Abstract
Most epithelial cells, besides their ubiquitous apical–basal polarity, are polarized within the plane of the epithelium, which is called planar cell polarity (PCP). Using Drosophila as a model, meaningful progress has been made in the identification of key PCP factors and the dissection of their intracellular molecular interactions. The long-range, global aspects of coordinated polarization and the overlying regulatory mechanisms that create the initial polarity direction have, however, remained elusive. Several recent publications have outlined potential mechanisms of how the global regulation of PCP might be controlled and how the distinct core factor groups might interact via frizzled, Van Gogh or flamingo. This review focuses on these exciting features and attempts to provide an integrated picture of these recent and novel insights.
Introduction
Most cell types require polarization to function properly. Neurons display axonal–dendritic polarization, mesenchymal cells need polarization to migrate directionally and epithelial cells are polarized along the apical–basal axis. Most epithelial cells are also polarized within the plane of the epithelium; this polarization is called planar cell polarity (PCP) [1–5]. Apical–basal (A/B) polarity and PCP are interconnected because core PCP proteins are localized at or near the position of adherens junctions and can bind or be bound by A/B determinants (Box 1). In Drosophila, PCP is evident in all adult tissues. For example, wing cells are polarized along the proximal–distal axis, body wall cells in the anterior–posterior axis and ommatidia in the eye within the dorsal–ventral axis [1–3]. In vertebrates, PCP is evident in both external features, such as scales in fish, feathers in birds, and hair in the fur of mammals, and in internal organs such as the inner ear [4,5]. Moreover, PCP signaling is required for the process of convergent extension of mesenchymal cells and neural tube cells during gastrulation and neurulation, respectively. During this process, cells move towards the midline and intercalate, leading to the extension of the body axis [6,7].
Box 1. Interactions between PCP and A/B determinants.
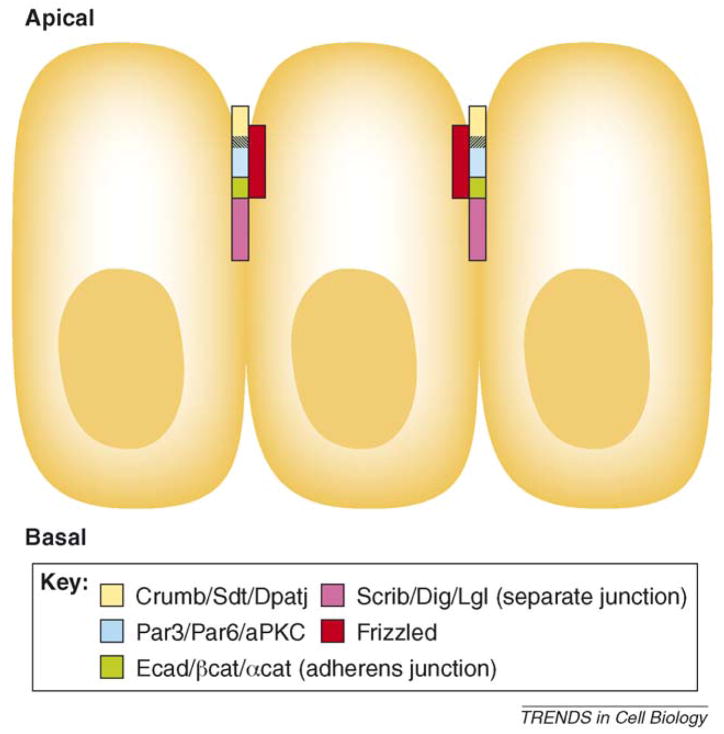
Epithelia are polarized in the apical–basal axis and manifest through several junctional complexes (Figure I), which include the Crumbs complex (consisting of Crumbs, Stardust and Patj), the Par complex (consisting of Par3/Bazooka, Par6, aPKC and Cdc42), the adherens junction (AJ) complex and the Dlg–Scrib–Lgl complex [93,94] (Figure I). AJs consist of E-cadherin, β-catenin and α-catenin, which in turn connect AJs to the actin network. All core PCP factors analysed, Fz (Figure I), Vang, Fmi, Dsh, Pk and Dgo overlap with AJs and Patj based on confocal microscopy. Previous work showed that electron microscopy (EM) is required to determine precise subcellular localization of Crumbs and Patj (current name instead of Dlt; Ref. [95]) relative to the other A/B determinants and, therefore, the precise location of the PCP factors needs to be verified by EM.
The core PCP proteins are concentrated in a small subcellular domain (‘subapical ring’), enabling efficient and specific interactions among these molecules. The functional importance of Fz subapical localization was studied in Drosophila [44]. Fz (also known as Fz1) is localized at AJs and Fz2 is evenly distributed along the apical–basal axis. These localization patterns are controlled by the cytoplasmic tails of the receptors [44]. Accordingly, an Fz1-1-2 chimera (with a Fz2 cytoplasmic tail) displays reduced PCP activity compared with wild-type Fz [44], because reduction of Fz localization at AJs decreases Fz PCP activity. aPKC is also implicated in modulating Fz PCP activity through the regulation of Fz phosphorylation [96], with dPatj recruiting aPKC to Fz [96]. Phosphorylation by aPKC inhibits Fz activity. Another apical protein Par3 (Bazooka in flies) antagonizes aPKC, thus protecting Fz from its negative effect [96].
The localization pattern of core PCP factors overlaps with E-cadherin localization along the apical–basal axis in several vertebrate tissues. In the developing mouse epidermis, E-cadherin is localized at the entire lateral membrane [18] and so does Fz6 and Vangl2 [18]. In the gut and intestinal endothelium, E-cadherin is localized at apical or sub-apical domains (similar to Drosophila epithelia) and Fz3 again colocalizes with E-cadherin at apical or sub-apical domains in these tissues [97]. Although there are variations in the localization patterns, Fz3/6 and Vangl2 usually colocalize with the E-cadherin domain along the apical–basal axis. Whether or not such localization of PCP molecules is functionally important in vertebrates needs to be addressed.
Figure I.
Apical localization of junctional protein complexes and PCP components.
Substantial progress has been made in the identification of key PCP factors, dissection of their intracellular interactions and insight into potential input into downstream effectors, which can be general or tissue specific (for detail on these features see Refs [1,8]). However, the long-range (global) aspects of coordinated cellular polarization and the overlying regulatory mechanisms that create the initial polarity direction or asymmetry have remained elusive. Two general models have been proposed. The first model proposes that polarity is initially determined in a small region within a tissue and then propagates across the tissue similar to ‘domino effect’ [9]. A second model proposes that there is a graded activity of a signal (or signals), which sets up a bias in each cell of a tissue. Several recent publications have tried to address how this might be achieved and how cells communicate polarity information to neighboring cells. These papers have re-ignited the discussions on how the global regulation of PCP is controlled and how the distinct core factor groups interact, and this review focuses on these features.
