Abstract
With advancing age, the prevalence of both stroke and non valvular atrial fibrillation (NVAF) is increasing. NVAF in old age has a high embolic potential if not anticoagulated. Oral anticoagulation therapy is cost effective in older people with NVAF due to their high base line stroke risk. The current stroke and bleeding risk scoring schemes have been based on complex scoring systems that are difficult to apply in clinical practice. Both scoring schemes include similar risk factors for ischemic and bleeding events which may lead to confusion in clinical decision making to balance the risks of bleeding against the risks of stroke, thereby limiting the applicability of such schemes. The difficulty in application of such schemes combined with physicians’ fear of inducing bleeding complications has resulted in under use of anticoagulation therapy in older people. As older people (≥75 years) with NVAF are all at high risk of stroke, we are suggesting a pragmatic approach based on a yes/no decision rather than a risk scoring stratification which involves an opt out rather an opt in approach unless there is a contraindication for oral anticoagulation. Antiplatelet agents should not be an alternative option for antithrombotic treatment in older people with NVAF due to lack of efficacy and the potential of being used as an excuse of not prescribing anticoagulation. Bleeding risk should be assessed on individual basis and the decision to anticoagulate should include patients’ views.
Keywords: atrial fibrillation, anticoagulation, older people
Non-valvular atrial fibrillation (NVAF) is the most common sustained cardiac arrhythmia [1]. It is recognised as a major risk factor for stroke, increasing the risk by 5-fold in comparison to that in individuals in normal sinus rhythm [2]. Randomized controlled trials have shown that prophylactic treatment with warfarin decreases the risk of stroke by 60% to 70% in patients with NVAF [3]. The major benefit of warfarin is seen in patients at highest risk for stroke such as older people (≥75 years) and those of any age with other risk factors such as hypertension, left ventricular dysfunction, diabetes mellitus, or previous transient ischemic attack (TIA) or stroke [4]. Current guidelines generally recommend oral anticoagulation therapy for patients with NVAF and at high stroke risk, antiplatelet therapy for those at low risk and either therapy for those with moderate risk [5]. Stroke risk as well as bleeding risk is assessed by stratification scoring schemes derived mainly from the control arms of the anticoagulation in NVAF clinical trials or from expert panel consensus. Age appears to be a consistent feature in both bleeding and stroke risk schemes. Therefore, the decision to anticoagulate older patients with NVAF for stroke prophylaxis is not very clear. This uncertainty combined with fear of inducing bleeding complications is reflected in clinical practice with under treatment of elderly patients [6]. This review investigates the current evidence for the stroke risk and benefits of oral anticoagulation in older people with NVAF, addresses physicians’ fear of using oral anticoagulation in the elderly and suggests a pragmatic approach in clinical practice.
Epidemiology of NVAF and stroke
NVAF is uncommon before the age of 60 years but the prevalence doubles with each decade of life affecting about 10% of older people 80–90 years old [7]. The prevalence of NVAF is projected to triple by 2050 [8]. For example, in the US it is estimated that 5.6 million people will have NVAF, of which 50% will be over 80 years old [9]. The progressive increase in prevalence is likely due to the improved survival of NVAF patients. Data from the Screening for Atrial Fibrillation in the Elderly (SAFE) trial showed a prevalence of NVAF of 6% in people aged 65–74, 12% in people aged 75–84, and 16% in people aged ≥85 [10]. The incidence of stroke is also age dependent and doubles for every successive decade after the age of 55 years reaching around 12% in white persons aged 75–84 years old and 17% in those >85 years of age [11]. NVAF is associated with a 5-fold increase in risk of ischemic stroke across all age groups and accounts for 15% to 20% of all strokes [12]. However, the attributable risk of stroke from NVAF increases significantly with age, from 1.5% for individuals aged 50 to 59 years to 23.5% for individuals aged 80 to 89 years with 40% of stroke in patients >80 years due to NVAF [2]. NVAF related stroke is associated with double mortality and more severe functional disability than stroke affecting patients in sinus rhythm [13]. In summary, age is a detrimental factor for the prevalence of both stroke and NVAF putting older people with NVAF at the highest risk of stroke compared with younger patients in sinus rhythm. Furthermore very old age is an independent predictor of both short and long term stroke outcomes. In the community based Copenhagen Stroke Study of 1,197 elderly stroke patients, very old age (≥85 years old) independently predicted short term mortality {odds ratio (OR) 2.5, 95% confidence interval (CI) 1.5 to 4.2}, and discharge to nursing home or in-hospital mortality (OR 2.7, 1.7 to 4.4). Five years after stroke old age predicted mortality or nursing home placement (OR 3.9, 2.1 to 7.3) and long term mortality {hazard ratio (HR) 2.0, 1.6 to 2.5}. Very old age was associated with more prevalent NVAF (37.4 % in those ≥85 years old vs 14.6% in those <85 years old, p <0.0001) while hypertension and diabetes were less often seen in the very old (25.3% vs 34.4%, p = 0.02 and 13.7% vs 22%, p = 0.01) suggesting that the more severe strokes among the very old are due to a shift from stroke caused by hypertensive arterial disease in the young towards cardioembolic strokes in the very old [14].
