Abstract
Introduction
Placental abruption is a serious condition that increases perinatal morbidity and mortality. Clinical prevention and treatment options are limited, especially in human preterm deliveries. Knowledge of the mechanisms that keep the placenta in place during pregnancy is critical for developing strategies for the prevention of abruption. Failure of physiological transformation of spiral arteries has been described as a major contributing factor of the placental abruption development. Baboons (Papio spp.) share striking similarities with humans in regard to placental structure, utero-placental blood flow, and fetal development; however, the mode of trophoblast invasion is shallow in baboons. This fact prompted the hypothesis that the incidence of placental abruption will be increased in baboons compared to humans.
Material and methods
Baboon placentas were collected between 2002 and 2008. Two independent veterinary pathologists evaluated the slides. A certified physician pathologist performed additional histology.
Results
Placental abruption was diagnosed in 22 baboons among 2,423 live births during the study period (0.9% prevalence). The most common clinical presentations were fetal demise and vaginal bleeding. The most common pathological findings were intraplacental hemorrhage with or without hematoma formation (86.4%). Other findings consisted of neutrophil infiltration (50%), decidual necrosis (22.7%), decidual vascular congestion and inflammation, villous congestion and retroplacental hemorrhage/hematoma (18.2%). These pathologic findings were the same for term and preterm deliveries.
Conclusion
This is the first systematic study of placental abruption in non-human primates, analyzing a large colony of baboons. Despite differences in trophoblast invasion, the clinical features observed in placental abruption affecting baboons resembled those reported in humans. The cluster of placental pathological findings in baboons also agreed with clinical reports, but the prevalence of these findings differed between baboons and humans. We discuss a mechanism of anti-abruption forces that offset shallow trophoblast invasion observed in baboons.
INTRODUCTION
Placental abruption is defined as the premature separation of a normally implanted placenta (1). In humans, the frequency of this pathology is approximately 0.6%–4% of live, term, singleton, pregnancies (2–5) and is associated with a high maternal and perinatal morbidity and mortality (1,2,4,6). Placental abruption has been extensively studied in humans with several theories proposed to explain its occurrence (7–9). The etiology is still poorly understood (10); consequently, both preventive and treatment measures are limited. The urgent need for identifying such measures is underscored by the fact that placental abruption is a contributing factor in 10% of preterm deliveries (11).
The placental structure and function of Old World non-human primates (NHP) greatly resembles that of humans, including the development of the villous tree, hemochorial placentation, intervillous space (IVS) circulation, and the structure of materno-fetal oxygen barrier (12, 13). Pioneering work by Ramsey et al. detailed hemodynamic parameters for IVS and uterine blood flow in the rhesus monkey (Macaca mulatta). These parameters bear striking similarities with humans, despite differences in the depth of trophoblast invasion (14, 15). Excellent reviews regarding differences and similarities in early placentation between human and NHP (M. mulatta and Papio spp.) describe features like early presence of intravascular trophoblast, superficial implantation and absence of decidual reaction in Old World NHP as compared to humans (12, 16, 17). Data describing premature placental separation in NHP is limited to a few cases of both experimental and spontaneous abruption (18–25). There are no published data available in any NHP regarding the incidence of placental abruption.
Failure of physiological transformation of spiral arteries has been recognized as one of the contributing factors to the pathophysiology of placental abruption in humans (9). Since physiologic trophoblast invasion into the uterine vessels is shallow in baboons (Papio spp.) as compared to humans (12), we hypothesized that baboons would have an increased incidence of placental abruption.
The study of spontaneous abruption in NHP may shed some light into the mechanism of such pathology in humans and answer the question: what keeps the placenta in place in the absence of deep trophoblast invasion? In this study we evaluated the prevalence, clinical features, and pathological findings of spontaneous placental abruption in baboons. We further compared our findings with existing human data to discuss potential anti-abruption mechanisms in naturally occurring shallow trophoblast invasion as seen in baboons.
MATERIALS AND METHODS
The baboon colony was maintained by the Texas Biomedical Research Institute at the Southwest National Primate Research Center (SNPRC) in San Antonio, Texas. The SNPRC’s Animal Care and Use Committee approved all animal procedures.
Gross evaluation of the placenta and fetus (if available) were performed by pathologists in cases of complicated pregnancies from 2002–2008 as part of the routine pathological examinations. Placental and fetal weights were recorded and fetal necropsies were performed when possible. Placental abruptions were diagnosed based on clinical and pathological features by a clinical veterinarian and a veterinary pathologist. Maternal age and parity, gestational age and clinical findings at diagnosis were abstracted from the pathology reports and from a computerized animal records database which is part of the Computerized Animal Management Program (CAMP) at the Texas Biomedical Research Institute (San Antonio TX). Placental samples were fixed in formalin and were processed conventionally and 5 µm sections stained with hematoxylin and eosin. Histology was performed by two independent veterinarians and a physician (each certified in pathology by the corresponding North American boards).