The Frizzled–Fmi core PCP group
PCP genes were originally identified in Drosophila based on loss-of-function phenotypes, which displayed irregular cellular hair orientations in the adult [10,11] and consequently were named, for example, frizzled, disheveled or prickle. PCP genes are found in two varieties: (i) core PCP genes (required in all tissues); and (ii) tissue-specific factors acting in one or a subset of tissues [1,3,8]. frizzled (fz), disheveled (dsh), Van Gogh (Vang; also known as strabismus [stbm]), prickle (pk), diego (dgo) and flamingo (fmi; also known as starry night [stan]) are core PCP factors of the Fz–Fmi group [2,3,8,12,13] (Table 1). Examples of tissue-specific PCP genes (i.e. in the Drosophila wing) include inturned (in) and multiple wing hair (mwh) [14–16]. These are thought to act downstream of core PCP factors and to participate in organizing cyto-skeletal networks.
Table 1.
Core PCP factors and their molecular features
| Drosophila gene | Mouse gene(s) | Molecular features and function(s) | Primary PCP Refs |
|---|---|---|---|
| Fz/Fmi core group | |||
| frizzled (fz) | Fz3, Fz6 | 7-pass transmembrane receptors; bind Wnt ligands and also activate Wnt–β-catenin signaling; interact with Dsh–Dvl proteins; recruit Dsh and Dgo to the plasma membrane; can co-IP with Fmi | [19,20,40,47,63] |
| disheveled (dsh) | Dishevelled-like 1–3 (Dvl1, Dvl2, Dvl3) | Cytoplasmic protein containing DIX, PDZ and DEP domains; recruited to membrane by Fz; binds to Fz, Pk, and Dgo (all species tested) and has also been shown to bind to Vang–Stbm in Xenopus | [64–70] |
| Van Gogh (Vang)/strabismus (stbm) | Vang-like 1 and 2 (Vangl1, Vangl2), Vangl2 also known as Looptail zebrafish mutant: trilobite (tri) | Novel four-pass transmembrane proteins; bind Pk, and Dgo (and Dsh in Xenopus); recruit Pk to the membrane; can co-IP with Fmi–Celsr | [18,21,30,41,53, 70–73] |
| prickle (pk) (also known as prickle-spiny legs) | Pk1 and Pk2 | Cytoplasmic protein with 3 LIM domains and a PET domain; recruited to the plasma membrane by Vang; can interact directly with Dsh, Vang and Dgo; competes with Dgo for Dsh binding. | [30,31,53,69, 74–76] |
| flamingo (fmi)/starry night (stan) |
Celsr1, Celsr2 and Celsr3 (Cadherin-EGF-LAG 7-pass G-type receptor 1) Zebrafish mutant: off-road (Celsr2) |
Multiple Cadherin repeats in extracellular domain, seven-pass transmembrane receptor features; capable of homophilic cell adhesion Can co-IP with Fz and Vang |
[18,33,47,77,78] |
| diego (dgo) | diversin (also known as ankyrin repeat domain 6; ankrd6); inversin (invs) | Cytoplasmic proteins containing 6 ankyrin repeats; recruited to membrane by Fz; bind Dsh, Vang and Pk; compete with Pk for Dsh binding | [62,69,79–81] |
| Fat/Ds core group | |||
| fat (ft) | Fat1, Fat2, Fat3, Fat4 (Fat-j) | Proto-cadherins with single pass transmembrane; binds Ds extracellularly (Drosophila); interacts with Atrophin in cytosol | [52,82–84,85] |
| dachsous (ds) | Dchs1, Dchs2 (more related genes possible) | Proto-cadherin with single pass transmembrane; binds Ft extracellularly | [52,82,83,86,87] |
| four-jointed (fj) | Fjx1 | Type II membrane protein; localized in Golgi; kinase, can phosphorylate Ft, Ds | [52,82,83,88,89] |
| approximated (app) | human DHHC9, DHHC14, DHHC18 | DHHC palmitoyltransferase; negative regulator of Ft | [90] |
| atrophin (atro),also known as Grunge (Gug) | Atrophin (human) | Transcriptional co-repressor; can bind to Ft cytoplamsic tail | [91,92] |
The core PCP genes are evolutionarily conserved and required for PCP establishment in all organisms tested, including mammals [2,4,5,12,17] (Table 1). Here, they regulate cell orientation in many contexts, such as in the skin [18,19], stereociliary bundles in sensory cells in the inner ear (e.g. see Refs [20–23]) and convergent extension processes of mesodermal and neural tube precursor cells (for reviews, see Refs [24,25]). For a discussion of vertebrate PCP features, see the review by Wang and Nathans [5].
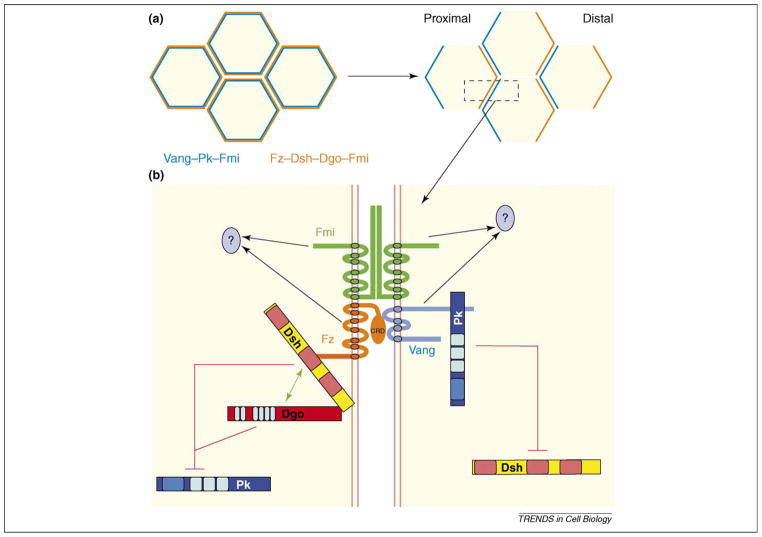
PCP proteins of the Fz–Fmi group are initially (preceding and during early stages of PCP signaling) localized within a subapical ‘ring’ at or near the adherens junctions in epithelial cells [3] (Box 1 and Figure 1a). At later stages, Fz, Dsh and Dgo become concentrated at the distal edge of wing cells [26–28] or the polar side of R3 cells in the eye [27,29], whereas Vang and Pk are concentrated to proximal sides of wing cells [30,31] or the equatorial side of R4 precursors in the eye [29,32] (Figure 1). Fmi co-localizes with both complexes in wing cells or at the R3–R4 border in the eye [33]. Equivalent polarization of core PCP molecules exists in vertebrate tissues in the respective axes [18,20,22,34,35]. These observations suggest that both polarized localization patterns of PCP molecules and associated signaling processes that lead to these patterns are evolutionarily conserved. In addition to the core group mentioned earlier, a second group centered on fat (ft), dachsous (ds) and four-jointed (fj) is required for PCP establishment (Table 1). The functional relationship of the Fat–Ds and Fz–Fmi groups is further described in Box 2.
