Abstract
OBJECTIVES
In chronic thromboembolic pulmonary hypertension (CTEPH), right ventricular (RV) dysfunction is associated with increased morbidity and mortality following pulmonary endarterectomy. Plasma brain natriuretic peptide (BNP) levels were previously shown to correlate with RV (dys)function. We hypothesized that BNP can be used as a non-invasive biomarker to identify patients at ‘high risk’ for postoperative morbidity and mortality.
METHODS
We studied the postoperative outcome in 73 consecutive patients. Patients were divided into three groups based on previously determined cut-off levels: BNP <11.5, indicating normal RV function (ejection fraction [EF] ≥45%), BNP >48.5 pmol/l, indicating RV dysfunction (right ventricular ejection fraction <30%) and BNP 11.5–48.5 pmol/l. Postoperative ‘bad outcome’ was defined as the presence of either residual pulmonary hypertension (PH) or (all-cause) mortality.
RESULTS
Plasma BNP >48.5 pmol/l was shown to be an independent predictor of ‘bad outcome’. Compared with BNP <11.5 pmol/l, BNP >48.5 pmol/l identified patients at higher risk for (all-cause) mortality (17 vs 0%; P = 0.009) and residual PH (56 vs 20%; P < 0.004). Also, the durations of mechanical ventilation and intensive care unit stay were significantly longer in patients with BNP >48.5 pmol/ml.
CONCLUSIONS
Plasma BNP levels may be of use as a non-invasive biomarker reflecting RV dysfunction, next to other well-recognized (invasive) parameters, for better preoperative risk stratification of CTEPH patients.
Keywords: Brain natriuretic peptide, Chronic thromboembolic pulmonary hypertension, Pulmonary endarterectomy, Right ventricular dysfunction, Biomarker
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) results from incomplete resolution of the vascular obstruction caused by pulmonary thromboembolism [1].
If left untreated, the prognosis in CTEPH is poor and proportional to the degree of pulmonary hypertension (PH) [2]. Advanced CTEPH leads to cardiac remodelling with a consequent impact on cardiac function [3]. As a result, death in most CTEPH patients is caused by progressive right ventricular (RV) failure. Pulmonary endarterectomy (PEA) is the therapy of choice for patients with surgically accessible CTEPH [1, 4, 5]. This intervention, however, does not come without potential risk, with mortality figures ranging from 5% to 24% of operated cases [1, 6, 7]. In these patients, preoperative RV dysfunction is associated with increased postoperative morbidity and mortality [7, 8]. Better preoperative identification of RV dysfunction and subsequent preoperative medical treatment may improve the postoperative outcome [9–11].
Cardiac magnetic resonance imaging (cMRI) is considered the most accurate method to quantify RV function in CTEPH patients [12]. cMRI, however, is not part of the routine work up in CTEPH patients; it requires specific expertise and is time consuming and expensive. Plasma brain natriuretic peptide (BNP) is secreted by the cardiac ventricles in response to wall stretching and volume overload [13, 14]. In CTEPH, BNP levels correlate with haemodynamic severity of disease [15–17]. Moreover, previously, using cMRI, we have demonstrated that plasma BNP levels can be used to identify RV dysfunction [18]. So, we hypothesized that BNP can be used as a non-invasive biomarker to identify patients at ‘high risk’ for increased postoperative morbidity and mortality. Therefore, using previously determined cut-off values [18], we studied postoperative haemodynamic and functional outcomes in relation to preoperative plasma BNP levels.
PATIENTS AND METHODS
Prospectively, we studied 73 consecutive patients (male 30; age 55 [16–78] years) diagnosed with CTEPH, referred to the Academic Medical Center of the University of Amsterdam. Diagnoses of CTEPH and cardiopulmonary haemodynamics were determined by pulmonary angiography and right heart catheterization [19]. PH was defined as mean pulmonary artery pressure (mPAP) >25 mmHg at rest or >30 mmHg during exercise. At inclusion, all patients received oral anticoagulants for at least 3 months. Patients with renal insufficiency (creatinine >115 μmol l−1), concomitant left-sided heart disease, uncontrolled systemic hypertension or uncontrolled diabetes mellitus were excluded. All patients included underwent PEA according to the protocol of the University of California San Diego [8]. All patients were operated upon by one surgeon (Jaap J. Kloek).
