Abstract
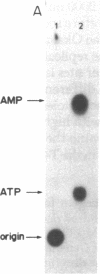
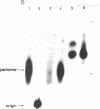
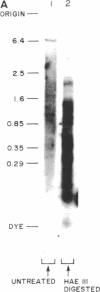
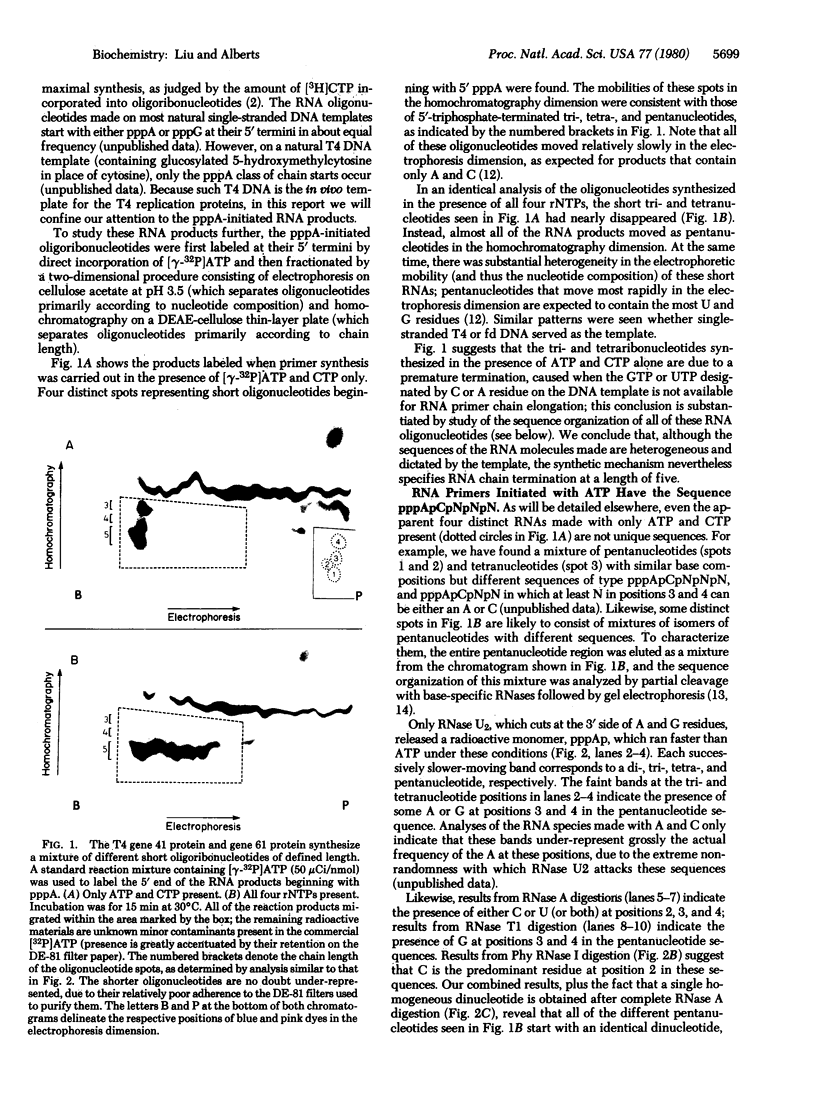
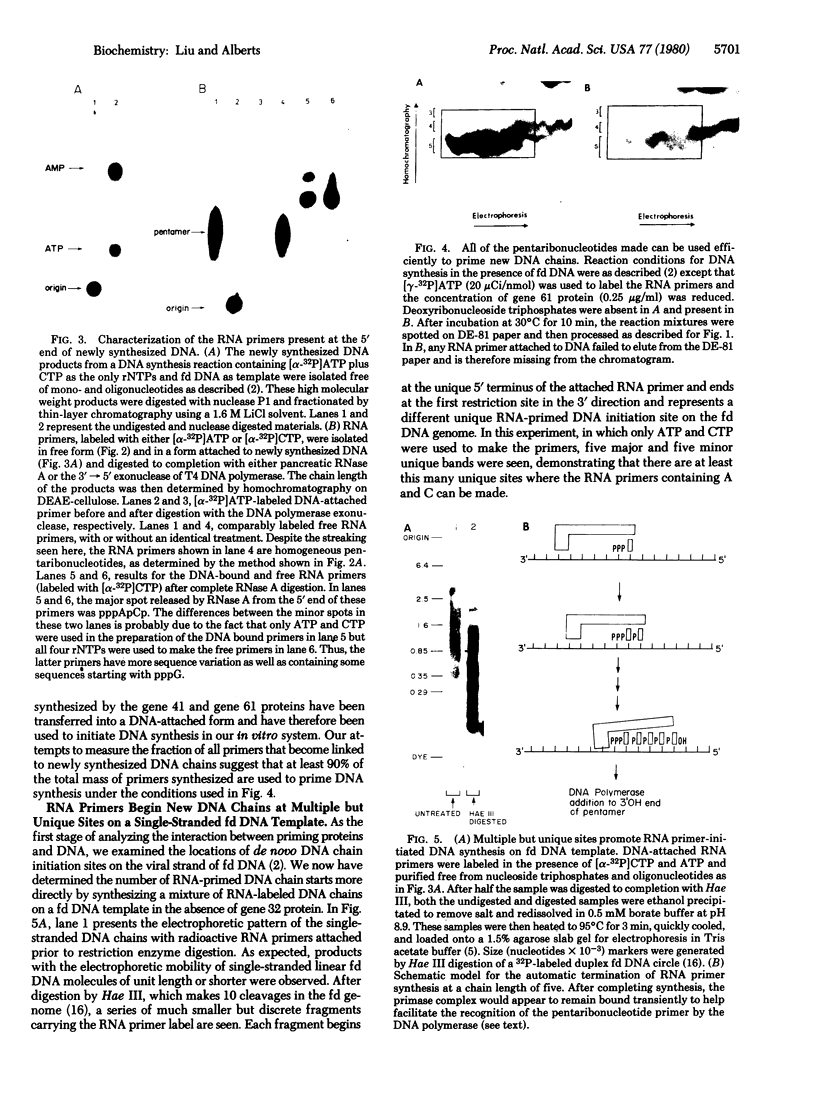
In the presence of single-stranded DNA, the bacteriophage T4 gene 41 and gene 61 proteins catalyze the synthesis of a group of pentaribonucleotides which are homogeneous in chain length but heterogeneous in nucleotide sequence. When single-stranded T4 DNA is used as template, a unique dinucleoside sequence, pppApC, is found at the 5' end of these pentaribonucleotides with the general sequence pppApCpNpNpN. In the presence of the remaining five T4 replication proteins, the pentaribonucleotides can be utilized with high efficiency to prime de novo DNA chain starts; as a result, the vast majority of them can be detected at the 5' end of newly made DNA molecules in an unaltered form. There are multiple, but specific, sites at which new DNA chains are primed in this way on a natural single-stranded DNA. Because identical RNA primers have been isolated from the 5' end of the Okazaki fragments made in T4-infected cells, we suggest that the T4 gene 41 and gene 61 proteins also make the pentaribonucleotides that prime de novo T4 DNA chain starts in vivo during lagging strand DNA synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R., Köster H. DNA chain length markers and the influence of base composition on electrophoretic mobility of oligodeoxyribonucleotides in polyacrylamide-gels. Nucleic Acids Res. 1979;6(6):2069–2087. doi: 10.1093/nar/6.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E. Pauses at positions of secondary structure during in vitro replication of single-stranded fd bacteriophage DNA by T4 DNA polymerase. Anal Biochem. 1980 Mar 15;103(1):127–139. doi: 10.1016/0003-2697(80)90246-8. [DOI] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Okazaki T. Structure of the RNA portion of the RNA-linked DNA pieces in bacteriophage T4-infected Escherichia coli cells. J Mol Biol. 1979 Dec 25;135(4):841–861. doi: 10.1016/0022-2836(79)90515-1. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Burke R. L., Hibner U., Barry J., Alberts B. Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):469–487. doi: 10.1101/sqb.1979.043.01.053. [DOI] [PubMed] [Google Scholar]
- McMacken R., Kornberg A. A multienzyme system for priming the replication of phiX174 viral DNA. J Biol Chem. 1978 May 10;253(9):3313–3319. [PubMed] [Google Scholar]