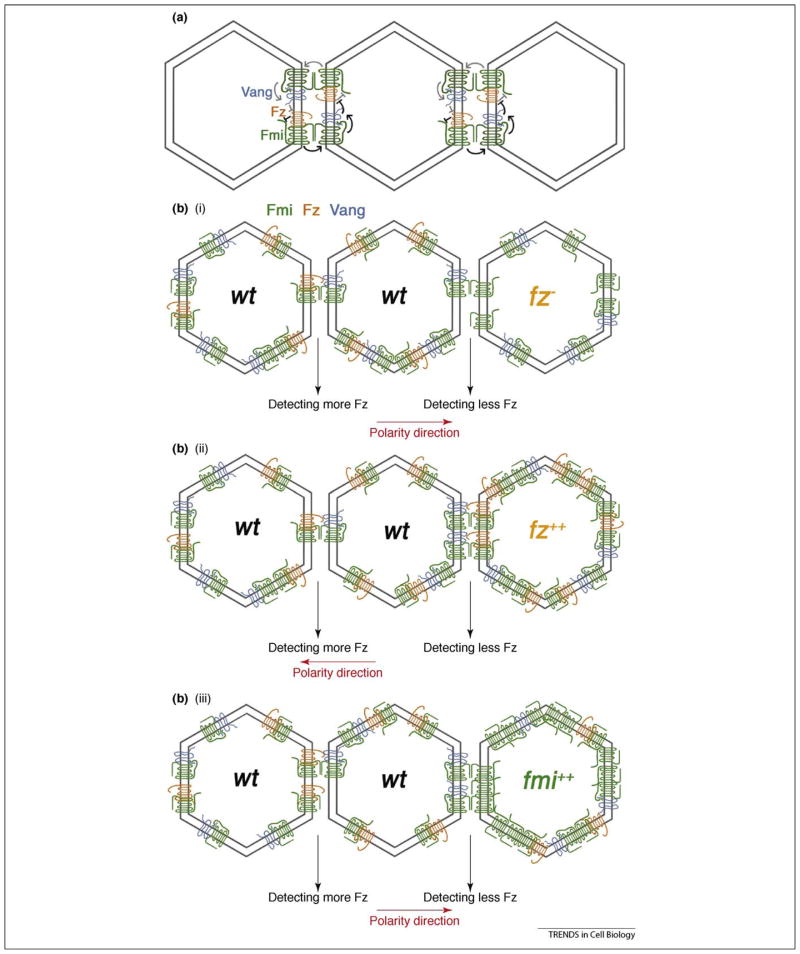
Figure 1.
Asymmetric localization of core PCP molecules using the fly wing as an example. (a) Top view of wing cells. At early pupal stages (left), the core PCP molecules are evenly distributed as a ring at and/or just apical to the adherens junctions (not illustrated). At later pupal stages (right), a Fz–Dsh–Dgo–Fmi complex is concentrated at distal edges of cells and the Vang–Pk–Fmi complex is concentrated at proximal edges of cells. (b) Schematic presentation of the Fz–Fmi-group PCP factors and their interactions. Fz is shown in orange with its interacting partners Dsh (yellow, with its specific domains DIX, PDZ and DEP [from left to right] in light red) and Dgo (red with its Ankyrin repeat domains in light blue). Fmi is shown in green. Vang is shown in light blue and its interacting partner Pk in dark blue (the PET and 3 LIM domains are indicated by lighter shades of blue). Fz can bind to Vang primarily via its CRD and Fmi displays homophilic interactions. Dsh and Dgo physically interact and can antagonize Pk (red lines on left); Pk antagonizes Dsh (red lines on right). The downstream effectors of non-autonomous signaling are unknown (black arrows with question marks).
Box 2. The relationship of the Ft–Ds and Fz–Fmi groups is complex.
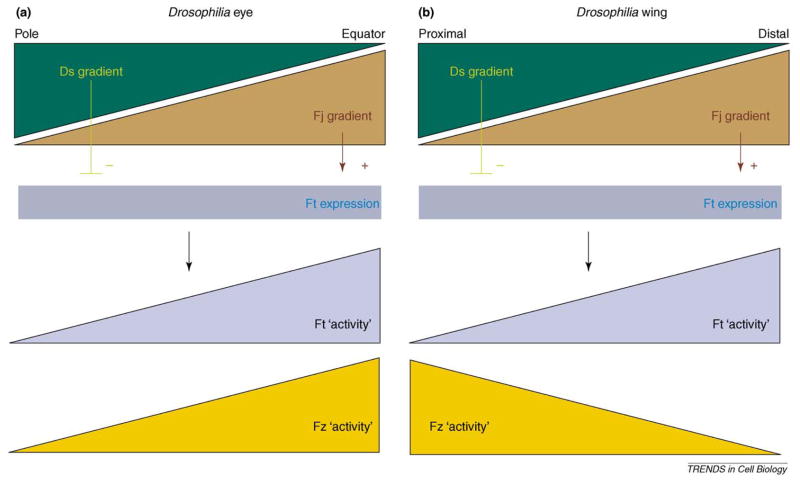
Originally, the Ft–Ds group was thought to act upstream of the Fz–Fmi core group, thereby influencing the asymmetry of the interactions among the Fz–Fmi-group proteins, as the polarization of Dsh, Pk and Fmi is altered in ft or ds mutants [82,83]. Ds and Fj are expressed in a graded fashion in the eye, wing and abdomen, and it was proposed that Ds and Fj act as inhibitors and activators of Ft activity, respectively, creating a Ft ‘activity’ gradient (Figure I). It was further suggested that Ft activity acts on Fz (and possibly other factors of the Fz–Fmi group) to generate their directed activation and localization pattern.