Study design
In a previous set of consecutive patients, we demonstrated that plasma BNP can be used as a parameter of RV function [18]. More particularly, using the receiver operating characteristic (ROC) analysis, we demonstrated that BNP < 11.5 pmol/l identified patients with normal RV function (RV ejection fraction [RVEF] ≥45%; area under the curve [AUC] 0.97), whereas BNP >48.5 pmol/l identified patients with distinct RV failure (RVEF <30%; AUC 0.91). Therefore, in the current study, patients were divided into three groups based on these previously determined cut-off values: Group I (BNP <11.5), Group II (11.5 ≤ BNP<48.5) and Group III (BNP >48.5).
Postoperatively, haemodynamic characteristics were determined on the first or second day after PEA, before removal of the Swan-Ganz catheter (Edwards Life-Sciences, Irvine, CA, USA). Postoperative ‘bad outcome’ was defined as either the presence of residual PH (mPAP >25 mmHg) or all-cause mortality. To assess short-term postoperative clinical outcome, we determined the duration of stay on the intensive care unit (ICU), the duration of mechanical ventilation (MV) and the number of different inotropics used in all surviving patients. RV function was routinely assessed by trans-thoracic echocardiography before PEA, and 2 weeks and 3 months after surgery, as described previously [20]. To assess systolic RV function, we determined the tricuspid annular plane systolic excursion and the systolic pulmonary artery pressure (sPAP) [20]. Overall RV function was assessed by the myocardial performance index [20]. To assess the functional outcome after PEA, a 6-min walk test was routinely performed in all patients before surgery, as well as 3 and 12 months following PEA according to the guidelines of the American Thoracic Society, as described previously [21, 22]. Plasma BNP levels were determined, as previously described, using an immunoradiometric assay (ShionoRIA BNP, Shionogi Pharmaceutical, Osaka, Japan) [18, 23]. To determine the plasma BNP level, blood samples were collected preoperatively during the diagnostic work up of CTEPH, before the initiation of any vasoactive medication.
Statistical analysis
All calculations were performed with a statistical package (SPSS 16; SPSS Inc., Chicago, IL, USA). Data are expressed as median (range), as indicated in the text. Haemodynamic parameters, functional and in-hospital characteristics and echocardiographic parameters between the three pre-defined groups of patients were analysed by the non-parametric Kruskall–Wallis test. In case of an overall statistical difference, the differences between groups were further analysed using the Mann–Whitney U-test. Preoperatively, the pulmonary vascular resistance is the most important predictor of outcome after PEA [7, 8]. So, using multiple logistic regression analysis, we studied the contribution of BNP >48.5 pmol/l and the total pulmonary resistance (TPR) in predicting postoperative ‘bad outcome’. The optimal cut-off point for the preoperative TPR, in predicting ‘bad outcome’ (i.e. residual PH [by definition mPAP >25 mmHg] or all-cause mortality), was identified by ROC curve analysis, using the highest likelihood ratio. The AUC is presented with a 95% confidence interval (CI). A P-value <0.05 was considered statistically significant.
RESULTS
Baseline patient characteristics
Baseline clinical and haemodynamic characteristics are summarized in Table 1.