- Morris C. F., Hama-Inaba H., Mace D., Sinha N. K., Alberts B. Purification of the gene 43, 44, 45, and 62 proteins of the bacteriophage T4 DNA replication apparatus. J Biol Chem. 1979 Jul 25;254(14):6787–6796. [PubMed] [Google Scholar]
- Morris C. F., Moran L. A., Alberts B. M. Purification of gene 41 protein of bacteriophage T4. J Biol Chem. 1979 Jul 25;254(14):6797–6802. [PubMed] [Google Scholar]
- Morris C. F., Sinha N. K., Alberts B. M. Reconstruction of bacteriophage T4 DNA replication apparatus from purified components: rolling circle replication following de novo chain initiation on a single-stranded circular DNA template. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4800–4804. doi: 10.1073/pnas.72.12.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G. RNA priming of DNA replication by bacteriophage T4 proteins. J Biol Chem. 1980 Mar 10;255(5):2176–2182. [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Kurosawa Y., Ogawa T., Seki T., Shinozaki K., Hirose S., Fujiyama A., Kohara Y., Machida Y., Tamanoid F. Structure and metabolism of the RNA primer in the discontinuous replication of prokaryotic DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):203–219. doi: 10.1101/sqb.1979.043.01.026. [DOI] [PubMed] [Google Scholar]
- Reichard P., Eliasson R., Söderman G. Initiator RNA in discontinuous polyoma DNA synthesis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4901–4905. doi: 10.1073/pnas.71.12.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Lanka E., Morelli G., Seiffert D., Yuki A. Bacteriophage-T7-induced DNA-priming protein. A novel enzyme involved in DNA replication. Eur J Biochem. 1977 Feb;72(3):543–558. doi: 10.1111/j.1432-1033.1977.tb11278.x. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Sinha N. K., Morris C. F., Alberts B. M. Efficient in vitro replication of double-stranded DNA templates by a purified T4 bacteriophage replication system. J Biol Chem. 1980 May 10;255(9):4290–4293. [PubMed] [Google Scholar]
- Southern E. M. An improved method for transferring nucleotides from electrophoresis strips to thin layers of ion-exchange cellulose. Anal Biochem. 1974 Nov;62(1):317–318. doi: 10.1016/0003-2697(74)90395-9. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Erickson J. M., Goulian M. Initiator RNA of nascent DNA from animal cells. J Mol Biol. 1979 Apr 25;129(4):531–545. doi: 10.1016/0022-2836(79)90467-4. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Jay E., Bahl C. P., Wu R. A reliable mapping method for sequence determination of oligodeoxyribonucleotides by mobility shift analysis. Anal Biochem. 1976 Jul;74(1):73–93. doi: 10.1016/0003-2697(76)90311-0. [DOI] [PubMed] [Google Scholar]