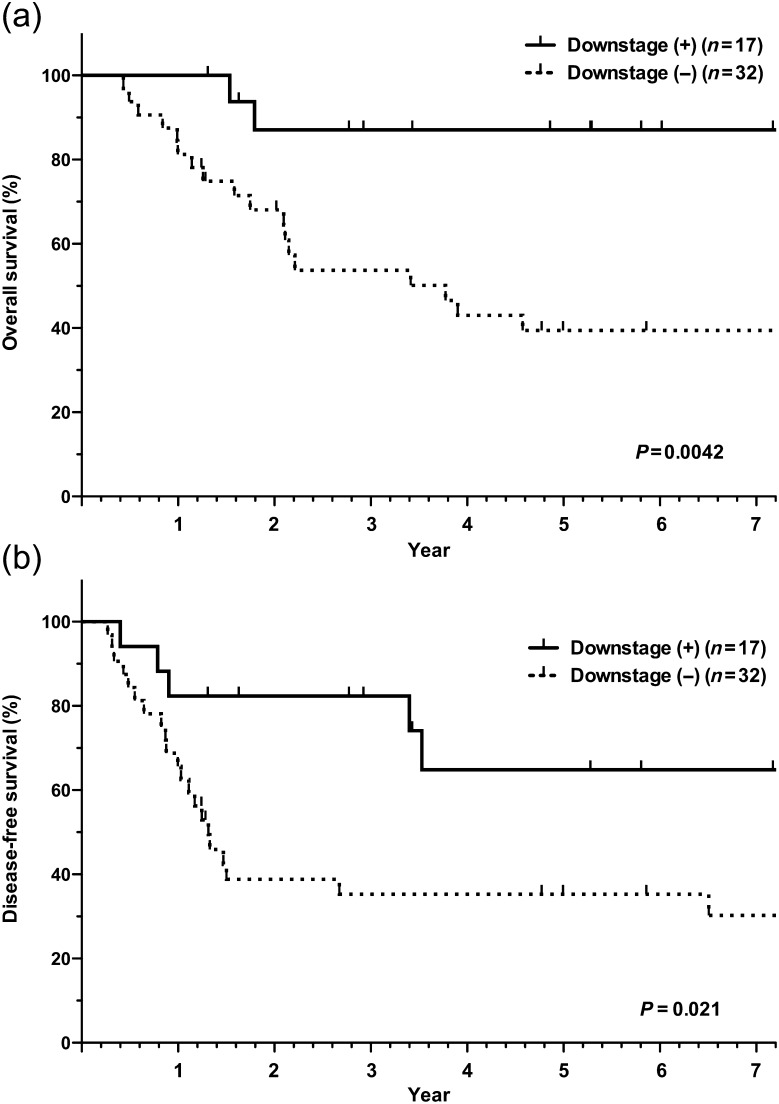
For the entire population, the 3-year and 5-year OS rates were 64.1 and 53.9%, respectively, and the 1-year and 2-year DFS rates were 70.0 and 53.1%, respectively. While no deaths directly related to induction therapy or surgery occurred, one chemotherapy patient who underwent a right pneumonectomy but ended up with an incomplete resection died of radiation pneumonitis during the post-operative radiotherapy period. The survival curves for the chemoradiotherapy and chemotherapy groups are shown in Fig. 1. Significant differences in OS (P = 0.0020) and DFS (P = 0.015) were noted between the chemoradiotherapy and chemotherapy groups. Furthermore, patients with pathological mediastinal downstaging after induction therapy had a significantly longer OS (P = 0.0042) and DFS (P = 0.021) than those without pathological mediastinal downstaging (Fig. 2; Table 2). Patients with pathological complete response tended to show a longer OS than those without pathological complete response (P = 0.092). In DFS, pathological complete response was significantly associated with favorable DFS (P = 0.034). Among the other factors that were examined (sex, histology and extent of pulmonary resection), no significant factors related to a favorable outcome were observed except pathological complete responders (OS, P = 0.092 and DFS, P = 0.034; Table 2). Adjuvant therapy after surgery did not affect the outcome of the patients.
Figure 1:
Survival curves stratified by induction therapy. (a) Overall survival; (b) disease-free survival. ChRT: chemoradiotherapy; ChT: chemotherapy.