Table 1:
Baseline functional and haemodynamic characteristics
| Characteristics | Patients (n = 73) |
|---|---|
| Age (years) | 55 (16–78) |
| Female/male | 43/30 |
| BMI | 28 (17–47) |
| HR (min−1) | 80 (60–100) |
| NYHA class, n | |
| II | 18 |
| III | 48 |
| IV | 7 |
| Resting haemodynamics | |
| mPAP (mmHg) | 40 (17–73) |
| CI (l min−1 m−2) | 2.5 (1–4) |
| PVR (dynes s cm−5) | 524 (114–2019) |
| TPR (dynes s cm−5) | 714 (189–2210) |
| mRAP (mmHg) | 7 (2–24) |
| PCWP (mmHg) | 10 (2–49) |
| SVO2 (%) | 64 (34–89) |
| SaO2 (%) | 92 (49–97) |
| 6-MWD (m) | 431 (171–637) |
| BNP (pmol/l) | 9 (0.1–187) |
Values are expressed as median (range).
BMI: body mass index; BNP: brain natriuretic peptide; CI: cardiac index; HR: heart rate; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; NYHA: New York Heart Association; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; SaO2: arterial oxygen saturation; SVO2: mixed venous oxygen saturation; TPR: total pulmonary resistance; 6-MWD: 6-min walking distance.
The three groups defined by the preset BNP cut-off values differed significantly with respect to the haemodynamic and functional characteristics (Table 2). Moreover, at baseline, the three groups differed significantly with respect to RV function (Table 3). Whereas patients with BNP levels <11.5 pmol/l had a rather well-preserved RV function, impairment of RV function was most pronounced in patients with BNP levels >48.5 pmol/l. Also, tricuspid regurgitation differed significantly between the groups.
Table 2:
Haemodynamic and functional characteristics of the patients (n = 73) according to the predefined BNP levels
| Group I BNP <11.5 (n = 40) |
Group II 11.5 ≤ BNP ≤ 48.5 (n = 15) |
Group III BNP >48.5 (n = 18) |
P-value |
|||
|---|---|---|---|---|---|---|
| I vs II | II vs III | I vs III | ||||
| Haemodynamics before PEA | ||||||
| mPAP (mmHg) | 32 (17–69) | 47 (22–73) | 50 (39–62) | 0.001 | NS | <0.0001 |
| CI (l min−1 m−2) | 2.8 (1.9–4.1) | 2.2 (1.6–3.8) | 1.8 (1–3.8) | 0.04 | NS | <0.0001 |
| TPR (dynes s cm−5) | 444 (189–1422) | 857 (251–1224) | 1186 (486–2210) | <0.0001 | 0.02 | <0.0001 |
| Functional class before PEA | ||||||
| 6-MWD pre PEA | 475 (259–637) | 417 (310–586) | 358 (40–508) | 0.05 | 0.05 | <0.0001 |
| Haemodynamics after PEA | ||||||
| mPAP (mmHg) | 21 (13–49) | 24 (15–49) | 26 (20–72) | NS | NS | 0.02 |
| CI (l min−1 m−2) | 2.4 (1.8–3.2) | 2.7 (1.8–4.5) | 2.6 (1.4–4.2) | NS | NS | NS |
| TPR (dynes s cm−5) | 395 (213–847) | 427 (211–726) | 444 (256–1440) | NS | NS | NS |
| Functional class after PEA | ||||||
| 6-MWD 3 months post-PEA | 511 (308–676) | 432 (333–575) | 513 (187–587) | NS | NS | NS |
| 6-MWD 1 year post-PEA | 514 (276–676) | 524 (376–822) | 528 (241–606) | NS | NS | NS |
| Δ6-MWD 1 year | 38 (–72–208) | 61 (–47–396) | 149 (48–309) | NS | NS | <0.0001 |
| Hospital characteristics | ||||||
| Duration of MV (h) | 19 (14–140) | 25 (11–70) | 69 (7–1080) | 0.7 | 0.06 | 0.01 |
| Duration of ICU stay (days) | 2 (1–9) | 3 (1–24) | 5 (1–28) | 0.2 | 0.4 | 0.02 |
| Number of inotropics used at ICU during first 24 h | 1 (0–3) | 1 (0–3) | 2 (1–3) | 0.005 | 0.003 | <0.0001 |
Values are expressed as median (range).