However, the slopes of the Ds–Fj gradients are opposite relative to the proposed Fz activity gradient in wing and eye tissues (Figure I), indicating that the relationship between the Ft–Ds and Fz–Fmi groups is more complicated (in the abdomen the slopes are inverted between the anterior and posterior compartment in each segment, further complicating the issue [98]). Moreover, the Ds or Fj gradients are only important for PCP establishment in the eye and not in the wing [86,99] because evenly expressed Ds largely rescues ds− mutant or ds− fj− double mutants in the wing (although minor PCP defects in the anterior proximal region are still present [99]). This could indicate that Ds and Fj gradients have minor roles in specifying wing PCP (the mild remaining PCP defects can be caused by non-optimal Ds expression levels in the rescue experiments, because even expression of Ds is driven by a heterologous promoter [tub-Gal4], which is often higher than endogenous levels). In addition, polarized localization of Fz–Fmi-group proteins is lost if any one factor of the Fz–Fmi group is mutant, suggesting that the core PCP factors within the Fz–Fmi group control their own polarization [3]; by contrast, in fat or ds mutants such polarized localization is still detected, albeit with abnormal orientation.
Recent work in the dorsal abdomen strongly demonstrates that the Ft–Ds and Fz–Fmi groups act in parallel to one another [12,52]. For example, overexpression and loss-of-function clones for fat or ds repolarize neighboring cells in fz−, fmi− or even double mutant fz− fmi− backgrounds [52]. Moreover, in the larval cuticle, PCP defects are only observed when both core factor sets are affected, suggesting that in this context they not only act in parallel but also redundantly [52]. A counter argument could arise from the fact that ft− clones seem not to repolarize neighbors in a fz− background in the wing [12,83]. However, this can be explained by the fact that fz− wings have such strong PCP disorganization that it is hard to detect repolarizing effects compared with tissues such as the abdomen which remain more organized in a fz− background [12]. Although the two core groups are not redundant in most tissues and mutations in a component of either core group are sufficient to generate robust PCP defects, double mutants for fmi− ds−, which affect both core groups, display much stronger phenotypes than either single mutant [52]. Because the double mutant effect seems to be additive, these data support the notion that the two groups act in parallel. More experimental data and discussion with regard to the relationship between the two core groups is presented elsewhere [12,52]. The downstream components of the Ft/Ds group are largely unknown. Atrophin can bind Ft and act downstream in regulating Fj expression and PCP signaling during R3–R4 precursor specification in the eye [91] and approximated, an Asp-His-His-Cys palmitoyltransferase, acts as an inhibitor of Ft signaling [90].
Figure I.
The relationship of Ds, Fj gradients and proposed Ft and Fz activity gradients in the eye and wing. (a) In the eye, Ds is expressed in a gradient that is highest at the poles, and low at the equator. Fj expression is highest at the equator and low at the poles. Thus, the Fz activity slope and Fj gradient are opposite to the Ds gradient. (b) In the wing, Ds is high proximally and low at the distal end and Fj is again the opposite. In contrast to the eye, however, the Fz activity slope is the same as Ds and opposite to Fj. Fz is expressed evenly in both eye and wing; a Fz activity gradient is inferred from genetic data. In summary, the slope of the Fz ‘activity’ gradient and Ds–Fj expression gradients (regulating Ft ‘activity’) have the relative opposite directions in the two tissues, suggesting that interactions between Fz–Fm-group and Ft–Ds-group factors, if any, must be more complicated than a simple one way regulatory input.
The genes within the Fz–Fmi group can be subdivided into cell autonomously and non-autonomously acting factors. Whereas dsh, pk, fmi or dgo mutant clones only affect PCP within the mutant cells [36–39], fz and Vang also affect the orientation of wild-type cells adjacent to the mutant tissue patch [39–41]. These observations suggested that Dsh, Pk and Dgo are only involved in receiving (or the interpretation of) PCP signals for establishing PCP within cells and do not send signals to neighboring cells. However, Fz and Vang are, in addition to their cell-autonomous roles, involved in cell-to-cell communication and propagation of PCP establishment, coordinating polarity at a more global level. The mechanism(s) of this Fz–Vang-mediated cell-to-cell communication process and the potential role of Fmi in it are discussed in the next section.
Non-autonomous Fz PCP signaling
Clonal analyses of the core PCP genes fz and Vang in the wing have revealed that polarity is not only altered within fz− or Vang− mutant clones but also in nearby wild-type cells [40,41]. Equivalent observations were made in the abdomen [39] and the eye [38,42], indicating that this behavior is a common feature of Fz and Vang and that they are involved non-autonomously in cell-to-cell communication. Similar non-autonomous effects of Fz have been observed in mammals, in which the phenotypic features of mosaic mouse Fz6− skin suggest non-autonomous cell–cell communication defects [19], indicating that the mechanism(s) regulating this are conserved.
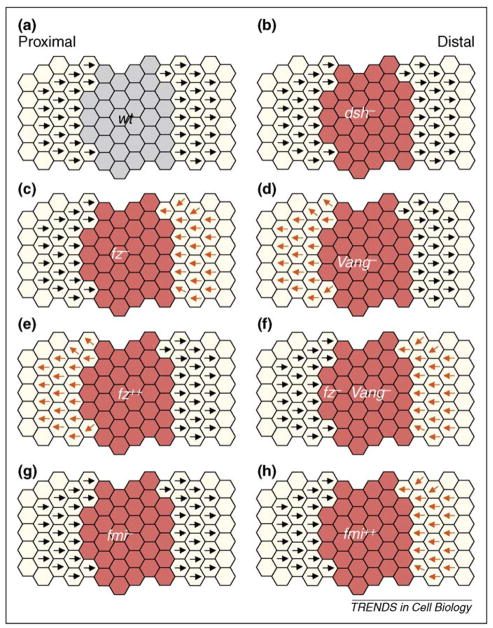
The fz− clones repolarize ~3–9 rows of wild-type cells in the wing or 2–4 rows in the abdomen with wild-type neighboring cells pointing towards mutant tissue [39,40]. This non-autonomous effect can be observed in all areas of the wing and abdomen [39,40]. Fz overexpression causes the opposite phenotype and wild-type neighboring cells reorient away from Fz-overexpressing cells [39,43,44] (Figure 2 shows schematic presentations of the non-autonomous behavior of distinct PCP factors). Based on these non-autonomous effects, Adler proposed that cells orient away from high and towards low Fz levels [43]. In wild-type flies, this is in the proximal–distal axis in the wing, the anterior–posterior axis in the abdomen and the equatorial–polar axis in the eye [2].
Figure 2.
