Abstract
Genetic testing of tumor susceptibility genes is now recommended in most patients with pheochromocytoma or paraganglioma (PPGL), even in the absence of a syndromic presentation. Once a mutation is diagnosed there is rarely follow-up validation to assess the possibility of misdiagnosis. This study prospectively examined the prevalence of von Hippel-Lindau (VHL) gene mutations among 182 patients with non-syndromic PPGLs. Follow-up in positive cases included comparisons of biochemical and tumor gene expression data in 64 established VHL patients, with confirmatory genetic testing in cases with an atypical presentation. VHL mutations were detected by certified laboratory testing in 3 of the 182 patients with non-syndromic PPGLs. Two of the 3 had an unusual presentation of diffuse peritoneal metastases and substantial increases in plasma metanephrine, the metabolite of epinephrine. Tumor gene expression profiles in these 2 patients also differed markedly from those associated with established VHL syndrome. One patient was diagnosed with a partial deletion by Southern blot analysis and the other with a splice site mutation. Quantitative polymerase chain reaction, multiplex ligation-dependent probe amplification, and comparative genomic hybridization failed to confirm the partial deletion indicated by certified laboratory testing. Analysis of tumor DNA in the other patient with a splice site alteration indicated no loss of heterozygosity or second hit point mutation. In conclusion, VHL germline mutations represent a minor cause of non-syndromic PPGLs and misdiagnoses can occur. Caution should therefore be exercised in interpreting positive genetic test results as the cause of disease in patients with non-syndromic PPGLs.
Keywords: pheochromocytoma, paraganglioma, von Hippel-Lindau syndrome, mutation testing, germline mutations, loss of heterozygosity
Introduction
A large proportion of pheochromocytomas and paragangliomas (PPGLs) have a hereditary basis due to germline mutations of numerous tumor susceptibility genes [1–3]. Mutations of the von Hippel-Lindau (VHL) gene, the neurofibromatosis gene, and the rearranged during transfection proto-oncogene (RET) represent well established hereditary causes of PPGLs. Mutations of genes encoding the 4 subunits of succinate dehydrogenase (SDH), the SDH complex assembly factor 2 [4], transmembrane protein 127 [5], and MYC associated factor X [6] represent more recently identified hereditary causes of PPGLs, bringing together a total of 10 genes in which germline mutations are now recognized as risk factors for the tumors.
Findings of germline mutations of tumor susceptibility genes among many patients with non-syndromic PPGLs have led to recommendations that all affected patients should be considered for genetic testing [1,2,7]. It remains unclear whether screening for germline mutations should include all genes or only a selection of genes according to other considerations. Whether misdiagnoses associated with genetic screening can occur is also unclear. The present study focused on screening for VHL mutations. We assessed the prevalence of unsuspected germline VHL mutations among patients with non-syndromic PPGLs, examined the clinical presentation of patients identified with mutations compared to established VHL syndrome and carried out follow-up testing to confirm the contribution of mutations to disease in patients with an atypical presentation.
Patients and Methods
Patients
The 246 patients with PPGLs were divided into 2 groups according to the absence or presence of a syndromic presentation or family history of VHL syndrome. Those with a non-syndromic presentation (i.e., no other tumors or stigmata indicating a specific syndrome) who underwent screening for VHL gene mutations included 103 females and 79 males and were aged between 7 and 83 years (median 43 years) at first diagnosis of tumors. Patients with established VHL syndrome included 29 females and 35 males and were aged between 6 and 75 years (median 29 years) at first diagnosis of tumors. Investigations were approved by intramural review boards of the contributing centers.
VHL mutation screening
Testing for VHL germline point mutations involved polymerase chain reaction (PCR) amplification of exons 1, 2, and 3 and their flanking sequences, followed by bidirectional DNA sequencing [8]. Structural rearrangements, including deletions, were initially screened for by Southern blot analysis [8]. In subsequent years deletions were screened for by multiplex ligation-dependant probe amplification (MLPA).
Catecholamine metabolite profiles
Results of biochemical testing for catecholamine excess in patients with non-syndromic PPGLs, who were identified with germline VHL mutations, were compared with biochemical profiles in the 64 patients with PPGLs in association with established VHL syndrome. Based on other studies [9,10], profiling was directed at measurements of plasma concentrations and urinary outputs of epinephrine and its O-methylated metabolite, metanephrine.