CI: cardiac index; mPAP: mean pulmonary pressure; MV: mechanical ventilation; NS: not significant; ICU: intensive care unit; TPR: total pulmonary resistance; 6-MWD: 6-min walking distance; Δ6-MWD: change from baseline in 6-MWD; the following inotropics were used in our ICU: Noradrenaline, dobutamine/dopamine, isoprenaline and milrinone.
Table 3:
Pre- and postoperative echocardiographic characteristics according to the predefined BNP levels
| Echocardiographic parameters | Group I BNP <11.5 (n = 40) |
Group II 11.5 ≤ BNP ≤ 48.5 (n = 15) |
Group III BNP >48.5 (n = 18) |
P-value |
||
|---|---|---|---|---|---|---|
| I vs II | II vs III | I vs III | ||||
| TAPSE | ||||||
| TAPSE before PEA | 23 (16–38) | 18 (13–30) | 14 (7–23) | 0.014 | 0.021 | <0.0001 |
| TAPSE 2 weeks after PEA | 13 (9–30) | 14 (10–17) | 11 (5–17) | NS | 0.037 | 0.043 |
| TAPSE 3 months after PEA | 16 (11–29) | 17 (12–27) | 14 (11–18) | NS | 0.042 | 0.015 |
| sPAP | ||||||
| sPAP before PEA | 52 (28–107) | 87 (60–116) | 94 (53–129) | 0.001 | NS | <0.0001 |
| sPAP 2 weeks after PEA | 32 (23–108) | 33 (25–53) | 40 (30–80) | NS | 0.045 | 0.078 |
| sPAP 3 months after PEA | 34 (25–108) | 32 (27–53) | 43 (22–107) | NS | 0.046 | 0.066 |
| MPI | ||||||
| MPI before PEA | 0.40 (0.14–0.87) | 0.64 (0.36–1.01) | 0.65 (0.40–1.47) | 0.005 | NS | <0.0001 |
| MPI 2 weeks after PEA | 0.30 (0.09–0.96) | 0.30 (0.19–0.58) | 0.64 (0.25–1.38) | NS | 0.011 | NS |
| MPI 3 months after PEA | 0.31 (0.11–0.62) | 0.30 (0.11–1.02) | 0.52 (0.25–0.84) | NS | NS | 0.001 |
| Tricuspid regurgitation | ||||||
| TR before PEA | 1 (0–2) | 2 (1–3) | 3 (1–3) | <0.0001 | NS | <0.0001 |
| TR 2 weeks after PEA | 0 (0–2) | 0 (0–3) | 2 (0–3) | NS | NS | 0.035 |
| TR 3 months after PEA | 0 (0–3) | 0 (0–2) | 1 (0–3) | NS | NS | NS |
Values are expressed as median (range).
TAPSE: tricuspid annular plane systolic excursion; sPAP: systolic pulmonary artery pressure; MPI: myocardial performance index; NS: not significant. The severity of the tricuspid regurgitation (TR) was classified as: 0, no TR; 1, mild; 2, moderate; 3, severe.
Outcome after pulmonary endarterectomy
In general, PEA resulted in substantial haemodynamic improvement. Postoperatively (n = 68), mPAP decreased from 40 (range 170–73 mmHg) to 23 mmHg (range 13–49 mmHg) and TPR from 714 (range 189–2210 dynes s cm−5) to 410 dynes s cm−5 (range 211–847 dynes s cm−5; both P <0.0001, Wilcoxon test); the CI did not change (2.5 [range 1–4.1 l min−1 m−2] and 2.4 l min−1 m−2 [range 1.4–4.5 l min−1 m−2], respectively; P = 0.57). Postoperatively, residual PH (mPAP >25 mmHg; range 26–55 mmHg) was observed in 21 patients, of whom 7 had a mPAP >30 mmHg. Five (6.8%) patients died, 3 due to progressive right heart failure caused by persistent pulmonary hypertension and 2 due to postoperative massive alveolar haemorrhage. In the surviving patients, the postoperative course was complicated in 7 by mild reperfusion lung injury for which they needed prolonged MV (median, 6 days; range 3–9).