Pregnancy was clinically diagnosed using standard criteria previously described for this animal colony. The first day of pregnancy was estimated as the day a female’s sexual swelling began to decrease minus two days (26). In cases where the cycle data were not available, pregnancy was dated by ultrasound examination under sedation. In our study, the gestational age at delivery confirmed by fetal biometric information obtained during ultrasound examinations in 6/19 animals (31.6%). Term and preterm pregnancies were categorized as previously described (26). Placental abruption is defined as premature (before birth) separation of the placenta from its normal implantation site (27). Clinical and/or pathological findings consistent with abruptio placentae were used to define these cases in the baboons. In order to facilitate comparison with human data, we excluded 3 cases of clinically and pathologically suspected placental abruption diagnosed before 88 days of gestation. This cutoff best simulates the 20 weeks’ gestation cutoff used to diagnose placental abruption in humans. Table 1A lists other fetal and maternal characteristics of analyzed cases. Table 1B describes cases in which abruption was suspected clinically and pathologically, but was excluded due to early gestational age (<88 days’ gestation).
Table 1.
A. Fetal and maternal characteristics in cases placental abruption in the baboons (Papio spp.) studied
| Case | Trimester | Maternal age (y) |
Parity 1 |
Clinical presentation |
Gestational age (days) |
Fetal gender |
Fetal weight (gram) |
Placental weight (gram) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 0 | IUFD | 90 | U | 96 | 54.4 |
| 2 | 2 | 9 | 2 | VB | 102 | U | N/A | 82 |
| 3 | 2 | 16 | 4 | LD and VB | 103 | U | N/A | N/A |
| 4 | 2 | 13 | 7 | VB | 110 | U | N/A | N/A |
| 5 | N/A | 8 | 0 | CS | 127 | U | N/A | N/A |
| 6 | 3 | 8 | 2 | CS | 128 | Male | 372 | N/A |
| 7 | 3 | 10 | 2 | Uterine prolapse | 148 | Male | 704 | 224 |
| 8 | 3 | 13 | 7 | IUFD | 155 | U | N/A | N/A |
| 9 | 3 | 12 | 4 | IUFD | 156 | Female | 567 | 240 |
| 10 | 3 | 15 | 5 | VB | 170 | Male | 875 | 317 |
| 11 | 3 | 12 | 5 | RP | 173 | Male | 1000 | 277 |
| 12 | 3 | 13 | 2 | IUFD | 178 | Male | 1018.6 | 201.10 |
| 13 | 3 | 14 | 2 | FH | 184 | Female | 446 | 120.58 |
| 14 | 3 | 9 | 4 | IUFD | 189 | Male | 982 | 282 |
| 15 | 3 | 7 | 0 | IUFD | Term | Female | 700 | 148.35 |
| 16 | 3 | 17 | 1 | IUFD | Term | U | 900 | N/A |
| 17 | 3 | 7 | 1 | IUFD | Term | U | N/A | N/A |
| 18 | 3 | 14 | 4 | LD | Term | U | 1248 | 328 |
| 19 | N/A | N/A | N/A | IUFD | Term | U | 706 | N/A |
| 20 | N/A | N/A | N/A | IUFD | Term | Female | 838 | 130 |
| 21 | N/A | N/A | N/A | MC/IUFD | N/A | Female | N/A | N/A |
| 22 | N/A | 18 | 8 | IUFD/MC | N/A | Female | 468 | 181.5 |
| B. Fetal and maternal characteristics in cases of suspected placental abruption excluded from the statistical analyses | |||||||
|---|---|---|---|---|---|---|---|
| Case | Maternal age (y) | Parity | Clinical presentation | Gestational age (days) |
Fetal gender | Fetal weight (gram) | Placental weight (gram) |
| 1 | N/A | N/A | Maternal death | 47 | N/A | N/A | 20.4 |
| 2 | 10 | 4 | IUFD | 70 | Male | 32 | 13.8 |
| 3 | 11 | 5 | Vaginal bleeding | 71 | N/A | N/A | N/A |
Number of offspring before abruption. U : Unknown, IUFD: Intrauterine fetal demise, FH: Fetal hypoxia; VB: Vaginal Bleeding; LD: Labor Dystocia, MC: Poor Maternal Conditions; CS: Finding during cesarean section, RP: retained placenta
IUFD: Intrauterine fetal demise
Placental efficiency was calculated as the ratio of fetal/placental weights (expressed as g•g−1), when these variables were available (28). SPSS was used for statistical analyses. Fisher’s exact test was utilized to compare dichotomous variables. A two-sided p-value below 0.05 was considered significant.
RESULTS
Prevalence and risk factors
A total of 2,423 singletons were delivered during the study period with placental abruption diagnosed in 22 baboons (0.9%). The mean gestational age at diagnosis of placental abruption was 143.8 ± 33.6 days. The mean maternal age was 10.3 ± 5.5 years, and the mean number of offspring before the abruption was 3.2 ± 2.5. Most abruptions (16 of 22) were diagnosed during the third trimester of the pregnancy. The mean fetal weight was 728 ± 299.7 grams, and the mean placental weight was 186.1 ± 97.6 grams. In 14 cases in which the fetal gender could be determined, 8 (57.1%) were male fetuses. Clinical presentations in the majority of abruptions (12/22) were intrauterine fetal demise (12 of 22) and/or vaginal bleeding (4 of 22).