Figure 2:
Survival curves stratified by pathological downstaging. (a) Overall survival; (b) disease-free survival.
Table 2:
Overall survival and disease-free survival rates according to clinicopathological factors
| Variables | N | OS |
DFS |
||||
|---|---|---|---|---|---|---|---|
| 3-year (%) | 5-year (%) | P | 1-year (%) | 2-year (%) | P | ||
| Induction therapy | |||||||
| ChRT | 35 | 74.8 | 67.1 | 0.0020 | 77.1 | 61.9 | 0.015 |
| ChT | 15 | 40 | 26.7 | 53.3 | 33.3 | ||
| Mediastinal downstage | |||||||
| (+) | 18 | 87.1 | 87.1 | 0.0042 | 82.4 | 82.4 | 0.021 |
| (−) | 31 | 53.7 | 39.4 | 65.6 | 38.8 | ||
| Sex | |||||||
| Male | 37 | 60.9 | 51.9 | 0.58 | 73.0 | 53.6 | 0.99 |
| Female | 13 | 75.2 | 60.2 | 61.5 | 53.9 | ||
| Histology | |||||||
| AD | 26 | 67.2 | 51.7 | 0.99 | 73.1 | 47.8 | 0.42 |
| Non-AD | 24 | 60.8 | 55.7 | 66.7 | 58.3 | ||
| Pulmonary resection | |||||||
| Lobectomy | 38 | 68 | 53.9 | 0.46 | 73.7 | 53.7 | 0.81 |
| Others | 11 | 54.6 | 54.6 | 63.6 | 54.6 | ||
| pCR | |||||||
| (+) | 8 | 85.7 | 85.7 | 0.092 | 87.5 | 87.5 | 0.034 |
| (−) | 41 | 61.4 | 49.7 | 68.3 | 47.8 | ||
ChRT: chemoradiotherapy; ChT: chemoradiotherapy; AD: adenocarcinoma; pCR: pathological complete response; P-value was calculated by log-rank test.
A multivariate analysis of all the patients considering induction therapy, pathological downstaging and pathological complete response showed that induction chemoradiotherapy and pathological downstaging were independent factors of favorable OS (chemoradiotherapy, P = 0.0099 and pathological downstaging, P = 0.039; Table 3). In addition, only induction chemoradiotherapy was an independent factor of favorable DFS (P = 0.042).
Table 3:
Multivariate analysis using Cox proportional hazard model
| Variables | Overall survival |
Disease-free survival |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Induction ChRT vs ChT | 0.31 | 0.13–0.75 | 0.0099 | 0.43 | 0.20–0.97 | 0.042 |
| Mediastinal downstaging (+) vs (−) | 0.19 | 0.010–0.94 | 0.039 | 0.61 | 0.18–1.64 | 0.35 |
| pCR (+) vs (−) | 1 | 0.039–25.4 | 1 | 0.25 | 0.012–1.7 | 0.17 |
ChRT: chemoradiotherapy; ChT: chemotherapy; HR: hazard ratio; CI: confidence interval; pCR: pathological complete response.
DISCUSSION
In our study, induction chemoradiotherapy and the downstaging of mediastinal lymph node metastasis were independent prognostic factors for N2/3 NSCLC patients who were treated with induction therapy. Our results indicated that induction chemoradiotherapy was associated with a significantly longer survival period than induction chemotherapy. The poor prognosis of patients receiving induction chemotherapy is thought to be due to the high rate of local recurrence, including the macroscopically incomplete resection of metastatic mediastinal lymph nodes. Indeed, the rationale for induction treatment in patients with locally advanced disease is to facilitate a complete surgical resection by reducing the quantity of cancer cells in the primary tumor and metastatic regional nodes and to eradicate possible micrometastases [16]. Patients with mediastinal lymph node metastasis, especially those with extra-nodal invasion, have a risk of incomplete resection, and induction chemoradiotherapy can prevent the survival of residual tumor cells near the resected margin, compared with chemotherapy. These facts strongly suggest that powerful induction therapy for local control may lead to an improvement in the prognosis of NSCLC patients with mediastinal lymph node metastasis. The absence of residual tumor cells obtained by the high pathological complete response with chemoradiotherapy might also contribute to avoiding a pneumonectomy. Regarding the identification of pathological downstaging as a prognostic factor in our study, many studies have reported similar results [17, 18].