Schematic illustrations displaying non-autonomous effects of clones of different genetic backgrounds in Drosophila wings. Orientation of cells surrounding mutant clones is indicated by arrows (black arrows for normal orientation and orange arrows for repolarized orientation). The mutant cells themselves (marked by red fields) show random orientation, which is not indicated by additional means. (a) Wild-type (wt) clone does not change polarity (for comparison). (b) dsh− mutant clone causes polarity defects within mutant cells but does not affect the polarity of neighboring cells (black arrows). (c) fz− mutant clone causes wild-type distal neighboring cells to reverse polarity and point toward the clone (orange arrows). (d) Vang− mutant clone causes proximal wild-type neighboring cells to reverse polarity and point away from mutant tissue (orange arrows). (e) Fz overexpressing clone (fz2+) causes neighboring cells to point away from clone (orange arrows). (f) fz−, Vang− double mutant clone causes neighboring wild-type cells to point towards mutant tissue (orange arrows; very similar to fz− mutant clones). (g) fmi− mutant clone has polarity defects inside mutant tissue but does not affect polarity of wild-type neighboring cells. (h) fmi overexpressing clone (fmi2+) causes wild-type neighboring cells to point toward the clone (orange arrows; similar to fz− mutant patches). See main text for discussion.
Vang− clones also display non-autonomous effects, but the reorientation of wild-type neighboring cells is in the opposite direction, with cells orienting away from mutant tissue [39,41] (Figure 2d). Similarly, in the mouse Vangl2 mutant, skin tissue also displays non-autonomous effects [18], supporting the notion that non-autonomous cell–cell communication mechanisms (affecting global PCP patterning) are evolutionarily conserved. Because fz−, Vang− double mutant clones (Figure 2f) display phenotypes very similar to fz− single mutant clones [45] (Figure 2c), it seems that the effect is mediated by cells reading Fz level information, rather than that of Vang (Fz level information rather than Vang levels seem to affect the orientation of neighboring wild-type cells).
The time requirement of non-autonomous Fz signaling precedes the time when the Fz–Fmi group core PCP molecules become asymmetrically localized [45], suggesting that Fz non-autonomous signaling has an instructive role in the global polarization of tissues. This is consistent with the observation that fz− or Vang− clones alter the localization behavior of all core PCP proteins [28,30,31,46,47]. Importantly, the non-autonomous Fz signaling process does not require Dsh (Figure 2b) or Dgo [37,38,45,46], suggesting that it uses distinct molecular mechanism(s) from the Fz–Dsh-mediated cell-autonomous PCP signaling.
What are the molecular mechanism(s) of Fz non-autonomous signaling?
Fz-mediated non-autonomous signaling also requires the third transmembrane factor of this core group, Fmi (aka Starry night [Stan]; Table 1). Although, fmi− mutant clones do not display non-autonomous effects on cellular orientation patterns [33,39] (Figure 2g), Fmi is required for the non-autonomous effects of Fz (because Fz-mediated non-autonomy is suppressed in a fmi− background [39]). The extracellular domain of Fmi can interact homophilically in trans [33] and the removal of Fmi from one cell causes the disappearance of Fmi from the contacting cell-membrane of a wild-type neighboring cell [33]. This suggests that the homophilic interaction across cell membranes stabilizes Fmi protein complexes just apical to or at adherens junctions on both sides of contacting cells (Box 1 and Figure 1b). Fmi is thus required for apical localization (or stabilization) of both Fz and Vang [27,28,30]. The reverse also applies, in that Fz and Vang are required for apical localization (or stabilization) of Fmi because its junctional localization is reduced in either single fz− or Vang− mutant clones, and even more so in fz− Vang− double mutant clones [48]. The mutual dependence of Fmi and Fz or Vang localization is also supported by the fact that Fmi can be co-immunoprecipitated with Fz [47] and that Celsr1 (a mouse Fmi orthologue; Table 1) can co-immunoprecipitate mVangl2 [18]. Collectively, these data suggest that Fmi (Celsr) interacts with Fz and Vang (mVangl2) to form stable complexes during PCP establishment.
From the genetic observations and the fact that Fmi interacts homophilically in trans, it was proposed that Fmi could transduce the polarizing information across cells [8,39,49]. In the model proposed by Lawrence and colleagues, Fz inhibits Fmi activity within the same cell and this is ‘transduced’ to the neighboring cell through a Fmi–Fmi bridge [39]. In the neighboring cell, this Fmi–Fmi interaction activates Vang, which in turn represses Fz there and this type of interaction spreads the polarity information from cell to cell. This ‘interaction loop’ can lead to a graded Fz activity across an entire field if a gradient of a factor X is proposed that would inhibit Fz [39]. This model is able to explain the fz−, Vang− and fmi− mutant phenotypes (for details, see Ref. [39]; Figure 3a). Because there is no experimental evidence ‘for’ or ‘against’ direct signaling mediated by the Fmi–Fmi bridge, it does not exclude the possibility that the ‘Fmi bridge’ functions simply to bring Fz and Vang in proximity at the opposite membranes across cells, thereby to enabling Fz to bind (or inhibit) Vang directly (another possibility is that Fmi is needed for proper apical localization of Fz and Vang near junctions; see earlier). A recent variation of the ‘Fmi–Fmi bridge’ model proposes that Fmi interacts homophilically in an asymmetric manner [47]. That is, Fmi that is part of an Fmi–Fz complex adopts a different conformation to Fmi itself (or with Vang) so as to ‘transduce’ the Fz levels between neighboring cells [47]. A problem with this model is that fmi− clones do not show a non-autonomous behavior analogous to fz and, therefore, the interaction mechanism must be (at least in part) different and more complex (i.e. a non-autonomous effect of Fmi is only observed when Fmi is overexpressed; Figure 2h). One possibility is that Fmi acts permissively in the process, being required in both the signal-sending and signal-receiving cell (see the next section). Another possibility is that Fmi is instructive, but only during the second phase of PCP establishment when the inter- and intra-cellular feedback loops stabilize the individual Fz- and Vang-based complexes on opposing membranes.
Figure 3.
Illustration of non-autonomous PCP signaling models. Top view of pupal wing cells. (a) Homophilic Fmi–Fmi intercellular bridge mediates the intercellular interaction between Fz and Vang [39]. This model proposes that Fz activates itself in the neighboring cell through an Fmi–Fmi intercellular bridge. Fz inhibits Fmi in the same cell and Fmi activates Vang in the neighboring cell. Vang antagonizes Fz activity. This regulation leads to the formation of a feedback loop. The interaction trails (following either black or grey signs in the panel) indicate that Fz activates Fz in neighboring cells. This would lead to an overall increase in Fz activity in the whole field. A gradient of ‘factor X’ is proposed to inhibit Fz activity. As a result, a Fz activity gradient is generated in response to this X-gradient. The model is based on data presented in Refs [39,50]. (b) A direct ‘Fz–Vang’ interaction model: (i) A wt cell in the middle ‘senses’ less Fz in a fz− mutant cell on right as compared to wt cell on left through the Fz–Vang interaction. Both Vang and Fz form complexes with Fmi. The Fmi–Fmi interaction brings Fz and Vang close to each other and stabilizes them at apical junctional regions, thus facilitating their interaction. (ii) A wt cell in the middle senses more Fz in the Fz overexpressing cell on the right as compared to wt cell on left. Polarity direction is adjusted accordingly. (iii) Fmi is overexpressed in right cell. Due to the large amount of Fmi in the apical junctional region, it outcompetes Fz–Fmi complexes to interact with Vang–Fmi and thus relatively less Fz is able to bind to Vang in the middle cell. As a consequence, Vang ‘detects’ less Fz in the Fmi-overexpressing cell as compared to wt cells (and polarity is changed accordingly), although absolute levels of Fz are not decreased in Fmi-overexpressing cells.