Pathological findings
Intraplacental hemorrhage was the most common histopathological finding (86.4%) among abruption cases. Other pathologic findings included: decidual and villous neutrophil infiltration (50%); decidual necrosis (22.7%); as well as decidual vascular congestion and inflammation, villous congestion, and retroplacental hematoma/hemorrhage (each of these affecting 18.2%). Figures 1 and 2 show macroscopic and microscopic pathological findings, respectively, related with these abruptions. One case displayed retroplacental hemorrhage with hemosiderin-laden macrophages and atherosis of placental bed spiral arteries (Fig. 3). Calculated placental efficiency was 3.9 ± 0.3 g•g−1 at term.
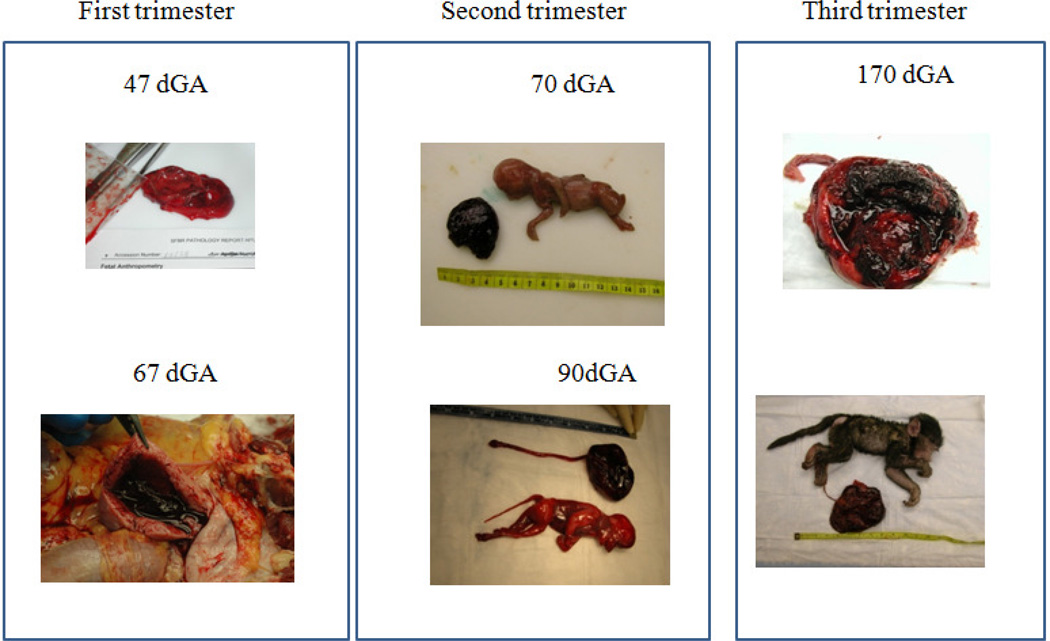
Figure 1.
Macroscopic presentation of placental abruption in baboons (Papio spp) at different gestational ages (dGA –Days of Gestation).
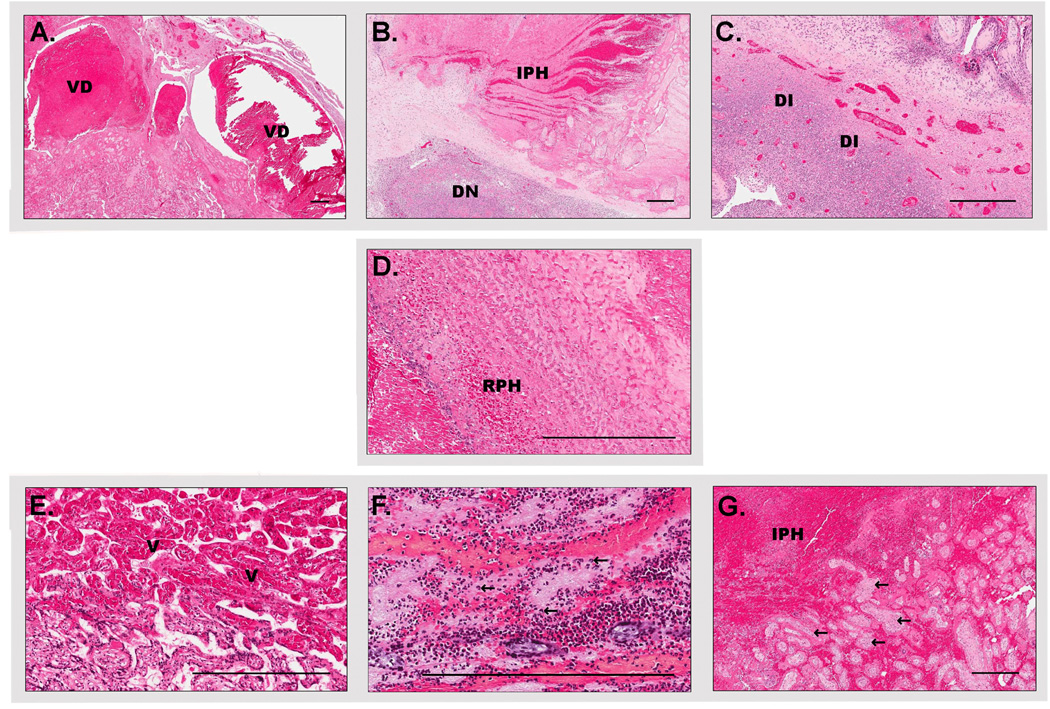
Figure 2.