Anticoagulation in older people (Table 1)
Table 1:
Anticoagulation in older people with NVAF
| • Efficacy of oral anticoagulation is maintained across all age groups even in the very old (≥85 years). |
| • Efficacy of oral anticoagulation is maintained across all CHADS2 scores even in those with low risk (CHADS2 score 1–2). |
| • The greatest net clinical benefit of oral anticoagulation is observed in those ≥85 years old due to their high base line stroke risk. |
| • Good quality of INR control is achievable regardless of age. |
| • Aspirin has very little benefit in stroke risk reduction in patients with NVAF and this benefit tends to be attenuated further in old age. |
| • Oral anticoagulation therapy remains superior to combination of antiplatelet agents. |
Clinical trials:
In the meta analysis by Hart et al of 29 clinical trials which included 28,044 patients (mean age 71 years), warfarin and antiplatelet agents reduced stroke risk by 64% (95% CI 49% to 74%) and 22% (6% to 35%) respectively in comparison to placebo. Warfarin was more effective than antiplatelets (relative risk reduction 39%, 22% to 52%) in the Birmingham Atrial Fibrillation Treatment of the Aged (BAFTA) study which included 973 elderly patients ≥75 years old, mean (SD) age 81.5 (4.2) years, annual risk of primary events which included ischemic stroke, systemic embolus or intra cranial hemorrhage (ICH) was 1.8% and 3.8% for patients on warfarin vs aspirin respectively {relative risk (RR) 0.48, 0.28 to 0.80, p=0·003, absolute risk reduction 2%, 0·7 to 3·2} [15]. There was no difference in the rates of all major hemorrhages (including ICH) between aspirin and warfarin treated groups (RR 0.96, 95% CI 0.53 to 1.75). Annual risk of extracranial hemorrhage was 1.4% for warfarin and 1.6% for aspirin (RR 0·87, 0·43 to 1·73). Importantly, efficacy of warfarin was similar in people aged ≥85 years vs younger people as well as in those with low stroke risk (CHADS2 score 1–2) vs those with high stroke risk (CHADS2 score 3–6) [16]. In an analysis of 13,559 adults with NVAF treated with oral anticoagulation, the net clinical benefit of warfarin was 0.68% per year (95% CI 0.34% to 0.87%) and was greatest for patients ≥85 years old (2.34% per year, CI 1.29% to 3.30%) [17]. The Warfarin versus Aspirin for Stroke Prevention in Octogenarians with atrial fibrillation study (WASPO) included a very old cohort of patients (mean age 83.9 years) with NVAF and showed that aspirin (300mg) was less tolerated with more adverse events (33% of patients vs 6%, p = 0.002) in comparison to adjusted dose warfarin. Furthermore, there were three cases of serious bleeding in the aspirin group as compared to none in the warfarin group. Of note good quality of anticoagulation control was achievable in this very elderly cohort, mean (SD) percentage of time in the target international normalized ratio (INR) range of 2.0–3.0 was 69% (17.7) [18]. The percentage of time spent in target INR has also been shown in another study to be similar in different age groups of patients with NVAF even in those ≥84 years old and it was not difficult to maintain INR in the therapeutic range as persons get older [19]. Warfarin remains superior to combination of more than one antiplatelet. Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE-W) study of 6706 participants (mean age 70 years) showed that even dual antiplatelet therapy was inferior to oral anticoagulation. There were 165 primary events in oral anticoagulation therapy arm (annual risk 3.93%) and 234 in the clopidogrel plus aspirin arm (annual risk 5.60%, RR 1.44, 95% CI 1.18 to 1.76, p=0.0003) [20]. Combination of antiplatelets and warfarin was associated with high risk of bleeding. In a study of 118,000 Danish patients with NVAF all combinations of warfarin, aspirin, and clopidogrel were associated with increased risk of bleeding particularly dual warfarin and clopidogrel therapy and triple therapy which were associated with a more than 3-fold higher risk of nonfatal and fatal bleeding with no additional benefit to prevention of ischemic stroke than was warfarin monotherapy [21].