Tumor gene expression profiling
PPGL tumor samples from 13 patients with established VHL syndrome and 2 of the 3 patients identified with VHL mutations and a non-syndromic presentation were prepared for microarray analysis according to Affymetrix protocols (Affymetrix, Inc). Gene expression intensities were calculated using Affymetrix AGCC software. For analysis of array data, the Partek Genomic Suite was used to RMA normalize (Robust Multichip Analysis), summarize and log transform the data. Hierarchical clustering was then carried out on genes showing more than 2 fold up or down regulation between the 2 groups.
Follow-up VHL gene testing
Follow-up testing of identified germline variants of the VHL gene in patients with non-syndromic PPGLs and an atypical presentation of disease involved analysis of DNA from blood and tumor samples. DNA was extracted using the automated Maxwell 16 system (Promega, Madison, MI, USA). For additional deletion analysis, real-time quantitative PCR (RQ-PCR) was performed as described elsewhere [8,11], using SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) in place of TaqMan probes, and run on an ABI Prism 7 000 Sequence Detection System (Applied Biosystems). All experiments were run in triplicate. MLPA was performed according to the manufacturer’s instructions with a commercially available probe mix (P016 VHL probe mix, MRC-Holland, Amsterdam, The Netherlands). An Agilent Custom High-Definition (Agilent, Santa Clara, CA, USA) comparative genomic hybridization (CGH) array was designed to investigate copy number of cancer genes as well as whole chromosomal regions, as previously described [11]. Bidirectional DNA sequencing was performed using the Big Dye Terminator v.1.1 Cycle Sequencing Kit (Applied Biosystems) according to manufacturer’s specifications and run on an ABI 3130 × 1 Genetic Analyzer.
Results
VHL mutation screening
Among the 182 patients with non-syndromic PPGLs tested for VHL germline mutations, 3 were identified by certified laboratory testing with mutations (Table 1). One patient (case 1) was identified with an established missense mutation. Southern blot analysis in the second patient (case 2) indicated a partial deletion characterized by an anomalous 9 kb band in addition to the half strength 9.7 kb wild type band. The third patient (case 3) had an exon 1 splice donor site mutation, IVS + 5 G > C. The certified diagnostic report stated: “this mutation results in abnormal splicing of the VHL pre-mRNA and has been reported previously in patients with VHL”. Evidently, designation as pathogenic was based on findings of the same gene variant in 2 previous patients with VHL syndrome, one with renal cell carcinoma and the other with a cerebellar hemangioblastoma.
Table 1.
Details of the 3 patients with non-syndromic PPGL and VHL mutations.
| Patient | Age* | Gender | Mutation | VHL protein | Mutation type |
|---|---|---|---|---|---|
| 1 | 8 | M | c.250 G>A | p.Val84Met | Missense |
| 2 | 24 | F | Partial deletion | Deletion | |
| 3 | 51 | M | IVS1+5 (G>C) | Splice site |
Age (years) at first diagnosis of pheochromocytoma
Clinical presentation
The single patient with the VHL missense mutation (case 1) had his first tumor diagnosed at age 8. He presented initially with a left adrenal pheochromocytoma, which was resected. Recurrent disease was diagnosed at age 26, at which time testing of the VHL gene was carried out.
The second patient (case 2) presented with bilateral adrenal pheochromocytomas, the first involving her left adrenal removed at age 23 and the second in her right adrenal removed at age 27. Recurrent disease was indicated 4 years later at age 31 with the onset of renewed signs and symptoms and consistently positive biochemical test results. The patient was referred to the NIH at age 33 at which time diffuse peritoneal metastases were revealed that were too extensive for surgical intervention.
Case 3 involved a male patient first diagnosed with a 6–7 cm left adrenal pheochromocytoma resected at age 51. He was later referred to the NIH where at age 72 surgical debulking was carried out revealing diffuse peritoneal metastases.
All 3 patients underwent thorough examination for other VHL clinical manifestations. Opthalmological examinations and imaging studies for lesions in the central nervous system, kidneys and organ systems failed to reveal any evidence of VHL syndrome.