A direct interaction between Fz and Vang
Recently, a different model was proposed in which a direct Fz–Vang interaction across cell membranes mediates the initial PCP direction [45,50]. In this model, the extracellular region of Fz, in particular, its cysteine-rich domain (CRD) physically interacts with the extracellular regions of Vang across cell membranes (Figure 1b). The resulting cellular orientation depends on the amount of available Fz to bind to Vang on neighboring cells [45]. This model is supported by several observations: (i) biochemical interaction between the FzCRD and Vang [45]; (ii) the Fz–Vang interaction can occur in trans across two cells in cell culture because Fz from one cell can recruit Vang from the neighboring cell to the contacting membrane [48]; and (iii) Fz non-autonomous signaling depends on FzCRD, because even a substitution with a different CRD (i.e. from Fz2) eliminates the non-autonomous signaling capability of Fz [45]. This model is supported by genetic data showing that the Fz non-autonomous activity is suppressed in Vang− backgrounds [39,41] and that a Fz isoform defective in its non-autonomous activity (by either deleting or replacing the CRD) cannot rescue the fz− null mutant PCP phenotype [45,51].
Despite these observations, Fmi is crucially required in this context, but how it participates mechanistically remains unresolved. It is likely that it stabilizes the Fz–Vang interaction through its homophilic interaction feature, or participates by other means, such as stabilizing the PCP protein complexes at apical junctional membranes. For example, in cell culture the presence of Fmi dramatically increases cell–cell contact associated with Fz–Vang localization [48]. Moreover, as mentioned earlier, Fmi is genetically required in both signal-sending (Fz) and signal-receiving cells (Vang) [39,52] and Fmi is required for correct subcellular localization of Fz and Vang near adherens junctions [27,28,30] (Box 1). This apical localization is important for the PCP function of Fz [44]. It is probable that junctional localization brings all PCP core components into a narrow band to increase signaling and interaction efficiency. Thus, the role of Fmi in the ‘Fz–Vang interaction model’ could mainly be to bring Fz and Vang to the right subcellular location or to help form Fmi–Fz and Fmi–Vang complexes that can then signal efficiently. In an ‘Fmi bridge signaling model’, the role of Fmi is to directly transduce the signal. For example, ‘Fz levels’ and the assembly of Fmi–Fz and Fmi–Vang complexes would modify the activity of Fmi, giving the homophilic interaction a direction [47]. Importantly, molecular experimental data would need to be provided to strengthen this model and, as mentioned earlier, fmi− mutant clones do not display non-autonomous effects (non-autonomous effects are only observed when Fmi is overexpressed). This indirectly supports the ‘Fz–Vang direct interaction model’. The observation that Fmi overexpression causes non-autonomous phenotypes similar to fz− can be explained through the ‘Fz–Vang interaction model’. When Fmi is overexpressed, its levels increase to much higher amounts relative to Fz at the subapical regions [48]. Under such conditions, Fmi outcompetes Fz–Fmi complexes and so Vang will detect less Fz on neighboring cells. Therefore, cell polarization will follow the lower levels of Fz detected by Vang and orient towards the Fmi-overexpressing cells (Figure 3b). Although this explanation is not a direct proof of the ‘Fz–Vang interaction model’, the Fmi-overexpression phenotype is compatible with it.
Because the protein complexes signaling across cell membranes contain both Fz and Fmi on one side and Vang and Fmi on the other (Figure 4), it is difficult to assess within the complex whether the Fz–Vang or an Fmi–Fmi interaction is more important for the transduction of polarity information or the instructive signal. Among components of one complex it is difficult to genetically determine the upstream or downstream relationships. One possibility to distinguish between the two mechanisms could be to look at downstream signaling events. However, at this juncture, no component is known that acts downstream of Fmi or Vang for the non-autonomous cell–cell communication aspect. Although Pk interacts with Vang in both biochemical and recruitment assays [30,53], Vang does not require Pk for non-autonomous activity. Strikingly, fz− non-autonomy is even enhanced in pk− mutant backgrounds [36,39], suggesting that Pk might compete for Vang binding with a ‘non-autonomous signaling’ effector (if pk were involved in non-autonomous signaling it would suppress the fz effect, as is the case for Vang− mutants). To further understand the intracellular signaling events of non-autonomous signaling, the identification of additional components in the pathway is required.
Figure 4.
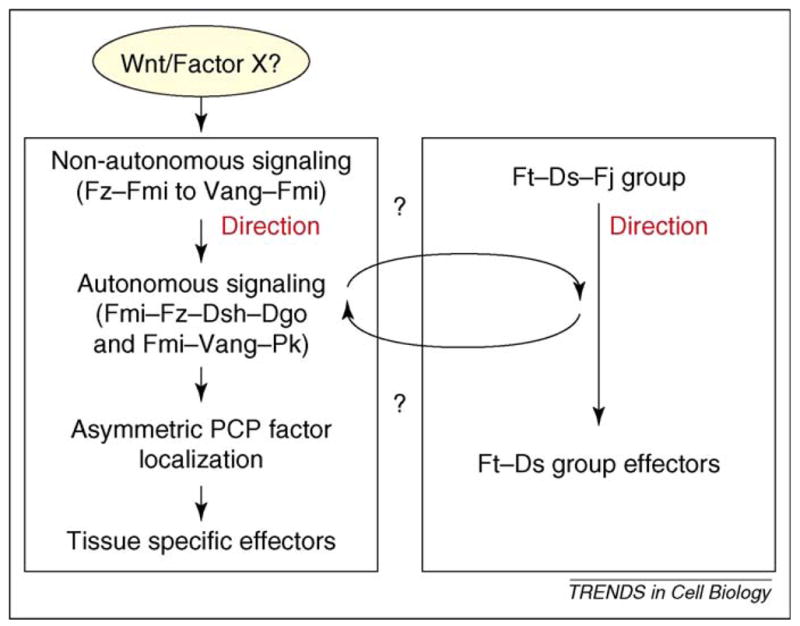
Summary of the relationships between the PCP signaling events. The diagram schematizes the relationships among the non-autonomous and autonomous Fz–Fmi core PCP signaling and Ft/Ds PCP signaling. The two core groups seem to act in parallel. The nature of Factor X to provide a polarization bias for the Fz–Fmi group (a Wnt or other) remains unknown.
