Deletion of early growth response gene Egr2 prevents anergy induction through diacylglycerol kinase α and restores Ras/MAPK signaling in T cells.
Abstract
T cell receptor engagement in the absence of costimulation results in a hyporesponsive state termed anergy. Understanding the transcriptional regulation of other T cell differentiation states has provided critical information regarding the biology of T cell regulation in vivo. However, the transcriptional regulation of T cell anergy has been poorly understood. Using the key anergy target gene diacylglycerol kinase (DGK) α as a focal point, we identified early growth response gene 2 (Egr2) as a central transcription factor that regulates the anergic state. Conditional Egr2 deletion in peripheral T cells abolishes induced expression of DGK-α and other anergy genes and restores Ras/MAPK signaling, IL-2 production, and proliferation upon attempted anergy induction. Using superantigen- and tumor-induced anergy models, we found that Egr2 is necessary for anergy induction in vivo. Collectively, our results implicate Egr2 as an essential transcriptional regulator of the T cell anergy program.
TCR engagement in the absence of costimulation results in a T cell hyporesponsive state characterized by defective IL-2 production and proliferation upon subsequent full rechallenge. This hyporesponsive state has been termed anergy and represents one important mechanism of controlling peripheral tolerance in vivo (Schwartz, 2003). The anergic state appears to result from increased expression of negative regulatory factors, which in turn mediate diminished TCR/CD28-induced signaling (Schwartz, 2003). This model is supported by the observation that protein synthesis inhibitor cycloheximide prevented anergy induction (Gajewski et al., 1995). Furthermore, a dominant-negative functional effect was observed upon fusing anergic cells with nonanergic T cells (Telander et al., 1999). Several anergy-associated factors have been identified, including diacylglycerol kinases (DGKs). Anergic T cells are characterized by defective Ras/MAP kinase signaling upon rechallenge with anti-CD3 and anti-CD28 mAbs, which is largely caused by up-regulation of DGK-α and DGK-ζ (Fields et al., 1996b; Zha et al., 2006; Zhong et al., 2008). DGKs phosphorylate DAG into phosphatidic acid, thus depleting the amount of DAG that otherwise could activate RasGRP1. Because RasGRP is the dominant form of RasGEF activating Ras in T cells, its failed activation blunts the Ras–MAP kinase–AP-1 pathway blocking T cell activation even in the presence of costimulation. Inasmuch as the up-regulated expression of DGKs in anergy occurs at the level of mRNA, study of the regulation of DGK-α expression would provide a starting point to investigate the transcriptional regulation of anergy-associated genes (Zha et al., 2006).
Calcium ionophores (such as ionomycin) can be sufficient to induce anergy, whereas CsA (cyclosporine A), which inhibits calcium/NFAT signaling, can prevent anergy induction, suggesting that NFAT is indispensible (Chai and Lechler, 1997). Macián et al. (2002) proposed that disproportionate activation of calcium/NFAT signaling over the Ras–MAP kinase–AP-1 pathway leads to anergy. The transcription factor early growth response gene 2 (Egr2) has also been suggested to contribute to anergy induction. Egr2 is a member of the Egr family that binds to DNA through highly conserved zinc finger domains (Gilardi et al., 1991). It has been demonstrated that Egr2 is induced early upon TCR engagement in a NFAT-dependent manner, and that overexpression of Egr2 inhibits T cell activation (Harris et al., 2004; Safford et al., 2005). However, the exact role of Egr2 in anergy is poorly understood. Here, we demonstrate that Egr2 is a major transcription factor that directly up-regulates DGK-α as well as other anergy-associated genes. Furthermore, Egr2 deletion prevents anergy induction in vitro and in vivo. Our data support the notion that Egr2 is an essential transcription factor of the anergy program, which drives functional alterations that characterize the anergic state.
RESULTS AND DISCUSSION
Egr2 directly regulates the expression of DGK-α upon anergy induction
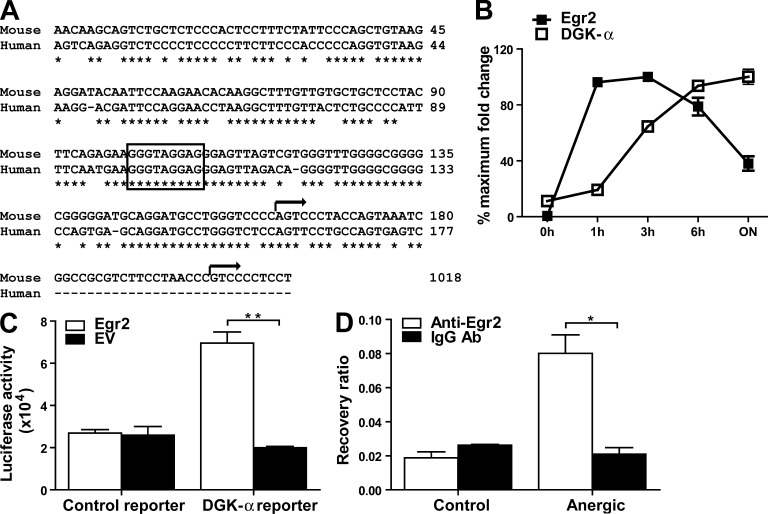
In the search for transcription factors that regulate DGK-α expression during anergy induction, we identified an Egr binding site located between −909 and −901 bp of mouse DGK-α transcriptional start site, and a corresponding binding site was found in the homologous region of human sequence (Fig. 1 A). This was of interest because our previous gene expression profiling analyses on anergic T cells identified Egr2 as a highly up-regulated transcriptional factor in the anergic state (Zha et al., 2006). Kinetic analysis upon anergy induction revealed that Egr2 mRNA peaked as early as 1–3 h after anti-CD3 mAb stimulation (Fig. 1 B). In comparison, DGK-α transcription occurred later, coinciding with the reported onset of hyporesponsiveness (Gajewski et al., 1995). The expression of both Egr2 and DGK-α was substantially lower in T cells fully activated with anti-CD3 + anti-CD28 mAbs (unpublished data). These observations suggest that Egr2 could be involved in the transcription of DGK-α in the anergic state.