Catecholamine metabolomic profiles
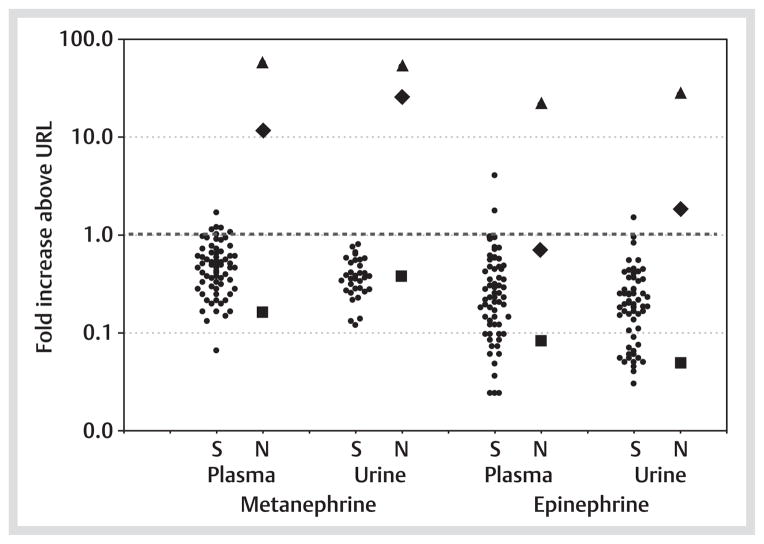
Two of the 3 patients (cases 2 and 3) with non-syndromic PPGLs who tested positive for VHL mutations showed increases in plasma and urinary metanephrine well outside the ranges for patients with PPGLs associated with established VHL syndrome (Fig. 1). Urinary outputs of epinephrine in these 2 patients were also elevated outside of the range of patients with established VHL syndrome.
Fig. 1.
Increases in plasma and urinary metanephrine and epinephrine above the upper limits of reference intervals (URL) in patients with syndromic (S) PPGLs due to established VHL syndrome (●) compared to the 3 patients (patients 1–3 in Table 1) with non-syndromic (NS) PPGLs who tested positive for VHL gene mutations (■ patient 1; ◆ patient 2; ▲ patient 3).
Tumor gene expression profiles
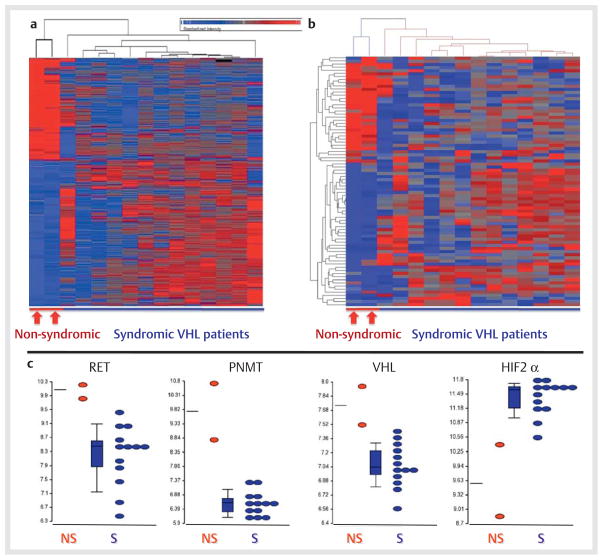
Hierarchical cluster analysis of microarray data supervised according to presence or absence of VHL syndrome indicated distinct gene expression profiles between tumors of the 13 patients with established VHL syndrome and the 2 with an atypical catecholamine metabolomic profile, who tested positive for VHL mutations (Fig. 2a). Hierarchical cluster analysis of a selection of 83 genes, established to distinguish tumors from patients with VHL and RET proto-oncogene mutations, again revealed highly distinct gene expression profiles between tumors of the 13 patients with established VHL syndrome and the 2 with the atypical catecholamine metabolomic profile (Fig. 2b). Among differentially expressed genes, the RET proto-oncogene, the phenylethanolamine N-methyltransferase (PNMT) gene and the VHL gene showed consistently higher expression in tumors from the 2 patients with a non-syndromic presentation than in those with established VHL syndrome, whereas the gene for hypoxia-inducible factor 2 alpha (HIF2α) showed lower expression in the 2 patients with a non-syndromic presentation than in those with established VHL syndrome (Fig. 2c).
Fig. 2.
Heat maps (a and b) and dot plots c derived from Affymetrix microarray data illustrating distinct gene expression profiles in PPGL tumor tissue samples from 13 patients with established VHL syndrome (S) compared to 2 patients (patients 2–3 in Table 1) with non-syndromic PPGLs (NS) who tested positive for VHL gene mutations and in whom catecholamine metabolite profiles (Fig. 1) indicated an atypical presentation with increases in plasma and urinary metanephrine.