Outcome after pulmonary endarterectomy in predefined brain natriuretic peptide groups
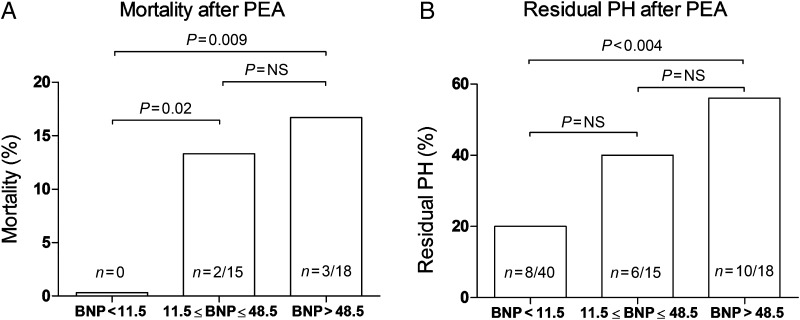
Haemodynamic and functional outcomes in the three predefined groups of patients are summarized in Table 2; mortality and residual PH are illustrated in Fig. 1.
Figure 1:
(A) Mortality in the three predefined BNP groups. (B) Number of patients with residual pulmonary hypertension in the three predefined BNP groups.
Patients with BNP <11.5 pmol/l (n = 40) had an excellent haemodynamic outcome, i.e. mean PAP normalized in all but 8 (20%) patients (median mPAP 29 mmHg; range 26–36 mmHg) and no deaths were observed in this group. In contrast, 10 of 18 patients (56%) with BNP levels >48.5 pmol/l had (by definition) residual PH (median mPAP 27 mmHg; range 26–72 mmHg). In this group, 3 (17%) patients died; 2 due to progressive right heart failure caused by severe persistent PH and 1 due to postoperative massive alveolar haemorrhage. In line, patients with BNP levels between 11.5 and 48.5 pmol/l had an intermediate outcome; in 6 of 15 patients (40%), residual PH was observed (median mPAP 28 mmHg; range 26–49 mmHg). Moreover, in this group, 2 (13%) deaths occurred; 1 due to progressive right heart failure and 1 due to postoperative massive alveolar haemorrhage.
In addition, short-term postoperative outcomes differed significantly between the three groups of patients (Table 2). Patients with BNP levels <11.5 pmol/l had an excellent short-term outcome. In contrast, in patients with BNP levels >48.5 pmol/l, both the duration of ICU stay and the duration of MV were significantly longer, and the number of different inotropics used was significantly larger (Table 2).
Postoperatively, the 6-min walk distance (6-MWD) improved significantly in all groups of patients (Table 2). Moreover, at 1 year, the 6-MWD did not differ between the three groups of patients. The change from baseline in 6-MWD between patients with preoperative BNP <11.5 pmol/l and those with BNP >48.5 pmol/l differed significantly (Table 2).
Postoperatively (Table 3), at 2 weeks and 3 months, RV function in the three groups still differed. Moreover, in line with the invasively measured direct postoperative mPAP, the echocardiographically assessed sPAP at 2 weeks and 3 months was significantly higher in the patients with BNP >48.5 pmol/l.
ROC curve analysis for TPR, predicting ‘bad outcome’ resulted in an AUC of 0.76 (95% CI 0.65–0.87; P <0.0001). The optimal cut-off point for TPR was determined by the highest likelihood ratio, leading to a cut-off >1023 dynes s cm−5.