Microphotographs of the main pathological findings of placental abruption in baboons (Papio spp.) A. Decidual vascular dilation (VD). B. Decidual necrosis (DN). C. Decidual inflammation (DI). D. Retroplacental hemorrhage (mild) (RPH). E. Villous congestion (V). F. Villous neutrophils (arrows) G. Intraplacental hemorrhage (IPH) and entrapped villi (arrows), (Scale bar 400 micrometers
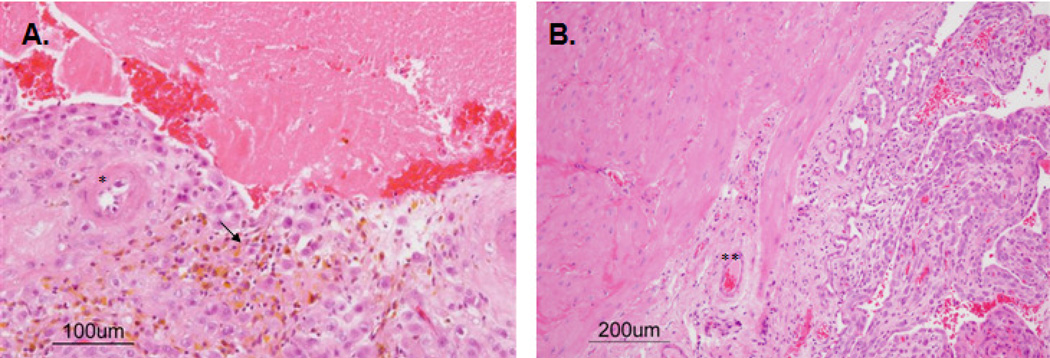
Figure 3.
Decidual-myometrial junction in placenta abruption (A) and non-abrupted baboon pregnancies (B). * Decidual artery arterial mural hypertrophy, **- intact decidual artery, arrow – hemosiderin laden macrophages.
Comparison of the prevalence of histopathologic findings between preterm pregnancies and term pregnancies revealed no significant differences between these two groups (Table 2).
Table 2.
Comparison of pathologic findings between preterm and term diagnosed placental abruption cases
| Pathologic Finding | Preterm n (%) | Term n (%) | p-value |
|---|---|---|---|
| Decidual vascular congestion | 1(11.1) | 2(18.2) | >0.99 |
| Decidual inflammation | 2(22.2) | 0 | 0.48 |
| Decidual necrosis | 3(33.3) | 1(9.1) | 0.59 |
| Villous congestion | 2(22.2) | 1(9.1) | 0.93 |
| Intraplacental hemorrhage | 9(100) | 10(90.9) | >0.99 |
| Neutrophil infiltration | 4(44.4) | 5(45.5) | >0.99 |
| Retroplacental hemorrhage | 3(33.3) | 2(18.2) | 0.92 |
| Total subjects* | 9 | 11 |
We were unable to obtain gestational age in 2 of the 22 subjects.
DISCUSSION
Prevalence and risk factors in baboon and humans
This is the first large study regarding placental abruption in NHP, considering the limited data available (Table 3). To the best of our knowledge, this is the first comprehensive study, describing spontaneous abruptio placentae in baboons (Papio spp.). Stark et al. reported abruptio placentae as a complication of chronic instrumentation in a baboon. Three cases of spontaneous abruption in baboons have been described by Benischke and Miller (19), and the rest of the studies referenced in Table 3 described placental abruption in NHP other than baboons.
Table 3.
Comparison of spontaneously occurring placental abruption previously reported in NHP.