Follow-up VHL gene testing
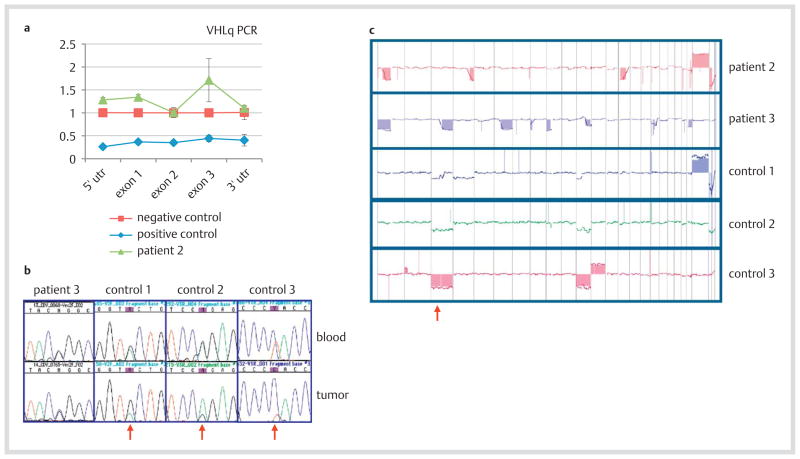
Array-based CGH of DNA extracted from blood cells of patient 2 failed to detect the partial VHL germline deletion predicted by the abnormal Southern blot (data not shown). RQ-PCR of the 3 VHL gene exons and the 5′ and 3′ untranslated flanking regions similarly failed to confirm the presence of a partial deletion (Fig. 3a); the same result was also indicated by diagnostic MLPA (not shown). Restriction digest experiments using long-range PCR and Eco RI and Ase I restriction enzymes indicated normal restriction patterns (data not shown).
Fig. 3.
VHL gene testing of blood and tumoral DNA. a Quantitative PCR (RQ-PCR) of blood DNA from patient 2 showed no loss in copy number in VHL or its flanking untranslated regions relative to a negative (wild type) control. The positive control is a patient with a known complete germline deletion of VHL. b Sequencing chromatographs of blood and tumor revealed germline mutations in patient 3 and 3 syndromic (control) VHL patients. Red arrows indicate diminished peaks of the wild type sequences and prevalence of mutant sequences in the tumors, revealing LOH in the control tumors but not in the tumor from patient 3. The VHL germline mutations are as follows: patient 3 (IVS1 + 5 G > C), control 1 (IVS2 + 3 A > G), control 2 (c.500 G > A; p.R167Q), control 3 (c.292 T > C; p.Y98H). c Array CGH genomic profiles of tumors from patients 2 and 3 and 3 control tumors. The third pane represents chromosome 3, and the red arrow denotes chromosome 3p. Patient 2 exhibits no loss of chromosome 3; patient 3 exhibits loss of 3q but not 3p. All 3 control tumors exhibit loss of 3p or a whole chromosome 3.
For patient 3, who was diagnosed with a splice site mutation, the variant was confirmed by repeat sequencing of the blood DNA. However, tumor DNA from this patient failed to reveal loss of heterozygosity (LOH), by virtue of the mutant and wild type sequences being present in equivalent amounts as the blood DNA control (Fig. 3b); in addition, no second hit point mutation was found in tumor DNA (data not shown). In contrast, tumor DNA from 3 syndromic VHL patients (controls 1–3) all displayed LOH of their wild type alleles (Fig. 3b).
Array CGH analysis was performed on the tumors from patients 2 and 3, as well as the 3 control patients (Fig. 3c). Although all five tumors displayed loss of some genomic regions, neither tumor from patient 2 nor 3 exhibited loss of chromosome 3p, which contains the VHL gene. In contrast, all 3 tumors from the control patients exhibited loss of chromosome 3p.
Discussion
Our findings that VHL mutations could not be confirmed as a cause of non-syndromic disease in 2 patients earlier diagnosed with VHL germline mutations illustrate a need for caution when interpreting genetic test results in the absence of other evidence for a mutation, particularly when clinical data are inconsistent with established genotype-phenotype relationships. The present data also support the findings of 4 earlier studies indicating a low prevalence of VHL germline mutations, from 1.6 to 3.5 %, among patients with non-syndromic PPGLs [2,3,12,13]. The higher prevalence of 11 % reported in the study of Neumann et al. [1] possibly reflects a founder effect associated with the Black Forest region of Germany.
