To the Editor—The 2012 International AIDS Conference was themed around the need for an “AIDS free generation.” The concept is that the HIV virus is not yet curable, but with medications, we can prevent those who are HIV-infected from developing complications of AIDS and opportunistic infections. Cryptococcal meningitis is one such opportunistic infection that causes 20%–25% of AIDS-related mortality in Africa [1]. Cryptococcal antigen (CrAg) can be detected in a subclinical phase, weeks prior to onset of symptomatic infection, and can be screened and preemptively treated with fluconazole to prevent overt cryptococcal meningitis. The new Food and Drug Administration–approved point-of-care CrAg lateral flow assay (LFA; Immy, Inc., Norman, OK) has fundamentally changed the cost-effectiveness of CrAg screening to prevent cryptococcal meningitis from occurring in those with subclinical infection. The CrAg LFA assay cost is $2.00 in resource limited regions and $5.00 in high-income countries, which translates to a probable real world cost of approximately $2.50–$5.00 in resource limited settings and $10 in high-income countries when including labor, shipping, and overhead costs. This screen and preemptive treatment strategy has the potential to vastly diminish and eliminate cryptococcal meningitis from occurring after antiretroviral therapy (ART) initiation and reduce the 20%–25% of early ART mortality caused by cryptococcosis [2–4].
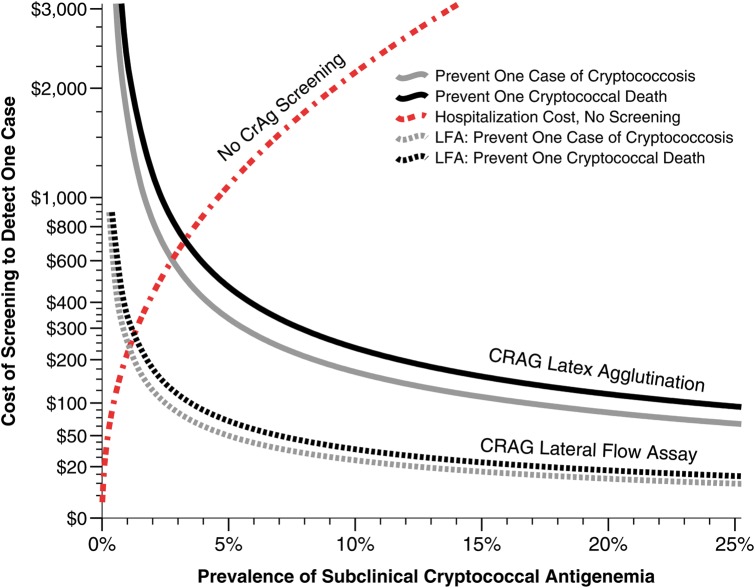
Previously Meya et al showed the value of CrAg screening in a prospective cohort, using the CrAg latex agglutination at a cost of $16.75 in Uganda [3]. Now with a real world cost of the CrAg LFA at $2.50, the cost per life saved with CrAg screening and pre-emptive fluconazole therapy is $39.73 in Uganda among persons with CD4<100 cells/μL, and $2.21 per quality adjusted life year saved [5]. In contrast to the cost of cryptococcal meningitis hospitalization, CrAg screening and targeted preemptive treatment of those CrAg+ is cost saving to healthcare systems at ≥1% CrAg+ prevalence (Figure 1).
But is this program only relevant to Africa? While early HIV testing is key, the US reality is that 38% of newly HIV diagnosed Americans received an AIDS diagnosis concurrently or within 1 year of their HIV diagnosis between 1996–2005 [6]. In North America, our opinion is CrAg screening should also be considered among those who do present with advanced AIDS and CD4<100. The US cost of cryptococcal meningitis hospitalization is extraordinarily high, in contrast to CrAg screening (approximately $10) followed by pre-emptive treatment with 10 weeks of fluconazole per World Health Organization guidelines ($30.24) at 800 mg daily for 2 weeks followed by 8 weeks of 400 mg daily [7]. The approximate cost of hospitalization for cryptococcosis from one Boston hospital including room and board ($700/day), liposomal amphotericin (approximately $350/day), and flucytosine (approximately $500/day) for 14 days is on average $50 000. For this cost, assuming that the LFA would cost $10 in high-income settings, one could screen approximately 5000 persons for asymptomatic antigenemia.
Thus while the prevalence of cryptococcal antigenemia among those with CD4 < 100 cells/μL is likely much lower in North America than in Sub-Saharan Africa or Asia, the cost of cryptococcal meningitis treatment is astronomically higher. CrAg screening is likely cost saving in the U.S. if screened population prevalence was >0.1%. Thus, US CrAg prevalence data are needed. Given the improved cost considerations, the US Department of Health and Human Services Opportunistic Infection guidelines should reexamine the benefit of routine pre-ART CrAg screening for those with CD4 < 100 or among persons hospitalized shortly after ART initiation [8]. While ideally in the future this population of late HIV presenters will be eliminated, among those who do present late, CrAg screening in the U.S. may not only be cost saving, but life saving.
Figure 1.
Cost of serum cryptococcal antigen screening using the latex agglutination versus the lateral flow assay based on asymptomatic prevalence. The figure displays the relative cost-effectiveness of cryptococcal antigen (CrAg) screening and preemptive fluconazole therapy based on the prevalence of antigenemia within a given population and outcomes from Kampala, Uganda. The costs to prevent one case of clinical cryptococcal meningitis (black lines) and to prevent one death (gray lines) are presented using latex agglutination (solid lines) versus lateral flow assay (dotted lines). Above a prevalence of approximately 1%, the cost of treatment of unmasking ART-associated cryptococcal meningitis is greater than the costs of screening with the lateral flow assay and treating with preemptive fluconazole. CrAg screening costs are based on $2.50 total lab test cost per lateral flow assay.
Notes
Financial support. D. B. has received institutional funding through National Institutes of Health grants U01AI089244, R21NS065713, K23AI073192.
Potential conflicts of interest. Both authors: No reported conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Park BJ, Wannwmuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 3.Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51:448–55. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965–72. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 5.Rajasingham R, Meya DB, Boulware DR. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J Acquir Immune Defic Syndr. 2012;59:e85–91. doi: 10.1097/QAI.0b013e31824c837e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Late HIV testing - 34 states, 1996–2005. MMWR Morb Mortal Wkly Rep. 2009;58:661–5. [PubMed] [Google Scholar]
- 7.WHO. Rapid advice: Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 8.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the national institutes of health, and the HIV medicine association of the infectious diseases society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]