| References | Species | n | Risk factors for abruption present |
Gestational age (days or trimester) |
Clinical features | Pathology | Spontaneous vs. artificially induced abruptio placentae |
|---|---|---|---|---|---|---|---|
| Present study | Papio spp. | 22 | Trauma, multiparity and humane euthanatia |
47–189 | Refer to tables 1 and 2 | Retro-placental clots | Spontaneous |
| 18 | Lion-tailed macaque |
1 | Multiparity | 3rd trimester | Maternal weakness, ataxia, pale mucous membranes, hypothermia and IUFD |
10 cm retroplacental blood clot |
Spontaneous |
| 19 | Mandrill, Gorilla | 2 | N/A* | Term | Stillbirths | N/A | Spontaneous |
| 20 | Gorilla | 1 | Preeclampsia | Term | Proteinuria documented in the second half of the pregnancy, stillbirth |
Small placenta, retroplacental hematomas |
Spontaneous |
| 21 | Macaca mulata | 6 | N/A | 100–120 | IUFD of all 6 fetuses | The experimental placental lobe had a dark brown color and showed areas of blood stagnation |
Experimental |
| 22, 23 | Macaca mulata | 7 | N/A | Near term | 2 cases of live births with severe asphyxia and with secondary brain injury 2 cases of incidental finding of placenta abruption on routine CS |
IUFD, retroplacental clots and signs of disseminated intravascular coagulation |
Spontaneous |
| 24 | Papio spp. | 3 | Chronic instrumentation of the fetus |
132–142 | IUFD , 3 fetuses | N/A | Complication of the experimental procedure |
| 25 |
Macaca fascicularis |
2 | N/A | > 140 | IUFD , 2 fetuses | N/A | Spontaneous |
Not available, IUFD: Intrauterine fetal demise
Based on the concept of shallow trophoblast invasion in baboon placentation (12, 16, 17), we anticipated an increased occurrence of placental abruption in this NHP. In contrast with our hypothesis, the prevalence of abruption in baboons in this colony was relatively low (0.9%) and comparable to that reported in human pregnancies (1,4,7,8). Various risk factors for placental abruption in humans have been previously described, including prior abruption (the strongest risk factor), acute trauma, smoking, male gender of fetus, preeclampsia, cocaine usage, maternal parity, and snake bites (29). Among these human risk factors, only maternal parity, male gender and acute trauma could be recognized in baboons. One of the limitations of our database relates to the history of placental abruption in the baboon population and documentation of premature rupture of fetal membranes. It is possible that baboons presenting with subtle or chronic clinical signs of abruption might have been misclassified with a different diagnosis, or missed, potentially leading to an underreporting of abruption cases. As in humans, it is possible that some occult abruptions might occur. If external bleeding is not apparent, and animal’s (maternal) well-being is not compromised, occult placental abruption may not be diagnosed. In these cases, animals could deliver preterm or at term. The fetal outcome would depend on placental compensatory capacities and the gestational age of the fetus. We are aware that in such cases the placenta abruption would not be noticed and would be recorded as a stillbirth or a normal delivery in the animal database.
Male gender of the offspring has been documented as another risk factor for placental abruption in humans. Similar to these data, in our study, 57% of fetuses delivered after abruptio placentae were males. Placental efficiency in our series was essentially the same to our published control animal data (30), suggesting an acute rather than a chronic event (such as absence of diminished uteroplacental blood flow during pregnancy).
Hypertension (including preeclampsia) and other chronic disorders comprise major risk factors for placental abruption in humans. Preeclampsia and abruption are thought to share pathophysiologic features, such as utero-placental ischemia (10). Data regarding preeclampsia in baboons are controversial. Some authors have reported the presence of experimental preeclampsia in these species (31,32). Others argue that pregnancy-related hypertension does not exist naturally in baboons due to their naturally shallow trophoblast invasion (33). In our colony, blood pressure as well as proteinuria is not continuously controlled; therefore, it was impossible to draw a conclusion in this regard. One baboon, included in this study, developed seizures with a subsequent clinical picture of placental abruption; however, a differential diagnosis of eclampsia was not assessed.
Pathological findings in baboons vs. humans
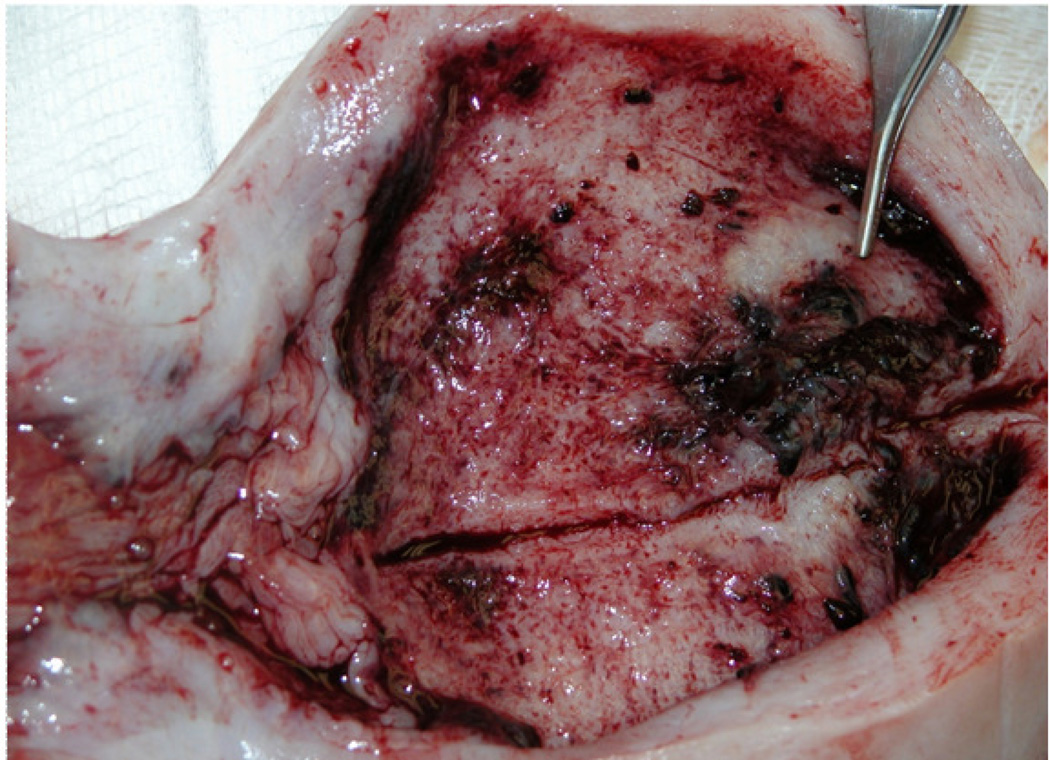
In human pregnancies, two clinical pathways of placental abruption have been proposed: acute (inflammation associated conditions) and chronic (diabetes mellitus, hypertension, smoking, etc.) (29). Pathological findings (such as presence of inflammation, determined by neutrophil infiltration) were noticed with the same frequency in preterm and term deliveries in this study. The differences between human and baboon data could be explained by differences in the incidence of infections between baboons and humans (26). Similar to reports in humans (34), we observed villous congestion with dilation and thrombosis of placental bed blood vessels in the abruption cases studied (Fig. 4).