Malignant disease is relatively rare in VHL-associated PPGLs, yet this was evident in 2 of the 3 patients with non-syndromic PPGLs who tested positive for VHL mutations. Interestingly, disease in both patients was characterized by high plasma concentrations and urinary outputs of metanephrine, indicating significant tumoral production of epinephrine. This finding is at variance with observations that PPGLs in VHL patients do not express the enzyme, PNMT, and consequently do not produce significant amounts of epinephrine or its O-methylated metabolite, metanephrine [14]. Tumor tissue from the 2 patients also revealed dissimilar gene expression profiles from those in patients with established VHL syndrome, further supporting the atypical presentation of disease in these 2 patients. The gene expression profiles were in line with those first described by Dahia et al. [15] in which differences in gene expression define 2 distinct cluster groups of tumors, also shown to be more simply distinguished by absence or presence of epinephrine production [9,16].
The atypical presentation in 2 of the patients with apparent VHL mutations strongly suggested that VHL inactivation was not responsible for their tumors. This suspicion was strengthened by considerations that PPGLs in VHL patients rarely occur due to deletions or splice site mutations, but are more usually due to missense mutations [17]. Findings that the partial VHL deletion indicated by Southern blotting could not be confirmed by MLPA or other techniques indicated that a VHL mutation was a highly unlikely cause of disease in that patient. In the other patient, the original splice variant was confirmed. Although diagnosed as pathogenic on the basis of the same variant in 2 unrelated patients with VHL clinical manifestations, a recent study has described the variant as likely non-functional [18]. It is therefore possible that either the variant should be reclassified as a nonfunctional polymorphism or has low disease penetrance. Either way, in the absence of LOH and a second hit somatic point mutation it is highly unlikely that the splice site variant was the cause of disease in our patient.
Interestingly another group carrying out mutation screening in patients with non-syndromic PPGLs also reported findings of VHL mutations in 2 patients with an atypical presentation of disease [19]. Both patents had novel mutations and like ours no other family history or manifestations suggestive of VHL syndrome. In one patient the tumor was indicated biochemically by large increases in urinary metanephrine, without increases in normetanephrine, again indicating atypical predominant production of epinephrine. As in our patient with the splice site variant, there was, however, no evidence of LOH in tumoral DNA, again raising doubt about whether the identified mutation was in fact responsible for the tumor.
Although commonly considered a “sacred cow”, this report establishes that the results of molecular diagnostic testing are not always infallible. The cases outlined here, along with reports of misdiagnoses associated with genetic screening for other conditions [20–23], indicate the need for some caution in the interpretation of mutation test results. Incorrect branding of a patient with a disease causing mutation, as in our 2 patients, may have undesirable consequences, including unnecessary anxiety for the patient and family members. Follow-up confirmation seems particularly prudent in situations where a familial or syndromic presentation of disease is unclear, such as in the present context of screening for mutations in patients with apparent sporadic PPGLs.
The importance of a reserved approach to interpretation of genetic test results is further illustrated by recent experience with another patient identified with a known pathogenic mutation of the SDH subunit B gene following genetic screening after presentation with a solitary epinephrine-producing adrenal tumor, but no evidence of a hereditary syndrome (Pacak, unpublished observations). Follow-up mutation testing failed to confirm that mutation, explaining why the mutation also could not be identified in 13 other family members who underwent genetic testing.
The other finding outlined here of a relatively low rate of germ-line VHL mutations among patients with non-syndromic PPGLs, although not in agreement with every study [1], raises some question about the appropriateness and cost-effectiveness of routine testing of this gene among all patients with PPGLs. As appropriately recommended by Cascon and colleagues age is an important consideration for testing of the VHL gene [24]. Thus, if testing of the VHL gene is considered in a patient with PPGL and no familial or clinical evidence of VHL syndrome, then it is best restricted to patients under 35 years of age [24,25] with tumors characterized by production of normetanephrine and without production of metanephrine [10].
Acknowledgments
This work was supported by the Deutsche Forschungsgesells-chaft and the intramural programmes of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Center for Cancer Research, National Cancer Institute, and the National Human Genome Research Institute, NIH.