Figure 1.
Egr2 is associated with DGK-α promoter upon anergy induction and can regulate DGK-α expression. (A) The homologous region of human and mouse DGK-α promoter sequences. The boxed site is a putative Egr2 binding site. The arrows represent the predicted transcriptional start sites of human and mouse DGK-α according to NCBI reference sequences. (B) pGL10 Th1 T cells were anergized with 1 µg/ml of immobilized anti-CD3, and Egr2 and DGK-α mRNA expression was assessed by qRT-PCR at the indicated time. (C) Jurkat cells were cotransfected with a control or a DGK-α reporter, and an EV or an Egr2-expressing vector, and luciferase activity was assessed 48 h later. (D) OVA-specific CAR Tg × Egr2flox/flox Th1 T cells were untreated (Control) or anergized with immobilized anti-CD3 mAb (Anergic) for 6–8 h and immunoprecipitated with an anti-Egr2 or a control IgG Ab, followed by qPCR using specific primers for the DGK-α promoter region. Data are presented as mean ± SD, and are representative of two to three independent experiments. *, P < 0.05; **, P < 0.01.
To determine whether Egr2 could induce the activity of DGK-α promoter, we cloned mouse DGK-α promoter region from −1054 to 192 bp, which contains the putative Egr binding site (−909 to −901 bp), into a luciferase reporter vector and cotransfected this with an Egr2-expressing vector into Jurkat cells. A control reporter (−54 to 190 bp), lacking the putative Egr binding site, and an empty expression vector (empty vector [EV]) were included as controls. As seen in Fig. 1 C, the luciferase activity of the DGK-α reporter increased in the presence of Egr2, suggesting that Egr2 is capable of driving DGK-α transcription. Next, we performed a ChIP assay, and the particular DGK-α promoter region was coprecipitated with an anti-Egr2 Ab in anergic cells (Fig. 1 D). Thus, upon anergy induction Egr2 associates with the DGK-α promoter and can directly regulate its expression.
Egr2 is necessary for the up-regulation of DGK-α upon anergy induction
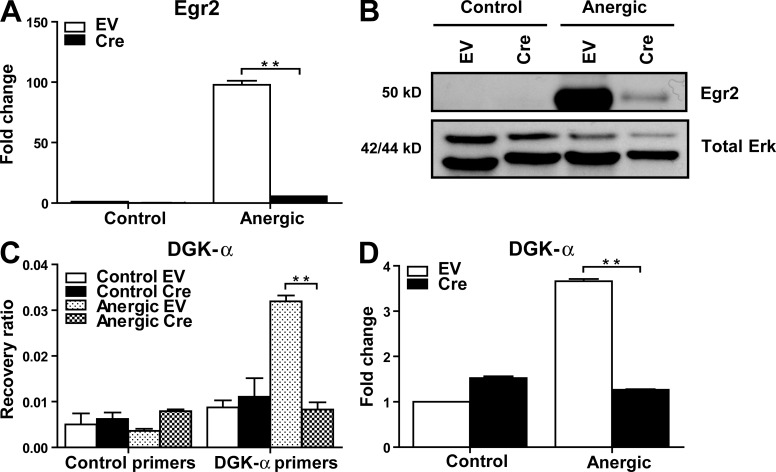
To determine the necessity of Egr2 in the expression of DGK-α upon anergy induction, we developed a system to be able to delete Egr2 conditionally from Th1 clones, to be able to use a monoclonal population of T cells, and to use the Th1 cellular context, which is the best characterized model for the anergy process (Schwartz, 2003). To achieve targeted gene deletion, we used a mouse that expresses Coxsackie/adenovirus receptor as a transgene (CAR Tg) in T cell lineage, along with a Cre-expressing adenovirus (Wan et al., 2000). Egr2flox/flox mice were interbred with CAR Tg mice to generate CAR Tg × Egr2flox/flox mice, from which OVA-specific Th1 cell clones were generated (Fitch et al., 2006). To delete Egr2, the Th1 T cell clones were transduced with the Cre adenovirus. As assessed by qRT-PCR and immunoblotting, the Egr2 expression normally induced during anergy induction by anti-CD3 mAb was reduced to minimal levels (Fig. 2, A and B).
Figure 2.
Egr2 deletion results in reduced DGK-α up-regulation upon anergy induction. (A and B) OVA-specific CAR Tg × Egr2flox/flox Th1 T cells were transduced with an EV or a Cre-expressing adenovirus (Cre) to delete Egr2. The cells were then untreated or anergized with immobilized anti-CD3 for 3–6 h, and deletion of Egr2 was confirmed by qRT-PCR (A) and immunoblotting (B). (C and D) Upon Egr2 deletion, the association of Egr2 with DGK-α was determined by ChIP Assay using primers specific for DGK-α and control primers for GJA5 (C). The up-regulation of DGK-α was assessed by qRT-PCR (D). Data are presented as mean ± SD and are representative of two to three independent experiments. **, P < 0.01.