Figure 4.
Dilated placental bed vessels in case of placental abruption in a baboon (Papio spp.
Although similar pathological features reported in human abruption cases were found in our study, the prevalence between the two species differed. In human abruptions, the most common features seen include retroplacental bleeding (between the placenta and maternal decidua) and poor trophoblast migration with inadequate spiral arteriole remodeling (35, 36, 37). In our baboons, the predominant characteristic was intraplacental hemorrhage (86.4%). Our findings regarding the absence of endovascular trophoblast invasion in the baboon myometrium agrees with the published data (38, 39).
What keeps the placenta in place in the baboon?
Since the depth of trophoblast invasion does not seem to play a critical role in the pathogenesis of placental abruption in baboons, other potential mechanisms must be explored. The increased prevalence of intraplacental hemorrhage in the baboons suggests the possibility that changes in IVS blood flow could contribute to abruption in these species. In a model of artificial placenta, Groeber described IVS blood flow as an essential component of “anti-abruptio forces” (40). IVS blood flow is diminished in human pregnancies complicated by preeclampsia (41) and intrauterine growth restriction (IUGR) (42). Idiopathic IUGR in the baboons has been reported (43). In our study, however 1 out of 10 animals with a recorded birth weight considered had IUGR (446 g at 184 days’ gestation) (26). Therefore the likely mechanism in the baboon could be associated with pathology of blood flow redistribution as suggested by Kiss and Tarjan (44). These authors described the subperitoneal plexus as a reservoir for blood shunted from the IVS through the arterio-venous anastomoses during uterine contractions. The increased contractility of the blood vessels or pathologic coagulation impairs this process and results in excessive blood volume within the IVS. This may explain the IVS hemorrhages in the baboon placenta.
Another theory, first suggested by Couvelaire in 1911 (45), links pathology of fetal capillaries to placental abruption, as quoted by Fisher (1964): “C’est dans le domaine des capillaries que se joue le drame” (46), or “It’s in the domain of the capillaries that the drama is played.” Whether these mechanisms are involved in placental abruption in the baboon remains to be elucidated.
In summary, additional research is needed to test the hypothesis that abnormalities in the intervillous space blood flow is an important pathophysiologic factor in placental abruption in baboons and that the regulation of the IVS may be important for pregnancy maintenance in presence of shallow trophoblast invasion.
The main strength of this study is its novelty in the field of the evolutionary pathology of abruption placentae. Baboons comprise an exemplary model for human pregnancy, because of the presence of a discoid hemochorial placenta with an analogous endocrine system. Our subjects were derived from a single baboon colony and all animals corresponded to the genus Papiosupporting the notion that these pathologic findings correspond to a homogeneous population. Lastly, three certified pathologists analyzed the samples independently thereby decreasing ascertainment bias.
Our study has several limitations inherent to its descriptive design. For example, the study does not include a control population. The pathology of the placentas as well as normal placental development for this baboon colony has been described elsewhere (26, 47). Pathologists were not blinded to the diagnosis of placental abruption. There were no possibilities for continuous blood pressure monitoring or urine analyses in this large baboon colony, which limited our study as well. Data were unavailable regarding history of abruption, a known risk factor for developing placental abruption in humans.
In conclusion, placental abruption in baboons appears to be a relatively rare event (≈1% of spontaneous singleton livebirths at term). Although the prevalence and clinical presentation of placental abruption share common patterns with those observed in human pregnancies, the pathological basis for abruption in baboons may be different. The regulation of the IVS blood flow may be important for pregnancy maintenance in shallow trophoblast invasion.