How is the Fz–Vang interaction regulated globally?
Assuming that the Fz–Fmi group acts in parallel to the Fat–Ds-group (Box 2), it remains unclear what upstream signaling events polarize the Fz–Vang (or Fz–Fmi–Vang–Fmi) interactions. Are there diffusible factors that could modify this interaction in a polar manner over a long range? Do these factors act on Fz or Vang or even on Fmi? No diffusible binding partners are known for either Vang or Fmi (Celsr) in any organism. For the Fz factors, an obvious candidate pool includes the Wnt family growth factors, which act as ligands for Fz receptors in the Wnt–β-catenin signaling pathway to regulate cell fate, growth and other biological processes [54]. This raises the intriguing possibility that Wnt(s) also interact with Fz(s) in the PCP pathway, but their role in PCP establishment is still being addressed.
In Drosophila, none of the Wnt family members has yet been linked to PCP. In vertebrates, however, Wnt11 and Wnt5 are required for the convergent extension during the gastrulation [55,56], suggesting that these Wnts have a crucial role in PCP establishment. Whether they function in a permissive or instructive manner during the process has not been clarified. Notably, the injection of Wnt11 RNA into very early zebrafish embryos rescued the Wnt11/silberblick loss-of-function phenotype, suggesting that the RNA does not need to be localized. Therefore, the (ubiquitous) presence of Wnt11 is sufficient and argues for a permissive role. Nevertheless, the absence of Wnt-11, -5 and -4 does lead to the loss of polarized Vang (Stbm) and Pk localization in developing zebrafish embryos [34].
By contrast, recent experiments with Wnt11 during somite patterning in chick embryos support the notion that Wnts are instructive in PCP establishment. It was shown that Wnt11 is expressed in the neural tube from where it orients the muscle fibers in neighboring somites in the anterior–posterior axis parallel to the neural tube [57] (all manipulations of Wnt11 expression were consistent with this interpretation). This also requires several core Fz–Fmi-group PCP molecules, suggesting that Wnt11 signals through the PCP core factors in orienting muscle fibers [57].
As mentioned earlier, in Drosophila it is unclear whether Wnt proteins contribute to PCP. Gain-of-function experiments with dWnt4 can alter cellular orientation of PCP in the wing [58,59], but loss-of-function alleles of dWnt4 do not show equivalent phenotypes. In the eye, an allelic combination of dWnt4 has been reported to show PCP defects (e.g. ommatidial chirality inversions) but these could be caused by an ectopic equator [59] similar to fj mutant clones in the eye [60]. However, because both the fj and ds gradients are under the transcriptional control of canonical Wnt–Wg-signaling in the eye, these defects could be caused by Wnt–β-catenin signaling rather than by a direct effect on PCP signaling [60]. Obviously, Wnts are not necessarily the only candidates and a novel Fz-binding factor might be discovered. In addition, in the abdomen, another secreted factor also seems to affect PCP in one compartment, namely Hedgehog (Hh) [58].
More work is needed to determine whether Wnts have an instructive role in PCP or function in PCP at all in Drosophila and how this might be related to the equivalent function in vertebrates, or whether other factors (either Hh or as yet unknown components) perform such roles.
Concluding remarks: relationship among non-autonomous, autonomous and Ft–Ds group PCP signaling
How are the distinct steps and aspects of PCP establishment linked and integrated at the cellular level? Several signaling events occur during PCP determination, which need to be coordinated (a schematic view of how these events might be connected is presented in Figure 4).
Non-autonomous Fz–Fmi group signaling seems to be upstream of the cell-autonomous feedback loops of the same core factors and their signaling to tissue specific effectors. Changing Fz or Vang levels (and thus their intercellular interactions) affects the direction of polarization, which is then propagated over some distance from cell-to-cell through local interactions. The factors acting cell-autonomously amplify these effects and non-autonomous repolarization is also detected by the altered localization of the autonomous core PCP factors such as Dsh and Pk [28,30,31,61]. Thus, non-autonomous PCP signaling affects the feedback loops (and direction) of cell-autonomous events. As such, the asymmetric localization of Fmi–Fz–Dsh–Dgo and Fmi–Vang–Pk complexes is a downstream signaling event (autonomous), serving also as the first visible read-out of PCP establishment. The asymmetric localization pattern is absent when cell-autonomous signaling is defective, such as in dsh− and/or pk− mutants [28,30,31,38,61].
Interestingly, in several cases in which cell-autonomous PCP factors are defective, the resulting phenotypic defects are less severe than expected from the aberrantly polarized localization of core PCP proteins. For example, PCP is less defective in pk− than in fz− animals in posterior compartments of the abdomen and several areas of the wing, suggesting that some aspects of PCP signaling occur in pk− and with defects in the asymmetric localization of core PCP factors [39]. Similarly, in dgo− wings the asymmetric localization of Fmi is lost, but cell orientation defects in dgo− mutants are much milder than the symmetrical Fmi localization would predict [62]. These observations suggest that the early non-autonomous signaling information can be interpreted and the feedback loops that reinforce the polarization are partially dispensable in certain tissues. As such, factors including Pk and Dgo only participate in feedback loops after the initial polarization has been established and the asymmetric localization of core Fz–Fmi-group proteins acts downstream and reinforces the PCP bias established earlier. PCP establishment can thus occur in some instances in the absence of asymmetric localization patterns of the Fz–Fmi-group proteins and that other (parallel) mechanisms of cell–cell communication exist. These might again argue that the Fat/Ds group acts in parallel to the Fz/Fmi group (Box 2).
In summary, PCP establishment does not seem to rely on a single signal transduction cassette or pathway. PCP generation is regulated by several core factor groups that show complex relationships. Importantly, our understanding has not reached a level that would suffice to make clear predictions of the molecular network among a given core group. Future work will need to establish the logic of the molecular interactions among the known players of the ‘global polarization’ in PCP establishment and dissect the molecular connections between the distinct PCP signaling processes (Box 3).
Box 3. Outstanding questions.
What are the instructive signal(s) for determining the direction of PCP polarization? How does this signal connect to the Fz, Vang or Fmi core PCP proteins or even the Ft–Ds PCP proteins?