Cre adenovirus-transduced T cell clones were anergized by immobilized anti-CD3 mAb. As shown in Fig. 2 (C and D), deletion of Egr2 abrogated detectable binding to DGK-α promoter, which, as expected, was associated with markedly reduced levels of DGK-α transcripts. Therefore, Egr2 is necessary for DGK-α up-regulation under anergizing conditions.
Egr2-deleted T cells are resistant to anergy induction in vitro
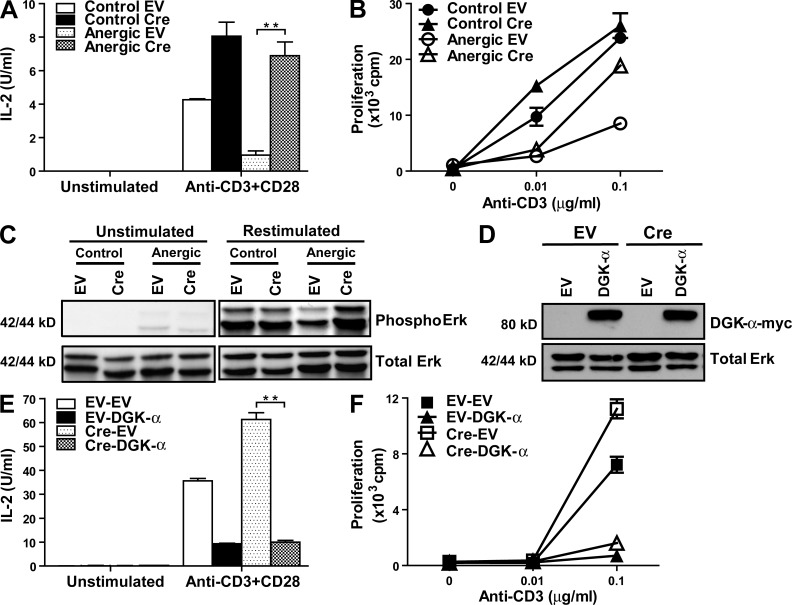
To determine whether Egr2 was essential for the functional characteristics of T cell anergy, CAR Tg × Egr2flox/flox T cell clones were transduced with the EV or Cre adenovirus and left untreated or anergized with immobilized anti-CD3 mAb. Upon rechallenge with anti-CD3 + anti-CD28 mAbs, the EV-transduced cells showed diminished IL-2 production and proliferation consistent with the hyporesponsive state of anergy (Fig. 3, A and B). However, with Egr2 deletion, the T cells remained active with nearly normal IL-2 secretion and proliferation. Of note, although the nonanergic control Cre-transduced cells produced a modestly increased amount of IL-2 upon anti-CD3 + anti-CD28 mAb stimulation compared with the control EV-transduced cells, the difference between the anergic Cre- and EV-transduced cells was more than sevenfold. Thus the restored IL-2 production found in the anergic Cre-transduced cells was not simply a result of hyperactivity; rather, Egr2 deletion rendered the cells less susceptive to anergy induction. Similar functional results were observed with additional independently derived CAR Tg × Egr2flox/flox Th1 clones (unpublished data). In agreement with these functional results, the blunted ERK phosphorylation normally observed in anergic cells was restored with Egr2 deletion (Fig. 3 C). Quantitative densitometry averaged over three independent experiments confirmed the statistical significance of this difference (P < 0.05).
Figure 3.
Egr2 deletion prevents anti-CD3 mAb-induced anergy in vitro. (A–C) EV- or Cre-transduced T cells were left untreated or anergized with anti-CD3. The cells were washed, rested for 1–2 d, and rechallenged with immobilized anti-CD3 + anti-CD28. IL-2 production was analyzed by ELISA (A), T cell proliferation by [3H] incorporation (B), and ERK phosphorylation by immunoblot (C). (D–F) Egr2-deleted T cells were transduced with an EV or a DGK–α–myc-expressing adenovirus. The overexpression of DGK-α was confirmed by immunoblot (D). IL-2 production (E) and T cell proliferation (F) upon stimulation with immobilized 1 µg/ml anti-CD3 + 1 µg/ml anti-CD28 were analyzed. Data are presented as mean ± SD and are representative of two to three independent experiments. **, P < 0.01. In B, the difference between anergic EV and anergic Cre at 0.1 µg/ml anti-CD3 is statistically significant (P < 0.01). In C, quantitative densitometry averaged over three independent experiments showed significant difference in Erk phosphorylation between anergic EV and anergic Cre during restimulation (P < 0.05). In F, there is a significant difference between EV-EV and Cre-EV (P < 0.01) but no significant difference between EV-DGK-α and Cre-DGK-α at 0.1 µg/ml of anti-CD3.
To further confirm the functional relationship between Egr2 and DGK-α, we reintroduced DGK-α into the Egr2-deleted T cells by transduction with a DGK-α–expressing adenovirus (Fig. 3 D). In fact, directed expression of DGK-α dominated over Egr2 deletion, resulting in reinhibition of IL-2 production and proliferation (Fig. 3, E and F). Thus, DGK-α is operational downstream of Egr2 and is one of the critical anergy-associated genes that contribute to T cell dysfunction.