Using logistic regression analysis, we studied the contribution of BNP >48.5 pmol/l and total pulmonary resistance (TPR) in predicting postoperative ‘bad outcome’, i.e. residual PH or all-cause mortality. Both BNP >48.5 and TPR >1023 had an equal odds ratio (OR) of 4.2 for predicting ‘bad outcome’ (95% CI 1.4–12.8; P = 0.01). When BNP >48.5 and TPR >1023 were both entered in the model, the OR was not statistically significant (OR = 2.6; 95% CI 0.7–9.5; P = 0.16).
COMMENT
This is the first study in CTEPH patients in which we demonstrated that plasma BNP levels may be of clinical use as a non-invasive parameter for preoperative risk stratification of CTEPH patients. The predefined cut-off levels were shown to reflect RV (dys)function; and plasma BNP >48.5 pmol/l was shown to be an independent predictor of ‘bad outcome’, i.e. residual pulmonary hypertension or all-cause mortality.
Using previously determined cut-off levels indicative of RV function as assessed by cMRI [18], we observed an excellent postoperative outcome without mortality in patients with BNP levels <11.5 pmol/l, indicating normal preoperative RV function (RVEF >45%). In contrast, in patients with BNP >48.5 pmol/l, indicating distinct RV dysfunction (RVEF <30%), postoperative outcome was significantly worse; residual pulmonary hypertension was observed in 56% and mortality in 17% of the patients. Moreover, short-term postoperative clinical outcome, i.e. the duration of MV, the duration of stay at the ICU and the number of different inotropics used, also differed significantly between these two groups. In addition, 2 weeks and 3 months after surgery, echocardiographic sPAP in patients with BNP >48.5 pmol/l was still (significantly) higher compared with the other groups of patients. The present findings confirm and extend our preliminary observations in a previous study, in a much smaller number of patients, on the usefulness of plasma BNP levels to predict and reflect preoperative RV dysfunction [18].
Patients with BNP levels >48.5 pmol/l represented haemodynamically and functionally the most severely affected patients. It has been demonstrated previously that severely affected patients, i.e. mPAP >50 mmHg, cardiac index <2.0 l min m−2, pulmonary vascular resistance >1000 dynes s cm−5 and/or New York Heart Association class IV disease, are at high risk for postoperative haemodynamic instability, progressive RV failure and death after PEA [7, 8]. Previously, BNP levels were shown to correlate closely with parameters reflecting the haemodynamic severity of disease in CTEPH patients [15–18]. In the present study, using logistic regression analysis we demonstrated that BNP >48.5 pmol/l was an independent predictor of postoperative ‘bad outcome’. Moreover, the predictive value of BNP >48.5 pmol/l was shown to be equivalent to the predictive value of TPR, which is currently used as the parameter of the severity of disease and risk stratification of CTEPH patients eligible for PEA. The predefined cut-off levels indeed reflected echocardiographically determined RV (dys)function in the patients studied. So, based on the present findings, BNP indeed appears a useful non-invasive biomarker to identify RV dysfunction and may therefore be of potential clinical use to better identify patients at ‘high risk’ for a more complicated postoperative course after PEA.
The combined use of BNP >48.5 pmol/l and TPR did not increase the predictive value for ‘bad outcome’. This might be due at least in part to the close correlation between BNP levels and TPR; there were only few patients with low TPR and BNP >48.5 pmol/l and high TPR and BNP ≤48.5 pmol/l, respectively, included in the present cohort. However, in contrast to TPR, BNP has several advantages; it is a very easy sampling procedure, and it can be used easily for follow-up and non-invasively to monitor the effects of medical treatment on RV function.