Our study showed that while no significant difference in the rate of distant metastasis was observed between the chemoradiotherapy group (34.3%) and the chemotherapy group (46.7%), the rate of distant relapse seems to be lower in the chemoradiotherapy group than in the chemotherapy group. Whether radiotherapy is necessary for patients receiving induction chemotherapy if the local disease lesion is controlled by surgical resection has been a point of discussion, since thoracic radiation does not seem to directly affect pre-existing micrometastases at distant sites. When considering distant metastasis, two possibilities should be taken into account: (i) the presence of micrometastasis before induction therapy, and (ii) the occurrence of micrometastasis during induction therapy and prior to surgical resection. The fact that the incidence of distant metastasis seemed to be lower in the chemoradiotherapy group implied that intensive local therapy may reduce the chance of micrometastasis during induction therapy. From this perspective, the addition of radiotherapy to chemotherapy may lower the rate of distant metastasis, compared with that for chemotherapy alone. The other possibility explaining the difference in the rates of distant metastasis is the difference in the chemotherapeutic regimens used for the induction therapies in our study.
We did not analyze the difference in surgical outcomes such as operating time and length of hospital stay between the chemotherapy and chemoradiotherapy groups. Our impression is that the surgery after chemoradiotherapy is technically more demanding compared with that after chemotherapy. For example, mediastinal lymph node dissection after a mediastinoscopy is often hard in cases after chemoradiotherapy. In addition, we basically use the greater omentum to cover anastomosis for sleeve bronchial resection after chemoradiotherapy. For these cases, operating time was longer in chemoradiotherapy than in chemotherapy. Regarding the usage of the omental flap to cover anastomosis, there may be the criticism that a laparotomy for omental harvest is too invasive. However, we intended to make a maximum effort to prevent anastomotic complication because the surgical outcome could be unfavorable when the bronchopleural fistula occurs in patients after induction therapy. Of note, the omental flap could not always prevent the bronchopleural fistula, suggesting the importance of appropriate surgery [19]. Recently, we have been using laparoscopic surgery to harvest the greater omentum in order to reduce the invasiveness of laparotomy.
To enable our study to be interpreted appropriately, its strengths and limitations need to be considered. A strength of the current report is that its subjects comprised consecutive patients who were prospectively cared for by the same treatment team according to preset institutional treatment policies. On the other hand, some of the inherent limitations of our study include its lack of a randomized design and chronological differences in the induction chemotherapy (1995–1999) and chemoradiotherapy (1999–2010) regimens and the discrepancy in sample size between the chemotherapy and chemoradiotherapy groups, especially the small number of the chemotherapy group. However, a randomized study comparing chemoradiotherapy followed by surgery with chemotherapy followed by surgery was closed due to the lack of interested participants (Radiation Therapy Oncology Group—0412). While the chronological difference may have affected the longer OS in the chemoradiotherapy group, compared with that in the chemotherapy group, the fact that a lower rate of incomplete resection, a favorable DFS time, and a more advanced disease stage were observed in the chemoradiotherapy group, compared with the chemotherapy group, seems to support the advantage of induction chemoradiotherapy in this patient population.
As one of the future directions of the induction therapy, identifying clinicopathological or biological factors that can be indicators of the induction regimen is critical for further improvement of therapeutic outcomes. Our sample size presented here was not large enough for this analysis, but data accumulation will lead to the establishment of a personalized induction therapy for NSCLC.
In conclusion, our results indicate that induction chemoradiotherapy is superior to induction chemotherapy with regard to the outcome of NSCLC patients with mediastinal lymph node metastasis.
Funding
There is no financial support for this study.
Conflicts of interest: Shinichi Toyooka and Katsuyuki Kiura had honorarium from Sanofi-Aventis. No other authors have conflict of interest to declare.
Acknowledgements
We thank Yuho Maki, Junichi Soh, Departments of Thoracic Surgery, Okayama University Hospital for collection of patient data and Hans Jiro Becker, University of Cologne, Cologne, Germany, for revising the English of our manuscript.