Clinical practice:
Warfarin is effective in ischemic stroke prevention for patients with NVAF in clinical practice as it was shown in clinical trials and the absolute increase in the risk of ICH is small. In a large observational study of 11,526 patients with NVAF (43% women, mean age 71 years), the annual rate of thrombotic event was 1.17% (95% CI 1.00 to 1.38) in patients receiving warfarin compared to 2.03% (1.79 to 2.30, P<.001) in those not receiving warfarin during a mean of 2.2 years of follow-up. Warfarin therapy was associated with a 51% (95% CI 39% to 60%) lower risk of thromboembolism and a reduced risk of all cause mortality (adjusted HR 0.69, 95% CI 0.61 to 0.77) compared with no warfarin therapy. Rate of ICH was low but significantly higher in those taking warfarin compared to those not taking warfarin (0.46% vs 0.23% respectively, P=.003, adjusted HR 1.97, 95% CI 1.24 to 3.13). However, warfarin therapy was not associated with an increased risk of non-intracranial major bleeding. It is likely that the efficacy of warfarin could be further improved as nearly two thirds of individuals sustaining an ischemic stroke while taking warfarin had an INR value below the target range of 2–3 [22]. In another study of 119,764 NVAF elderly Medicare patients, mean (SD) age 79.3 (8.6) years, there was no increase in the annual rate of hemorrhagic stroke (0.5% vs 0.6%) and a slightly elevated but not significant risk of a major bleed (7.6% vs 7.5%) in the warfarin treated group (70,057 patients) in comparison to non warfarin users (49,707 patients) after 2.1 years of follow up [23]. Oral anticoagulation has also shown benefits in long term stroke outcomes in addition to the known short term benefits. In a prospective population based study, therapeutic INR was associated with improved late survival after ischemic stroke (adjusted 2 year OR for mortality was 0.08, 95% CI 0.01 to 0.78, P=0.03). Functional outcome was dependent on quality of anticoagulation control. An INR of 2 to 3 at ischemic stroke onset was associated with good functional outcome (modified Rankin Scale score=0 to 2) at 1 year (adjusted OR=4.8, 95% CI 1.45 to 23.8, P=0.04) [24].