Acknowledgements
We thank Dr. Graham Burton and Dr. A. Enders for the helpful discussions and encouragement. We appreciate Dr. K. Benirschke for the critical review of the manuscript. We acknowledge the wonderful help of Dr. L. Gomez in the reading of this paper. We gratefully acknowledge the help and dedication of Mrs. M. Silva, M. Hohmann, C. Snider, Mr. A. Perez, S. Chambers, and the many excellent animal caretakers, technicians and veterinarians of The Southwest National Primate Center, whose dedication to animal care and science made this work possible. We are grateful to Dr. A. Schenone for editing of the manuscript. This research was partially supported by NCRR grant P51 RR013986 to the Southwest National Research Primate Research Center and unrestricted support from the Le Bonheur Chair of Excellence in Pediatric Endocrinology (to R.F.); this work was conducted in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR014578 and C06 RR015456. R.F. discloses unrelated research support over the past 3 years from Juvenile Diabetes Research Foundation International grant 1-2011-597, NIH grant R21 HD059292, NIH grant U01 DK085465, Gabrielle’s Angel Foundation, MacroGenics, Eli Lilly & Co., Pfizer, Novo Nordisk, Diamyd Therapeutics AB, Tercica, and Bristol-Myers Squibb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunningham FG, Williams JW. Williams Obstetrics (22/e) New York: McGraw-Hill Professional; 2005. [Google Scholar]
- 2.Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011;90:140–149. doi: 10.1111/j.1600-0412.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 3.Raymond EG, Mills JL. Placental abruption. Maternal risk factors and associated fetal conditions. Acta Obstet Gynecol Scand. 1993;72:633–639. doi: 10.3109/00016349309021156. [DOI] [PubMed] [Google Scholar]
- 4.Ananth CV, Berkowitz GS, Savitz DA, Lapinski RH. Placental abruption and adverse perinatal outcomes. JAMA. 1999;282:1646–1651. doi: 10.1001/jama.282.17.1646. [DOI] [PubMed] [Google Scholar]
- 5.Ananth CV, Getahun D, Peltier MR, Smulian JC. Placental abruption in term and preterm gestations: evidence for heterogeneity in clinical pathways. Obstet Gynecol. 2006;107:785–792. doi: 10.1097/01.AOG.0000207560.41604.19. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen S, Irgens LM, Bergsjo P, Dalaker K. Perinatal mortality and case fatality after placental abruption in Norway 1967–1991. Acta Obstet Gynecol Scand. 1996;75:229–234. doi: 10.3109/00016349609047092. [DOI] [PubMed] [Google Scholar]
- 7.Dommisse J, Tiltman AJ. Placental bed biopsies in placental abruption. Br J Obstet Gynaecol. 1992;99:651–654. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernischke K, Kaufmann P, Baergen RN. Pathology of the Human Placenta (5/e) New York: Springer Science+Business Media; 2006. [Google Scholar]
- 9.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Han CS, Schatz F, Lockwood CJ. Abruption-associated prematurity. Clin Perinatol. 2011;38:407–421. doi: 10.1016/j.clp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter AM. Comparative studies of placentation and immunology in non-human primates suggest a scenario for the evolution of deep trophoblast invasion and an explanation for human pregnancy disorders. Reproduction. 2011;141:391–396. doi: 10.1530/REP-10-0530. [DOI] [PubMed] [Google Scholar]
- 13.Samson JE, Mari G, Dick EJ, Jr, Hubbard GB, Ferry RJ, Jr, Schlabritz-Loutsevitch NE. The morphometry of materno–fetal oxygen exchange barrier in a baboon model of obesity. Placenta. 2011;32:845–851. doi: 10.1016/j.placenta.2011.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsey EM, Corner GW, Donner MW, Stran HM. Radioangiographic studies of circulation in the maternal placenta of the rhesus monkey: preliminary report. Proc Natl Acad Sci USA. 1960;46:1003–1008. doi: 10.1073/pnas.46.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey EM, Corner GW, Jr, Donner MW. Serial and cineradioangiographic isualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol. 1963;86:213–225. doi: 10.1016/0002-9378(63)90434-4. [DOI] [PubMed] [Google Scholar]
- 16.Houston ML. The development of the baboon (Papio sp.) placenta during the fetal period of gestation. Am J Anat. 1969;126:17–29. doi: 10.1002/aja.1001260103. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey EM, Houston ML, Harris JW. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol. 1976;124:647–652. doi: 10.1016/0002-9378(76)90068-5. [DOI] [PubMed] [Google Scholar]
- 18.Calle PP, Ensley PK. Abruptio placentae in a lion-tailed macaque. J Am Vet Med Assoc. 1985;187:1275–1276. [PubMed] [Google Scholar]
- 19.Benirschke K, Miller CJ. Anatomical and functional differences in the placenta of primates. Biol Reprod. 1982;26:29–53. doi: 10.1095/biolreprod26.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Benirschke K, Adams FD. Gorilla diseases and causes of death. J Reprod Fertil Suppl. 1980;28:139–148. [PubMed] [Google Scholar]
- 21.Myers RE. The pathology of the rhesus monkey placenta. Acta Endocrinol Suppl (Copenh) 1972;166:221–257. doi: 10.1530/acta.0.071s221. [DOI] [PubMed] [Google Scholar]