Egr2 deletion impairs anergy induction in vivo
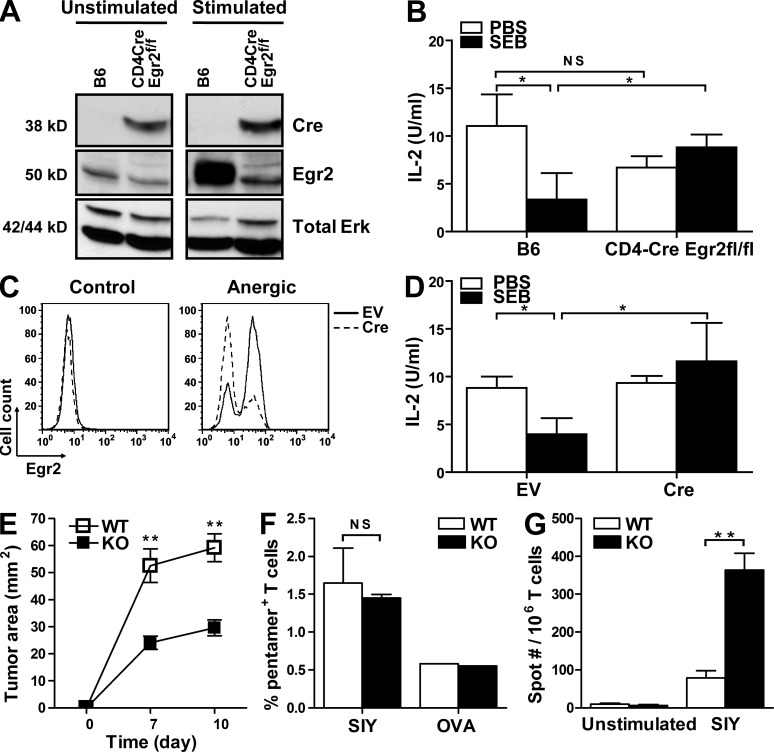
We chose a well established model of superantigen-induced anergy to examine whether the Egr2 pathway was important for anergy induction in vivo (Kawabe and Ochi, 1990; Rellahan et al., 1990). Two methods were used. In the first, CD4-Cre × Egr2flox/flox mice were generated by interbreeding CD4-Cre Tg mice and Egr2flox/flox mice, and the lack of Egr2 expression in T cells from these mice was confirmed by immunoblot (Fig. 4 A). CD4-Cre × Egr2flox/flox mice and control mice were intraperitoneally injected with staphylococcal enterotoxin B (SEB) or PBS. 7 d later, splenic T cells were isolated, comparable numbers of Vβ8+ T cells were restimulated with SEB ex vivo, and IL-2 production was measured. Consistent with the superantigen-induced anergy model, T cells from SEB-treated control mice produced significantly less IL-2 than those obtained from PBS-treated counterparts (Fig. 4 B). In contrast, there was no reduction of IL-2 by T cells from conditionally Egr2-deficient mice treated with SEB, indicating that Egr2-deficient T cells were resistant to superantigen-induced anergy in vivo. This effect was not simply an artifact of Cre expression in T cells because CD4-Cre Tg mice remained susceptible to SEB-induced anergy induction (unpublished data).
Figure 4.
Egr2 deletion leads to resistance to SEB-induced anergy in vivo and enhanced anti-tumor immune response. (A) Deletion of Egr2 gene in T cells from CD4-Cre × Egr2flox/flox was confirmed by immunoblot. (B) CD4-Cre × Egr2flox/flox and control B6 mice were intraperitoneally injected with SEB (20 µg/mouse) or PBS. 7 d later, equal numbers of Vβ8+ cells were stimulated with SEB and T cell–depleted irradiated splenocytes. IL-2 production was assessed 48 h later. (C) Cre adenovirus-mediated Egr2 deletion was confirmed by intracellular staining/flow cytometry. (D) EV- or Cre-transduced cells were adoptively transferred into OT-1 TCR Tg × Rag2−/− mice, which were intraperitoneally injected with SEB or PBS the next day. Rechallenge was conducted as described in B. (E–G) CD4-Cre × Egr2flox/flox and control B6 mice were implanted with 2 × 106 B16. SIY cells subcutaneously. Tumor size was measured on the indicated days (E). After 14 d, the mice were sacrificed and the percentage of SIY-specific CD8+ T cells was determined by SIY-Kb pentamer staining. OVA-Kb pentamer was used as a negative control (F). The SIY-specific functional T cell response was analyzed by IFN-γ ELISPOT from splenocytes. Data are represented as mean ± SEM, and are representative of two to three independent experiments. n = 5–7 mice per group per experiment. *, P < 0.05; **, P < 0.01; n.s., not statistically significant.
Because of a concern that Egr2 deletion, even late in thymic development, using Cre driven by the CD4 promoter might yield an abnormal peripheral T cell compartment, a second method was used. T cells from CAR Tg × Egr2flox/flox mice were purified, and Egr2 was deleted in vitro using the Cre-expressing adenovirus. After verification of Egr2 deletion by intracellular flow cytometry (Fig. 4 C), T cells were adoptively transferred into OT-1 TCR Tg × Rag2−/− mice. The rationale for using these recipients is to block homeostatic proliferation of the transferred T cells, which itself could interrupt or reverse anergy but without inducing immune-mediated regression of CAR-expressing T cells (Brown et al., 2006; Kline et al., 2008). In addition, the β chain of OT-1 TCR is Vβ5, which does not respond to SEB superantigen (Vβ8). 1 d after adoptive transfer, recipient mice were injected intraperitoneally with SEB, and 7 d later T cells were isolated and analyzed as described in the previous section. As with the CD4-Cre model, Cre-transduced T cells did not show reduced IL-2 production after SEB treatment in vivo (Fig. 4 D), confirming the importance of Egr2 for anergy induction in this model.