PEA is the therapy of first choice in CTEPH patients with surgically accessible thrombi [1, 7, 8]. In most patients, PEA can be performed with an acceptable mortality risk and results in clinical improvement and often near-normalization of pulmonary haemodynamics [7, 8]. A significant proportion of patients with CTEPH who undergo PEA, however, are in a haemodynamically unstable condition in the preoperative period, to the point that risks from surgery in general are significantly increased [7, 8]. In general, medical pretreatment is not indicated in clinically stable patients with surgically accessible CTEPH [24]. However, selected patients with more complex variants of CTEPH might benefit from medical pretreatment [9, 25]. Individual factors predictive of a beneficial response and whether such a response will have a positive impact on postoperative morbidity or mortality still remain to be established. In our view, the current findings point to a potential role of plasma BNP as a non-invasive parameter reflecting RV function to identify ‘high-risk’ patients and subsequently monitor the effect of medical treatment on RV function in these patients prior to PEA.
We also studied whether functional outcome at 1 year differed between the three pre-defined groups of patients. Despite the fact that compared with the patients with BNP levels <11.5 pmol/l, residual pulmonary hypertension was observed more frequently in the BNP >48.5 pmol/l group, 6-MWD at 1 year did not differ between the three groups of patients. In fact, postoperative functional outcome, as expressed by the change from baseline in 6-MWD 1 year after PEA, was even significantly better in the BNP >48.5 group when compared with the patients with BNP <11.5 pmol/l. So, although patients with increased BNP levels are at risk of a more complicated postoperative course after PEA, in the end, the patients who survive a PEA may in fact benefit more from surgical treatment.
There are some limitations to this study that need to be addressed. The first is the moderate sample size. Furthermore, although, based on the present study, BNP levels >48.5 pmol/l, shown previously to be indicative of RV dysfunction [18], appear to be a useful non-invasive parameter for risk stratification of CTEPH patients, the usefulness of BNP in daily clinical practice still needs to be proven. In particular, the usefulness of BNP levels to monitor the effects of medical treatment of ‘high-risk’ patients needs to be proven. In the present study, most of the clinically more severely affected patients were treated preoperatively while waiting for surgery with epoprostenol, bosentan and/or sildenafil. The effects of medical treatment on BNP levels were not determined. In addition, the blood samples for BNP analyses were obtained before the initiation of any vasoactive medication.
In conclusion, we demonstrated that plasma BNP levels may be of use as a non-invasive biomarker reflecting RV dysfunction, next to other well-recognized (invasive) parameters such as TPR, for better preoperative risk stratification of CTEPH patients. However, additional studies in larger numbers of patients are warranted to determine the exact usefulness of plasma BNP in the identification of ‘high-risk’ patients, and on its role in monitoring the medical pretreatment in selected patients prior to PEA.
FUNDING
Sulaiman Surie was supported by a non-restricted research grant from Actelion Pharmaceuticals bv, Woerden, Netherlands. The authors had full control of the design of the study, methods used, outcome parameters, analysis of data and production of the written report.
Conflict of interest: none declared.
REFERENCES
- 1.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345:1465–72. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 2.Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151–8. doi: 10.1378/chest.81.2.151. [DOI] [PubMed] [Google Scholar]
- 3.Reesink HJ, Marcus JT, Tulevski II, Jamieson S, Kloek JJ, Vonk NA, et al. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg. 2007;133:58–64. doi: 10.1016/j.jtcvs.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Archibald CJ, Auger WR, Fedullo PF, Channick RN, Kerr KM, Jamieson SW, et al. Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 1999;160:523–8. doi: 10.1164/ajrccm.160.2.9808109. [DOI] [PubMed] [Google Scholar]