What, if any, are the molecular links between the Fz–Fmi PCP pathway and the Ft–Ds PCP group?
What are the effectors downstream of Vang, Fz or Fmi in non-autonomous PCP signaling?
How does Fz mediated non-autonomous PCP signaling connect to autonomous signaling molecularly?
What are the molecular events that lead to the asymmetric localization of Fz–Dsh–Dgo and Vang–Pk complexes?
Update
After this article was accepted for publication, a paper has been published showing that knockdown of Vangl2 expression, Fz3 or Vangl2 overexpression in Xenopus has non-autonomous effects on PCP that are very similar to the non-autonomous effects in Drosophila. This further suggests that the non-autonomous PCP signaling mechanism is largely conserved. See Mitchell, B. et al. (2009) The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 19, 1–6
Acknowledgments
The authors would like to apologize to those colleagues whose work could not be cited here owing to space limitations.
References
- 1.Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 2.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 3.Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- 4.Karner C, et al. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 6.Tada M, et al. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–260. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 7.Wallingford JB, et al. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 8.Klein TJ, Mlodzik M. PLANAR CELL POLARIZATION: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 9.Fanto M, McNeill H. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- 10.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 11.Adler PN. The genetic control of tissue polarity in Drosophila. Bioessays. 1992;14:735–741. doi: 10.1002/bies.950141103. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence PA, et al. Planar cell polarity: one or two pathways? Nat Rev. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 14.Adler PN, et al. The Drosophila tissue polarity gene inturned functions prior to wing hair morphogenesis in the regulation of hair polarity and number. Genetics. 1994;137:829–836. doi: 10.1093/genetics/137.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strutt D, Warrington SJ. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development. 2008;135:3103–3111. doi: 10.1242/dev.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, et al. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics. 2008;180:219–228. doi: 10.1534/genetics.108.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons M, Mlodzik M. Planar cell polaritysignaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo N, et al. Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci U S A. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, et al. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montcouquiol M, et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 22.Montcouquiol M, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 25.Myers DC, et al. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–455. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- 26.Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das G, et al. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- 28.Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 29.Strutt D, et al. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr Biol. 2002;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- 30.Bastock R, et al. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 31.Tree DR, et al. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 32.Rawls AS, Wolff T. Strabismus requires Flamingo and Prickle function to regulate tissue polarity in the Drosophila eye. Development. 2003;130:1877–1887. doi: 10.1242/dev.00411. [DOI] [PubMed] [Google Scholar]
- 33.Usui T, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 34.Ciruna B, et al. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin C, et al. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adler PN, et al. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev. 2000;96:197–207. doi: 10.1016/s0925-4773(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Adler PN. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics. 2002;160:1535–1547. doi: 10.1093/genetics/160.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence PA, et al. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- 40.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 41.Taylor J, et al. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng L, et al. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]
- 43.Adler PN, et al. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, et al. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2004;2:E158. doi: 10.1371/journal.pbio.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen WS, et al. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Garrec JF, et al. Establishment and maintenance of planar epithelial cell polarity by asymmetric cadherin bridges: a computer model. Dev Dyn. 2006;235:235–246. doi: 10.1002/dvdy.20617. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence PA, et al. Planar cell polarity: A bridge too far? Curr Biol. 2008;18:R959–R961. doi: 10.1016/j.cub.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CM, et al. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc Natl Acad Sci U S A. 2004;101:15961–15966. doi: 10.1073/pnas.0407103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casal J, et al. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenny A, et al. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 55.Heisenberg CP, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 56.Tada M, Smith JC. Xwnt11 is a targetof Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 57.Gros J, et al. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2009;457:589–593. doi: 10.1038/nature07564. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence PA, et al. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development. 2002;129:2749–2760. doi: 10.1242/dev.129.11.2749. [DOI] [PubMed] [Google Scholar]
- 59.Lim J, et al. Control of planar cell polarity by interaction of DWnt4 and four-jointed. Genesis. 2005;42:150–161. doi: 10.1002/gene.20142. [DOI] [PubMed] [Google Scholar]
- 60.Zeidler MP, et al. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr Biol. 1999;9:1363–1372. doi: 10.1016/s0960-9822(00)80081-0. [DOI] [PubMed] [Google Scholar]
- 61.Shimada Y, et al. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 62.Feiguin F, et al. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 63.Vinson CR, et al. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- 64.Krasnow RE, et al. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development. 1995;121:4095–4102. doi: 10.1242/dev.121.12.4095. [DOI] [PubMed] [Google Scholar]
- 65.Boutros M, et al. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 66.Axelrod JD, et al. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallingford JB, et al. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenny A, et al. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- 70.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 71.Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- 72.Jessen JR, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 74.Gubb D, et al. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeuchi M, et al. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 76.Carreira-Barbosa F, et al. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 77.Das G, et al. The atypical cadherin Flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Dev Cell. 2002;2:655–666. doi: 10.1016/s1534-5807(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 78.Curtin JA, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 79.Schwarz-Romond T, et al. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the β-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moeller H, et al. Diversin regulates heart formation and gastrulation movements in development. Proc Natl Acad Sci U S A. 2006;103:15900–15905. doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang CH, et al. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 83.Ma D, et al. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 84.Saburi S, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 85.Hou R, Sibinga NE. Atrophin proteins interact with the fat1 cadherin and regulate migration and orientation in vascular smooth muscle cells. J Biol Chem. 2009;284:6955–6965. doi: 10.1074/jbc.M809333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 87.Rock R, et al. Expression of mouse dchs1, fjx1, and fat-j suggests conservation of the planar cell polarity pathway identified in Drosophila. Dev Dyn. 2005;234:747–755. doi: 10.1002/dvdy.20515. [DOI] [PubMed] [Google Scholar]
- 88.Strutt H, et al. Cleavage and secretion is not required for Four-jointed function in Drosophila patterning. Development. 2004;131:881–890. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- 89.Ishikawa HO, et al. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matakatsu H, Blair SS. The DHHC Palmitoyltransferase approximated regulates fat signaling and Dachs localization and activity. Curr Biol. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fanto M, et al. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- 92.Hou R, Sibinga NE. Atrophin proteins interact with the Fat1 cadherin and regulate migration and orientation in vascular smooth muscle cells. J Biol Chem. 2009;284:6955–6965. doi: 10.1074/jbc.M809333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tepass U, et al. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 94.Humbert PO, et al. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 2006;16:622–630. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 95.Tanentzapf G, et al. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J Cell Biol. 2000;151:891–904. doi: 10.1083/jcb.151.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Djiane A, et al. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 97.Matsuyama M, et al. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet. 2009;5:e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Casal J, et al. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- 99.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]