To further examine the functional role for Egr2 in vivo, CD4-Cre × Egr2flox/flox mice and control mice matched for age and sex were injected subcutaneously with B16.SIY (SIY-expressing B16 melanoma cells). This is a tumor model system in which anergy appears to be one mechanism of immune escape in vivo (Kline et al., 2008). As shown in Fig. 4 E, tumor growth was significantly slowed in Egr2-deleted mice. Although the frequency of SIY-specific T cells in CD4-Cre × Egr2flox/flox and control mice were similar (Fig. 4 F), Egr2 deletion led to markedly augmented SIY-specific IFN-γ production ex vivo assessed by ELISPOT (Fig. 4 G). These results argue for increased functional capacity of the endogenously activated anti-tumor T cells and indicate that T cell–intrinsic Egr2 expression can contribute to tumor escape from immune destruction in vivo. We did not observe any obvious evidence of spontaneous autoimmunity in CD4-Cre × Egr2flox/flox mice up to 12 mo of age (unpublished data).
In the present study, we demonstrate that Egr2 directly regulates the expression of DGK-α in anergic T cells. Furthermore, conditional deletion of Egr2 in T cells abolished anergy-induced DGK-α expression indicating the necessity of Egr2 in this process. In contrast, transduction of DGK-α back into the Egr2-deleted T cells resulted in decreased IL-2 production and T cell proliferation. These data suggest that DGK-α acts downstream of Egr2 and is one of the critical anergy-associated genes responsible for maintaining the anergic state. Besides DGK-α, multiple anergy-associated genes have been identified previously including DGK-ζ, Cbl-b, Itch, GRAIL, Tob1, and Dtx1, which also contribute to T cell–intrinsic hyporesponsiveness and the regulation of peripheral tolerance. In fact, using ChIP assay, we found that Egr2 bound the regulatory regions of these additional anergy-associated genes (unpublished data), and conditional deletion of Egr2 prevented their up-regulation as determined by qRT-PCR (the fold increase upon anergy induction of these genes was compared in EV and Cre adenovirus-infected CAR Tg × Egr2flox/flox T cells, and the results are DGK-ζ: 5.64 ± 0.08 vs. 2.19 ± 0.01; Cbl-b: 5.62 ± 0.05 vs. 1.65 ± 0.01; Itch: 6.54 ± 0.47 vs. 3.41 ± 0.18; Tob1: 2.56 ± 0.11 vs. 1.07 ± 0.01; Dtx1: 2.80 ± 1.16 vs. 0.73 ± 0.65; all P values <0.05). We have also performed gene expression profiling of control versus Egr2-deleted T cells and a genome-wide ChIP-SEQ analysis, which have identified 46 genes that represent the identifiable Egr2 transcriptome in T cell anergy (unpublished data). The functional importance of several of these new candidates is currently being characterized. Thus, Egr2 is a major transcriptional regulator of the full anergy program.
T cell anergy can be viewed as a differentiation state of activated T cells, and previous studies had suggested that anergic T cells are not inert but can have additional functional roles, such as the ability to function as suppressor cells (Chai et al., 1999). The identification of transcriptional regulators for other T cell differentiation states (e.g., Tbet for Th1, GATA-3 for Th2, RORγT for Th17, etc.) has provided tremendous information about the biology of those subsets in immune models in vivo. The identification of Egr2 as a central factor for anergic T cells should similarly place studies of the anergic state on firmer footing for in vivo studies, and pursuit of additional gene targets of Egr2 might identify molecules that could be used as markers for anergic T cells for ex vivo identification.
Egr2 is the first molecule in our hands which, when deleted from or blocked in peripheral T cells, results in anergy resistance in anti-CD3 induced in vitro anergy model as well as in the superantigen SEB-induced in vivo anergy models. Increased activities of protein tyrosine kinase Fyn, and Rap1 GTPase, which, driven by guanine nucleotide exchange factor C3G bound to adapter protein CrkL, have been described in anergic T cells (Gajewski et al., 1994; Boussiotis et al., 1997). However, Fyn- and CrkL-deficient T cells remained subject to anergy induction, arguing that those pathways are dispensable (Fields et al., 1996a; Zha et al., 2006). Primary T cells from Cbl-b knockout and PTEN conditional knockout mice had been reported to be hyperresponsive and relatively anergy resistant (Bachmaier et al., 2000; Suzuki et al., 2001). However, introduction of a dominant-negative form of Cbl-b, or conditional deletion of PTEN directly in the peripheral T cells, led to hyperactivation but intact anergy susceptibility (Zha and Gajewski, 2007; unpublished data). Thus, not every genetic manipulation of peripheral T cells that can lead to increased activation results in anergy resistance, and these observations together argue more strongly for the particular importance of the Egr2 pathway.
In the anergy models using immobilized anti-CD3 or SEB, Egr2 deletion prevented anergy induction. However, Ramón et al. (2010) recently reported that CD4-Cre × Egr2flox/flox mice did not demonstrate increased immune responses against minor histocompatibility antigens, Toxoplasma gondii infection, or lymphocytic choriomeningitis virus. These data suggest that anergy is not a dominant mechanism of immune dysfunction in those models, and support the notion that Egr2 is not simply a global negative regulator of T cell activation. It will be of interest to examined the role of Egr2 and its target genes in other in vivo models in which anergy is thought to contribute to peripheral tolerance, such as costimulation blockade-induced immune suppression for solid organ transplantation (Alegre and Najafian, 2006).
MATERIALS AND METHODS
Mice and cells.