References
- 1.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:202S–20S. doi: 10.1378/chest.07-1362. doi:10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 2.Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, 3rd, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–92. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 3.Eberhardt W, Wilke H, Stamatis G, Stuschke M, Harstrick A, Menker H, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase II trial. J Clin Oncol. 1998;16:622–34. doi: 10.1200/JCO.1998.16.2.622. [DOI] [PubMed] [Google Scholar]
- 4.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–9. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 5.Kiura K, Ueoka H, Segawa Y, Tabata M, Kamei H, Takigawa N, et al. Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer. Br J Cancer. 2003;89:795–802. doi: 10.1038/sj.bjc.6601217. doi:10.1038/sj.bjc.6601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol. 2010;28:3299–306. doi: 10.1200/JCO.2009.24.7577. doi:10.1200/JCO.2009.24.7577. [DOI] [PubMed] [Google Scholar]
- 7.van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–50. doi: 10.1093/jnci/djk093. doi:10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Swann RS, Rusch VW, Turrisi AT, 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. doi:10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins K, Chino JP, Marks LB, Ready N, D'Amico TA, Clough RW, et al. Preoperative chemotherapy versus preoperative chemoradiotherapy for stage III (N2) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75:1462–7. doi: 10.1016/j.ijrobp.2009.01.069. doi:10.1016/j.ijrobp.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 10.Date H, Kiura K, Ueoka H, Tabata M, Aoe M, Andou A, et al. Preoperative induction chemotherapy with cisplatin and irinotecan for pathological N(2) non-small cell lung cancer. Br J Cancer. 2002;86:530–3. doi: 10.1038/sj.bjc.6600117. doi:10.1038/sj.bjc.6600117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama H, Ueoka H, Kiura K, Tabata M, Kozuki T, Tanimoto M, et al. Preoperative concurrent chemoradiotherapy with cisplatin and docetaxel in patients with locally advanced non-small-cell lung cancer. Br J Cancer. 2004;90:979–84. doi: 10.1038/sj.bjc.6601624. doi:10.1038/sj.bjc.6601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyooka S, Kiura K, Takemoto M, Oto T, Takigawa N, Fujiwara T, et al. Long-term outcome of induction chemoradiotherapy with docetaxel and cisplatin followed by surgery for non-small cell lung cancer with mediastinal lymph node metastasis. Interact CardioVasc Thorac Surg. 2012;14:565–9. doi: 10.1093/icvts/ivs028. doi:10.1093/icvts/ivs028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shien K, Toyooka S, Kiura K, Matsuo K, Soh J, Yamane M, et al. Induction chemoradiotherapy followed by surgical resection for clinical T3 or T4 locally advanced non-small cell lung cancer. Ann Surg Oncol. 2012;19:2685–92. doi: 10.1245/s10434-012-2302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. doi:10.1097/00000421-198212000-00014. [PubMed] [Google Scholar]
- 15.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. doi:10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 16.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–92. doi: 10.1056/NEJMra035536. doi:10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 17.Bueno R, Richards WG, Swanson SJ, Jaklitsch MT, Lukanich JM, Mentzer SJ, et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg. 2000;70:1826–31. doi: 10.1016/s0003-4975(00)01585-x. doi:10.1016/S0003-4975(00)01585-X. [DOI] [PubMed] [Google Scholar]
- 18.Jaklitsch MT, Herndon JE, 2nd, DeCamp MM, Jr, Richards WG, Kumar P, Krasna MJ, et al. Nodal downstaging predicts survival following induction chemotherapy for stage IIIA (N2) non-small cell lung cancer in CALGB protocol #8935. J Surg Oncol. 2006;94:599–606. doi: 10.1002/jso.20644. doi:10.1002/jso.20644. [DOI] [PubMed] [Google Scholar]
- 19.Toyooka S, Soh J, Shien K, Sugimoto S, yamane M, Oto T, et al. Sacrificing the pulmonary arterial branch to the spared lobe is a risk factor of bronchopleural fistula in sleeve lobectomy after chemoradiotherapy. Eur J Cardiothorac Surg. 2012 doi: 10.1093/ejcts/ezs323. doi:10.1093/ejcts/ezs323. [DOI] [PubMed] [Google Scholar]