- 5.Kramm T, Mayer E, Dahm M, Guth S, Menzel T, Pitton M, et al. Long-term results after thromboendarterectomy for chronic pulmonary embolism. Eur J Cardiothorac Surg. 1999;15:579–83. doi: 10.1016/s1010-7940(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 6.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1122–7. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 7.Dartevelle P, Fadel E, Mussot S, Chapelier A, Herve P, de PM, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637–48. doi: 10.1183/09031936.04.00079704. [DOI] [PubMed] [Google Scholar]
- 8.Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–62. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 9.Bresser P, Fedullo PF, Auger WR, Channick RN, Robbins IM, Kerr KM, et al. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:595–600. doi: 10.1183/09031936.04.00020004. [DOI] [PubMed] [Google Scholar]
- 10.Bresser P, Pepke-Zaba J, Jais X, Humbert M, Hoeper MM. Medical therapies for chronic thromboembolic pulmonary hypertension: an evolving treatment paradigm. Proc Am Thorac Soc. 2006;3:594–600. doi: 10.1513/pats.200605-115LR. [DOI] [PubMed] [Google Scholar]
- 11.Reesink HJ, Surie S, Kloek JJ, Tan HL, Tepaske R, Fedullo PF, et al. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2010;139:85–91. doi: 10.1016/j.jtcvs.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Kreitner KF, Kunz RP, Ley S, Oberholzer K, Neeb D, Gast KK, et al. Chronic thromboembolic pulmonary hypertension – assessment by magnetic resonance imaging. Eur Radiol. 2007;17:11–21. doi: 10.1007/s00330-006-0327-x. [DOI] [PubMed] [Google Scholar]
- 13.Boomsma F, van den Meiracker AH. Plasma A- and B-type natriuretic peptides: physiology, methodology and clinical use. Cardiovasc Res. 2001;51:442–9. doi: 10.1016/s0008-6363(01)00195-x. [DOI] [PubMed] [Google Scholar]
- 14.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–22. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 15.Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998;31:202–8. doi: 10.1016/s0735-1097(97)00452-x. [DOI] [PubMed] [Google Scholar]
- 16.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–70. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 17.Nagaya N, Ando M, Oya H, Ohkita Y, Kyotani S, Sakamaki F, et al. Plasma brain natriuretic peptide as a noninvasive marker for efficacy of pulmonary thromboendarterectomy. Ann Thorac Surg. 2002;74:180–4. doi: 10.1016/s0003-4975(02)03654-8. [DOI] [PubMed] [Google Scholar]
- 18.Reesink HJ, Tulevski II, Marcus JT, Boomsma F, Kloek JJ, Vonk NA, et al. Brain natriuretic peptide as noninvasive marker of the severity of right ventricular dysfunction in chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2007;84:537–43. doi: 10.1016/j.athoracsur.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Auger WR, Fedullo PF, Moser KM, Buchbinder M, Peterson KL. Chronic major-vessel thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology. 1992;182:393–8. doi: 10.1148/radiology.182.2.1732955. [DOI] [PubMed] [Google Scholar]
- 20.Surie S, Bouma BJ, Bruin-Bon RA, Hardziyenka M, Kloek JJ, van der Plas MN, et al. Time course of restoration of systolic and diastolic right ventricular function after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Am Heart J. 2011;161:1046–52. doi: 10.1016/j.ahj.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Reesink HJ, van der Plas MN, Verhey NE, van Steenwijk RP, Kloek JJ, Bresser P. Six-minute walk distance as parameter of functional outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;133:510–6. doi: 10.1016/j.jtcvs.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 22.ATS Committee on Proficieny Standards for Clinical Pulmonary Function Laboratories statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 23.Tulevski II, Hirsch A, Sanson BJ, Romkes H, van der Wall EE, van Veldhuisen DJ, et al. Increased brain natriuretic peptide as a marker for right ventricular dysfunction in acute pulmonary embolism. Thromb Haemost. 2001;86:1193–6. [PubMed] [Google Scholar]
- 24.Jensen KW, Kerr KM, Fedullo PF, Kim NH, Test VJ, Ben-Yehuda O, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120:1248–54. doi: 10.1161/CIRCULATIONAHA.109.865881. [DOI] [PubMed] [Google Scholar]
- 25.Bresser P, Surie S. Medical therapy for chronic thromboembolic pulmonary hypertension. Multidiscip Resp Med. 2008;3:434–9. [Google Scholar]