Egr2flox/flox and CD4-Cre Tg mice were gifts from H. Singh and F. Gounari (University of Chicago, Chicago, IL), respectively. CAR Tg mice expressing the extracellular domain of CAR under control of a Lck promoter/CD2 enhancer were generated as previously described (Wan et al., 2000). All three mouse strains have been backcrossed with C57BL/6 mice for more than eight generations. C57BL/6 mice were purchased from Taconic. All mice were housed in pathogen-free conditions at the University of Chicago, and all animal protocols were approved by the Institutional Animal Care and Use Committee. To generate CAR Tg × Egr2flox/flox Th1 clones, CAR Tg × Egr2flox/flox mice were immunized in the hind footpads with chicken OVA (Sigma-Aldrich) emulsified in complete Freund’s adjuvant (Sigma-Aldrich). 7 d later, the draining lymph nodes were harvested, and CD4+ Th1 cell clones were derived and maintained as previously described (Gajewski and Fitch, 1990; Fitch et al., 2006).
Luciferase reporter vector construction and analysis.
The mouse DGK-α promoter region from −1054 to 192 bp containing a putative Egr2 binding site (−909 to −901 bp) was amplified from genomic DNA by PCR using the following primers: forward, 5′-ATCACTCGAGTTCCAGCTGTCAACGCTTCCTTCT-3′; and reverse, 5′-CTATGGTACCTCTCTCTCTGACCCTCACTGGACC-3′. As control, the region from −54 to 190 bp was amplified using the following primers: forward, 5′-ATCGACTCGAGCCAGCTGTCAACGCT-3′; and reverse, 5′-TCGTAGGTACCCAGTGTGTCGTCAGGA-3′. The fragments were then cloned into a pGL4luc2 vector (Promega) via KpnI and XhoI sites (underlined) to make DGK-α and control luciferase reporter vectors. A plasmid encoding Egr2 was a gift from J. Powell (Johns Hopkins School of Medicine, Baltimore, MD). Jurkat cells (12 × 106 cells in 300 µl of RPMI1640 with 10% FBS) were cotransfected with 10 µg EV or Egr2-expressing vector, along with 10 µg control or DGK-α luciferase reporter vector by electroporation (300 V and 800 µF) using a Gene Pulser II electroporator (Bio-Rad Laboratories). 16 h later, the cells were lysed in Glo Lysis Buffer (Promega), and luciferase activity was measured by Bright-Glo Luciferase Assay System (Promega).
Adenovirus transduction.
Cre- and DGK-α–expressing adenoviruses were generated as previously described (Zha et al., 2006, 2008). For T cell transduction, cells were suspended at the high density of 10 × 106/ml in DMEM with 2% FBS, incubated with an EV or the Cre adenovirus at 37°C for 50 min, transferred to DMEM with 10% FBS, and cultured for another 16 h at low density of 106/ml.
Anergy induction in vitro and in vivo.
In vitro anergy was induced by treating cells overnight with 1 µg/ml of immobilized anti-CD3 mAb (145-2C11; BioXCell). The cells were then harvested, washed, and rested for 1–3 d before analysis or rechallenge. T cell rechallenge was performed using 1 µg/ml anti-CD3 + 1 µg/ml anti-CD28 (PV-1; BioXCell) mAbs, either immobilized to plastic plates or bound to beads (Dynabeads; Invitrogen). IL-2 production was assessed at 24 h by ELISA, and proliferation was measured at 48 h by [3H] thymidine incorporation.
In vivo anergy was induced by superantigen SEB using two different models. In the first model, CD4-Cre × Egr2flox/flox or control mice were injected intraperitoneally with SEB (20 µg/mouse; Toxin Technology) or PBS. 7 d later, total T cells were purified from spleens using a Pan T cell Isolation kit (Miltenyi Biotec). The number of Vβ8+ cells was calculated by multiplying total number of T cells by percentage of Vβ8+ cells determined by flow cytometry. For rechallenge, 25 × 103 Vβ8+ cells were stimulated with 10 µg/ml SEB and 250 × 104 irradiated T cell–depleted splenocytes. IL-2 production was assessed at 48 h by ELISA. In the second model, total T cells from CAR Tg × Egr2flox/flox mice were purified, transduced with the EV or Cre-expressing adenovirus, and cultured for 3 d with 1 µg/ml IL-7 (R&D Systems). 5 × 106 cells were then adoptively transferred into OT-1 TCR Tg × Rag2−/− mice. The next day, the mice were treated by SEB or PBS injection, and 7 d later the T cell collection and rechallenge were performed as described.
ChIP assay.
ChIP assays were conducted according to the manufacturer’s protocol (Millipore). In brief, 2.5 × 106 cells were lysed in 500 µl SDS lysis buffer, and cellular DNA was sheared with six sets of 15-s pulses plus a 60-s rest using a Misonix Sonicator 3000 (Qsonica). For immunoprecipitation, 200 µl of cell lysate supernatant was diluted fivefold in ChIP dilution buffer, and an anti-Egr2 Ab was added at 1:100 (Covance). SYBR green qPCR was conducted using primers specific for DGK-α promoter (forward, 5′-CCCGCCCCAAACCCACGACTAACT-3′; reverse, 5′-TGCTCTCCCACTCCTTTCTATTCC-3′). Primers specific for GJA5 were used as controls (forward, 5′-ACCATGGAGGTGGCCTTCA-3′; reverse, 5′-CATGCAGGGTATCCAGGAAGA-3′).
qRT-PCR.