- 22.Myers RE. Cystic brain alteration after incomplete placental abruption in monkey. Arch Neurol. 1969;21:133–141. doi: 10.1001/archneur.1969.00480140033003. [DOI] [PubMed] [Google Scholar]
- 23.Myers RE, Fujikura T. Placental changes after experimental abruptio placentae and fetal vessel ligation of rhesus monkey placenta. Am J Obstet Gynecol. 1968;100:946–951. [PubMed] [Google Scholar]
- 24.Stark RI, Daniel SS, James LS, MacCarter G, Morishima HO, Niemann WH, et al. Chronic instrumentation and longterm investigation in the fetal and maternal baboon: tether system, conditioning procedures and surgical techniques. Lab Anim Sci. 1989;39:25–32. [PubMed] [Google Scholar]
- 25.Sesbuppha W, Chantip S, Dick EJ, Jr, Schlabritz-Loutsevitch NE, Guardado-Mendoza R, Butler SD, et al. Stillbirths in Macaca fascicularis. J Med Primatol. 2008;37:169–172. doi: 10.1111/j.1600-0684.2007.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlabritz-Loutsevitch NE, Moore CM, Lopez-Alvarenga JC, Dunn BG, Dudley D, Hubbard GB. The baboon model (Papio hamadryas) of fetal loss: maternal weight, age, reproductive history and pregnancy outcome. J Med Primatol. 2008;37:337–345. doi: 10.1111/j.1600-0684.2008.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creasy RK, Resnik R, Jay D, editors. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice (6/e) Philadelphia PA: Saunders/Elsevier; 2009. [Google Scholar]
- 28.Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ. Placental efficiency and adaptation: endocrine regulation. J Physiol. 2009;587((Pt 14)):3459–3472. doi: 10.1113/jphysiol.2009.173013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen TT, DeFranco EA, Stamilio DM, Chang JJ, Muglia LJ. A population-based study of race-specific risk for placental abruption. BMC Pregnancy Childbirth. 2008;8:43. doi: 10.1186/1471-2393-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, et al. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30:752–760. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 32.Cavanagh D, Rao PS, Tung KS, Gaston L. Eclamptogenic toxemia: the development of an experimental model in the subhuman primate. Am J Obstet Gynecol. 1974;120:183–196. doi: 10.1016/0002-9378(74)90360-3. [DOI] [PubMed] [Google Scholar]
- 33.Ramsay MM, Tame JD, Winter JA, Carbone LG, Schlafer DH, Nathanielsz PW. Proteinuric hypertension in a pregnant baboon: was this pre-eclampsia? J Med Primatol. 1997;26:207–212. doi: 10.1111/j.1600-0684.1997.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 34.Elsasser DA, Ananth CV, Prasad V, Vintzileos AM. Diagnosis of placental abruption: relationship between clinical and histopathological findings. Eur J Obstet Gynecol Reprod Biol. 2010 Feb;148(2):125–130. doi: 10.1016/j.ejogrb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dommisse J, Tiltman AJ. Placental bed biopsies in placental abruption. Br J Obstet Gynaecol. 1992 Aug;99(8):651–654. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011 Jun;25(3):313–327. doi: 10.1016/j.bpobgyn.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ananth CV, Oyelese Y, Prasad V, Getahun D, Smulian JC. Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol. 2006;128:15–21. doi: 10.1016/j.ejogrb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Enders AC, King BF. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am J Anat. 1991;192:329–346. doi: 10.1002/aja.1001920403. [DOI] [PubMed] [Google Scholar]
- 39.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 40.Groeber WR. Antiabruption dynamics of the intervillous circulation in an artificial uterus. Am J Obstet Gynecol. 1966;95:640–647. doi: 10.1016/s0002-9378(16)34739-1. [DOI] [PubMed] [Google Scholar]
- 41.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunelli R, Masselli G, Parasassi T, De Spirito M, Papi M, Perrone G, et al. Intervillous circulation in intra-uterine growth restriction. Correlation to fetal well being. Placenta. 2010;31:1051–1056. doi: 10.1016/j.placenta.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Brans YW, Kuehl TJ, Hayashi RH, Andrew DS, Reyes P. Amniotic fluid in baboon pregnancies with normal versus growth-retarded fetuses. Am J Obstet Gynecol. 1986;155:216–219. doi: 10.1016/0002-9378(86)90114-6. [DOI] [PubMed] [Google Scholar]
- 44.Kiss F, Tarjan G. [On the pathogenesis of uteroplacental apoplexy] Zentralbl Gynakol. 1960;82:1757–1772. [PubMed] [Google Scholar]
- 45.Couvelaire Traitement chirurgical des hémorrhagies utéro-placentaires avec décollement du placenta normalement inséré. Ann Gynécol. 1911;8:591. [Google Scholar]
- 46.Fischer H. [On the problem of premature detachment of the placenta] Z Arztl Fortbild (Jena) 1964;58:736–738. [PubMed] [Google Scholar]
- 47.Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, Jenkins S, Hubbard G, Burton GJ, Nathanielsz P. Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (Papio sp) Placenta. 2007;28:783–793. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]