References
- 1.Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, Januszewicz A, Eng C, Smith WM, Munk R, Manz T, Glaesker S, Apel TW, Treier M, Reineke M, Walz MK, Hoang-Vu C, Brauckhoff M, Klein-Franke A, Klose P, Schmidt H, Maier-Woelfle M, Peczkowska M, Szmigielski C. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- 2.Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, Chamontin B, Delemer B, Giraud S, Murat A, Niccoli-Sire P, Richard S, Rohmer V, Sadoul JL, Strompf L, Schlumberger M, Bertagna X, Plouin PF, Jeunemaitre X, Gimenez-Roqueplo AP. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23:8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 3.Cascon A, Pita G, Burnichon N, Landa I, Lopez-Jimenez E, Montero-Conde C, Leskela S, Leandro-Garcia LJ, Leton R, Rodriguez-Antona C, Diaz JA, Lopez-Vidriero E, Gonzalez-Neira A, Velasco A, Matias-Guiu X, Gimenez-Roqueplo AP, Robledo M. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J Clin Endocrinol Metab. 2009;94:1701–1705. doi: 10.1210/jc.2008-2756. [DOI] [PubMed] [Google Scholar]
- 4.Bayley JP, Kunst HP, Cascon A, Sampietro ML, Gaal J, Korpershoek E, Hinojar-Gutierrez A, Timmers HJ, Hoefsloot LH, Hermsen MA, Suarez C, Hussain AK, Vriends AH, Hes FJ, Jansen JC, Tops CM, Corssmit EP, de Knijff P, Lenders JW, Cremers CW, Devilee P, Dinjens WN, de Krijger RR, Robledo M. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–372. doi: 10.1016/S1470-2045(10)70007-3. [DOI] [PubMed] [Google Scholar]
- 5.Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, Lechleiter JD, Sass M, Aronin N, Schiavi F, Boaretto F, Opocher G, Toledo RA, Toledo SP, Stiles C, Aguiar RC, Dahia PL. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42:229–233. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, Landa I, Leandro-García LJ, Letón R, Honrado E, Ramos-Medina R, Caronia D, Pita G, Gómez-Graña A, de Cubas AA, Inglada-Pérez L, Maliszewska A, Taschin E, Bobisse S, Pica G, Loli P, Hernández-Lavado R, Díaz JA, Gómez-Morales M, González-Neira A, Roncador G, Rodríguez-Antona C, Benítez J, Mannelli M, Opocher G, Robledo M, Cascón A. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43:663–667. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- 7.Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, Mannelli M, McNicol AM, Tischler AS. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. Nat Clin Pract Endocrinol Metab. 2007;3:92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- 8.Hattori K, Teranishi J, Stolle C, Yoshida M, Kondo K, Kishida T, Kanno H, Baba M, Kubota Y, Yao M. Detection of germline deletions using real-time quantitative polymerase chain reaction in Japanese patients with von Hippel-Lindau disease. Cancer Sci. 2006;97:400–405. doi: 10.1111/j.1349-7006.2006.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, Mannelli M, Friberg P, Grebe SK, Timmers HJ, Bornstein SR, Lenders JW. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18:97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, Bornstein SR, Tiebel O, Adams K, Bratslavsky G, Linehan WM, Pacak K. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–420. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benhammou JN, Vocke CD, Santani A, Schmidt LS, Baba M, Seyama K, Wu X, Korolevich S, Nathanson KL, Stolle CA, Linehan WM. Identification of intragenic deletions and duplication in the FLCN gene in Birt-Hogg-Dube syndrome. Genes Chromosomes Cancer. 2011;50:466–477. doi: 10.1002/gcc.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brauch H, Hoeppner W, Jahnig H, Wohl T, Engelhardt D, Spelsberg F, Ritter MM. Sporadic pheochromocytomas are rarely associated with germline mutations in the vhl tumor suppressor gene or the ret protooncogene. J Clin Endocrinol Metab. 1997;82:4101–4104. doi: 10.1210/jcem.82.12.4454. [DOI] [PubMed] [Google Scholar]