Total RNA was purified using an RNeasy Mini kit (QIAGEN). RNA was reverse transcribed by M-MLV RT (Invitrogen). The primers and probes were purchased from IDT and Roche, and Applied Biosystems. qRT-PCR used primers and probes specific for Egr2 (forward, 5′-CTACCCGGTGGAAGACCTC-3′; reverse, 5′-AATGTTGATCATGCCATCTCC-3′) and DGK-α (forward, 5′-CTGGGCACTGGAAATGATCT-3′; reverse, 5′-AATCTTTCTCAAATTCTCACCTTCATA-3′). Relative RNA abundance was determined based on control 18S RNA (Hs99999901_s1; Applied Biosystems).
Immunoblot analysis.
Equal numbers of T cells were resuspended in ice-cold lysis buffer containing 50 mM Tris, pH 7.6, 5 mM EDTA, 150 mM NaCl, 0.5% Triton X-100, 1 mM PMSF, 10 mM NaF, 1 mM Na3VO4, and 1× protease inhibitor mixture (Roche). After a 30-min incubation on ice, the cells were spun for 10 min at top speed at 4°C, and supernatant was collected. The cellular lysate was loaded into 10% Tris-HCL gels (Bio-Rad Laboratories), separated by SDS-PAGE, and transferred to PVDF membranes (Millipore). Proteins were detected using primary antibodies against Egr2 (1:1,000), phospho-ERK (1:1,000; Cell Signaling Technology), total ERK (1:1,000; Invitrogen), and Myc-tag (1:1,000; Cell Signaling Technology). Secondary antibodies were HRP-linked anti–mouse IgG or anti–rabbit IgG (1:3,000; GE Healthcare). Detection was performed using an ECL Detection kit (GE Healthcare).
Flow cytometry and intracellular staining.
Antibodies specific for CD3, CD4, CD8, and Vβ8 were purchased from BD, eBioscience, and BioLegend. For Egr2 intracellular staining, cells were permeated for 1 h (Foxp3 Buffer Set; eBioscience), stained with the anti-Egr2 rabbit polyclonal Ab (1:100) for 1 h at 25°C, followed by a secondary antibody Alexa Fluor 647 goat anti–rabbit (1:100; Invitrogen) for 1 h at 4°C.
Tumor implantation and IFN-γ ELISPOT.
B16. SIY tumor cells were washed three times with DPBS, and 2 × 106 cells were injected subcutaneously in 100 µl DPBS at the flank. Tumor size was measured twice per week, and tumor area was calculated by multiplying the longest diameter and the shortest diameter of the tumor. 14 d later, the mice were sacrificed, and the percentage of SIY-specific CD8+ T cells was determined by SIY-Kb pentamer staining according to the manufacturer’s protocol (Proimmune). An irrelevant pentamer OVA-Kb was used as a negative control. To determine functional tumor antigen-specific immune responses by ELISPOT, splenocytes were plated at 1 × 106 cells/well and stimulated overnight with medium alone or 80 nM SIY peptide. IFN-γ–producing cells were assessed using the manufacturer’s protocol (mouse IFN-γ ELISPOT kit; BD).
Statistical analysis.
Data from independent groups were analyzed using a Student’s t test. Western blot data were analyzed using ANOVA after digital densitometry.
Online supplemental material.
Table S1 shows ChIP Assay primers used to confirm Egr2 association. Table S2 shows qRT-PCR primers and probes used to confirm dependency on Egr2 for expression in anergy.
Supplementary Material
Acknowledgments
We thank Dr. Harinder Singh and Dr. Fotini Gounari (University of Chicago, Chicago, IL) for providing Egr2flox/flox and CD4-Cre Tg mice; Dr. Jonathan Powell (Johns Hopkins School of Medicine, Baltimore, MD) for providing an Egr2 plasmid; Fenge Gao, Mihir Vohra, and Long Zhang for technical assistance; and Drs. Justin Kline, Maria-Luisa Alegre, Mercedes B. Fuertes, and Robbert M. Spaapen for critical review of the manuscript.
This work was supported by R01 AI080745 and R01 CA118153 from the National Institutes of Health.
The authors have no competing financial interests.
Footnotes
Abbreviation used:
- CAR Tg
- Coxsackie/adenovirus receptor transgenic
- CsA
- cyclosporine A
- DGK
- diacylglycerol kinase
- Egr2
- early growth response gene 2
- EV
- empty vector
- SEB
- staphylococcal enterotoxin B
References
- Alegre M.L., Najafian N. 2006. Costimulatory molecules as targets for the induction of transplantation tolerance. Curr. Mol. Med. 6:843–857 10.2174/156652406779010812 [DOI] [PubMed] [Google Scholar]
- Bachmaier K., Krawczyk C., Kozieradzki I., Kong Y.-Y., Sasaki T., Oliveira-dos-Santos A., Mariathasan S., Bouchard D., Wakeham A., Itie A., et al. 2000. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 403:211–216 10.1038/35003228 [DOI] [PubMed] [Google Scholar]
- Boussiotis V.A., Freeman G.J., Berezovskaya A., Barber D.L., Nadler L.M. 1997. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 278:124–128 10.1126/science.278.5335.124 [DOI] [PubMed] [Google Scholar]
- Brown I.E., Blank C., Kline J., Kacha A.K., Gajewski T.F. 2006. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J. Immunol. 177:4521–4529 [DOI] [PubMed] [Google Scholar]
- Chai J.G., Lechler R.I. 1997. Immobilized anti-CD3 mAb induces anergy in murine naive and memory CD4+ T cells in vitro. Int. Immunol. 9:935–944 10.1093/intimm/9.7.935 [DOI] [PubMed] [Google Scholar]
- Chai J.G., Bartok I., Chandler P., Vendetti S., Antoniou A., Dyson J., Lechler R. 1999. Anergic T cells act as suppressor cells in vitro and in vivo. Eur. J. Immunol. 29:686–692 [DOI] [PubMed] [Google Scholar]
- Fields P., Fitch F.W., Gajewski T.F. 1996a. Control of T lymphocyte signal transduction through clonal anergy. J. Mol. Med. 74:673–683 10.1007/s001090050071 [DOI] [PubMed] [Google Scholar]
- Fields P.E., Gajewski T.F., Fitch F.W. 1996b. Blocked Ras activation in anergic CD4+ T cells. Science. 271:1276–1278 10.1126/science.271.5253.1276 [DOI] [PubMed] [Google Scholar]
- Fitch F.W., Gajewski T.F., Hu-Li J. 2006. Production of TH1 and TH2 cell lines and clones. Curr. Protoc. Immunol. Chapter 3:3: 13 [DOI] [PubMed] [Google Scholar]
- Gajewski T.F., Fitch F.W. 1990. Anti-proliferative effect of IFN-gamma in immune regulation. IV. Murine CTL clones produce IL-3 and GM-CSF, the activity of which is masked by the inhibitory action of secreted IFN-gamma. J. Immunol. 144:548–556 [PubMed] [Google Scholar]
- Gajewski T.F., Qian D., Fields P., Fitch F.W. 1994. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc. Natl. Acad. Sci. USA. 91:38–42 10.1073/pnas.91.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T.F., Fields P., Fitch F.W. 1995. Induction of the increased Fyn kinase activity in anergic T helper type 1 clones requires calcium and protein synthesis and is sensitive to cyclosporin A. Eur. J. Immunol. 25:1836–1842 10.1002/eji.1830250707 [DOI] [PubMed] [Google Scholar]
- Gilardi P., Schneider-Maunoury S., Charnay P. 1991. Krox-20: a candidate gene for the regulation of pattern formation in the hindbrain. Biochimie. 73:85–91 10.1016/0300-9084(91)90079-G [DOI] [PubMed] [Google Scholar]
- Harris J.E., Bishop K.D., Phillips N.E., Mordes J.P., Greiner D.L., Rossini A.A., Czech M.P. 2004. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J. Immunol. 173:7331–7338 [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. 1990. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J. Exp. Med. 172:1065–1070 10.1084/jem.172.4.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline J., Brown I.E., Zha Y.Y., Blank C., Strickler J., Wouters H., Zhang L., Gajewski T.F. 2008. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin. Cancer Res. 14:3156–3167 10.1158/1078-0432.CCR-07-4696 [DOI] [PubMed] [Google Scholar]
- Macián F., García-Cózar F., Im S.H., Horton H.F., Byrne M.C., Rao A. 2002. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 109:719–731 10.1016/S0092-8674(02)00767-5 [DOI] [PubMed] [Google Scholar]
- Ramón H.E., Cejas P.J., LaRosa D., Rahman A., Harris J.E., Zhang J., Hunter C., Choi Y., Turka L.A. 2010. EGR-2 is not required for in vivo CD4 T cell mediated immune responses. PLoS ONE. 5:e12904 10.1371/journal.pone.0012904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellahan B.L., Jones L.A., Kruisbeek A.M., Fry A.M., Matis L.A. 1990. In vivo induction of anergy in peripheral Vβ8+ T cells by staphylococcal enterotoxin B. J. Exp. Med. 172:1091–1100 10.1084/jem.172.4.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford M., Collins S., Lutz M.A., Allen A., Huang C.T., Kowalski J., Blackford A., Horton M.R., Drake C., Schwartz R.H., Powell J.D. 2005. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat. Immunol. 6:472–480 10.1038/ni1193 [DOI] [PubMed] [Google Scholar]
- Schwartz R.H. 2003. T cell anergy. Annu. Rev. Immunol. 21:305–334 10.1146/annurev.immunol.21.120601.141110 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Yamaguchi M.T., Ohteki T., Sasaki T., Kaisho T., Kimura Y., Yoshida R., Wakeham A., Higuchi T., Fukumoto M., et al. 2001. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 14:523–534 10.1016/S1074-7613(01)00134-0 [DOI] [PubMed] [Google Scholar]
- Telander D.G., Malvey E.N., Mueller D.L. 1999. Evidence for repression of IL-2 gene activation in anergic T cells. J. Immunol. 162:1460–1465 [PubMed] [Google Scholar]
- Wan Y.Y., Leon R.P., Marks R., Cham C.M., Schaack J., Gajewski T.F., DeGregori J. 2000. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc. Natl. Acad. Sci. USA. 97:13784–13789 10.1073/pnas.250356297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Y., Gajewski T.F. 2007. An adenoviral vector encoding dominant negative Cbl lowers the threshold for T cell activation in post-thymic T cells. Cell. Immunol. 247:95–102 10.1016/j.cellimm.2007.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Y., Marks R., Ho A.W., Peterson A.C., Janardhan S., Brown I., Praveen K., Stang S., Stone J.C., Gajewski T.F. 2006. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat. Immunol. 7:1166–1173 10.1038/ni1394 [DOI] [PubMed] [Google Scholar]
- Zha Y., Shah R., Locke F., Wong A., Gajewski T.F. 2008. Use of Cre-adenovirus and CAR transgenic mice for efficient deletion of genes in post-thymic T cells. J. Immunol. Methods. 331:94–102 10.1016/j.jim.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X.P., Guo R., Zhou H., Liu C., Wan C.K. 2008. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol. Rev. 224:249–264 10.1111/j.1600-065X.2008.00647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.