- 13.Pigny P, Cardot-Bauters C, Do Cao C, Vantyghem MC, Carnaille B, Pattou F, Caron P, Wemeau JL, Porchet N. Should genetic testing be performed in each patient with sporadic pheochromocytoma at presentation? Eur J Endocrinol. 2009;160:227–231. doi: 10.1530/EJE-08-0574. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhofer G, Walther MM, Huynh TT, Li ST, Bornstein SR, Vortmeyer A, Mannelli M, Goldstein DS, Linehan WM, Lenders JW, Pacak K. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab. 2001;86:1999–2008. doi: 10.1210/jcem.86.5.7496. [DOI] [PubMed] [Google Scholar]
- 15.Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nose V, Li C, Stiles CD. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, Munson PJ, Mannelli M, Goldstein DS, Elkahloun AG. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxiadriven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Rel Cancer. 2004;11:897–911. doi: 10.1677/erc.1.00838. [DOI] [PubMed] [Google Scholar]
- 17.Zbar B, Kishida T, Chen F, Schmidt L, Maher ER, Richards FM, Crossey PA, Webster AR, Affara NA, Ferguson-Smith MA, Brauch H, Glavac D, Neumann HPH, Tisherman S, Mulvihill JJ, Gross DJ, Shuin T, Whaley J, Seizinger B, Kley N, Olschwang S, Boisson C, Richard S, Lips CHM, Linehan WM, Lerman M. Germline mutations in the Von Hippel-Lindau disease (VHL) gene in families from North America, Europe, and Japan. Hum Mutat. 1996;8:348–357. doi: 10.1002/(SICI)1098-1004(1996)8:4<348::AID-HUMU8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Young AC, Craven RA, Cohen D, Taylor C, Booth C, Harnden P, Cairns DA, Astuti D, Gregory W, Maher ER, Knowles MA, Joyce A, Selby PJ, Banks RE. Analysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinoma. Clin Cancer Res. 2009;15:7582–7592. doi: 10.1158/1078-0432.CCR-09-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ercolino T, Becherini L, Valeri A, Maiello M, Gagliano MS, Parenti G, Ramazzotti M, Piscitelli E, Simi L, Pinzani P, Nesi G, DeglInnocenti D, Console N, Bergamini C, Mannelli M. Uncommon clinical presentations of pheochromocytoma and paraganglioma in two different patients affected by two distinct novel VHL germline mutations. Clin Endocrinol (Oxf) 2008;68:762–768. doi: 10.1111/j.1365-2265.2007.03131.x. [DOI] [PubMed] [Google Scholar]
- 20.Solano AR, Dourisboure RJ, Weitzel J, Podesta EJ. A cautionary note: false homozygosity for BRCA2 6174delT mutation resulting from a single nucleotide polymorphism masking the wt allele. Eur J Hum Genet. 2002;10:395–397. doi: 10.1038/sj.ejhg.5200821. [DOI] [PubMed] [Google Scholar]
- 21.Ramus SJ, Harrington PA, Pye C, Peock S, Cook MR, Cox MJ, Jacobs IJ, DiCioccio RA, Whittemore AS, Piver MS, Embrace Easton DF, Ponder BA, Pharoah PD, Gayther SA. Screening for the BRCA1-ins6kbEx13 mutation: potential for misdiagnosis. Mutation in brief #964. Online. Hum Mutat. 2007;28:525–526. doi: 10.1002/humu.9493. [DOI] [PubMed] [Google Scholar]
- 22.Choi JH, Velayati A, Stubblefield BK, Orr-Urtreger A, Gan-Or Z, Tayebi N, Sidransky E. False-positive results using a Gaucher diagnostic kit – RecTL and N370S. Mol Genet Metab. 2010;100:100–102. doi: 10.1016/j.ymgme.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miolo G, Crovatto M, Manno M, Pivetta B, Tessitori G, Picci L. Heterozygous variant at nucleotide position 875+11A > T in exon 6A cystic fibrosis transmembrane conductance regulator gene induces 852del22 mutation false-positivity by line probe assay. Fertil Steril. 2011;95(1121):e1121–e1124. doi: 10.1016/j.fertnstert.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Cascon A, Lopez-Jimenez E, Landa I, Leskela S, Leandro-Garcia LJ, Maliszewska A, Leton R, de la Vega L, Garcia-Barcina MJ, Sanabria C, Alvarez-Escola C, Rodriguez-Antona C, Robledo M. Rationalization of genetic testing in patients with apparently sporadic pheochromocytoma/paraganglioma. Horm Metab Res. 2009;41:672–675. doi: 10.1055/s-0029-1202814. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhofer G, Timmers H, Lenders JW, Bornstein SR, Tiebel O, Mannelli M, King KS, Vocke C, Linehan WM, Bratslavsky G, Pacak K. Age at diagnosis of pheochromocytoma differs according to catecholamine phenotype and tumor location. J Clin Endocrinol Metab. 2011;96:375–384. doi: 10.1210/jc.2010-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]