Cost:
NVAF patients with stroke are typically older (averaging approximately 75 years) with large cerebral infarctions leading to more severe strokes, worse functional outcomes and higher early mortality compared with ischemic stroke patients in sinus rhythm [25]. They have 50% higher probability of remaining disabled or handicapped and direct costs are higher (€10,192 vs. €9374, P<0.01 for the year 2001) than stroke patients without NVAF [26]. Therefore, anticoagulation therapy is likely to be cost effective. In 973 patients (>75 years old) with NVAF of a randomised controlled study in the community, warfarin was cost effective compared to aspirin. Total costs were lower in the warfarin group (difference −£165, 95% CI −£452 to £89). The primary event rate was lower in the warfarin group (0.05 versus 0.1), and the quality adjusted life years score was higher (difference 0.02, 95% CI −0.07 to 0.11) [27]. In the aforementioned study of Medicare beneficiaries of 119,764 NVAF patients (mean age 79.3 years), warfarin use was independently associated with lower total medical costs, averaging $9836 per patient per year in 2006 in comparison to patients not taking warfarin (P<0.0001) during an average follow-up of 2.1 years [23]. Costs of warfarin-related major bleeding complications seem to be modest as well. The average annual cost per patient for management of bleeding complication was reported as $19 in the USA (year 2000–2003) [28], £47.30 in the UK (year 1999–2000) [29] and €15 in Germany (year 2004) [30].
Anticoagulation control:
The recommended INR target is 2 to 3. An INR <2 is associated with increased risk of ischemic events while an INR of >3 is associated with increased risk of bleeding. In hospitalized patients with stroke, an INR <2.0 on admission was independently associated with increased odds of severe stroke (OR 1.9, 95% CI 1.1 to 3.4) and mortality (HR 3.4, 95% CI 1.1 to 10.1) compared to INR ≥2.0 [31]. The benefit of oral anticoagulation above antiplatelet therapy appears to be dependent on quality of anticoagulation control and the time spent in the therapeutic range (TTR) in a proportional way. In a post hoc analysis of the ACTIVE-W trial, a wide variation in TTRs resulted in different efficacy of oral anticoagulation. For patients at centres achieving below the median TTR (65%) no treatment benefit was demonstrated between clopidogrel plus aspirin versus warfarin (RR of vascular events 0.93, 95% CI 0.70 to 1.24, p = 0.61). However, for patients at centres with a TTR above the study median, warfarin had a marked benefit reducing vascular events by >2 fold (RR 2.14, 95% CI 1.61 to 2.85, P<0.0001). Mean TTR also varied between countries from 46% to 78% with a corresponding relative risk 0.6 to 3.6 (clopidogrel plus aspirin versus warfarin). The authors concluded that a TTR of a minimum 58% would be needed for warfarin to be beneficial over antiplatelet therapy [32].
Risk stratification schemes
Stroke risk:
There are more than a dozen published schemes for stratifying stroke risk in patients with NVAF. At the core of existing schemes are 4 features that have been independently and consistently associated with stroke in atrial fibrillation patients: previous stroke or TIA, hypertension, advanced age, and diabetes [33]. There is wide variation of the risk estimation between these schemes with a 5 to 7 fold variation in the fraction of patients categorized as being at low or high risk among the schemes. In an analysis of 12 published schemes, the fractions of patients categorized by the different schemes as low risk varied from 9% to 49% and those categorized as high risk varied from 11% to 77%. Differences among these schemes lead to inconsistent stroke risk estimates for many atrial fibrillation patients, resulting in confusion among clinicians and non uniform use of anticoagulation [34]. While risk stratification schemes have been derived from patients enrolled in clinical trials, an updated version of the CHADS2 (Congestive cardiac failure, Hypertension, Age >75 years, Diabetes mellitus, Stroke or TIA) scheme (CHA2DS2-VASc) was tested in 1,084 real world cohort of patients which included other risk factors commonly encountered in every day clinical practice. This revised CHADS 2 provides some refinement with the addition of vascular disease, Age 65 to 74 years and female Sex to a risk factors based scheme. (Table 2) It has also highlighted the high stroke risk associated with age ≥75 with a score of 2 equal to the score of a history of stroke or TIA. The refined scheme classified only 15.1% as intermediate risk group, 9.2% as low risk and the majority (75.7%) as high risk as compared to 61.9%, 20.4% and 17.7% respectively classified by the classic CHADS 2 scheme. The impact of the new refined scoring scheme is that the majority of patients with NVAF will be eligible for oral anticoagulation. In a Multicenter, observational, cross-sectional study to determine the impact of the new scheme CHA2DS2-VASc and of the new recommendations for oral anticoagulation in a contemporary sample of patients with NVAF, the percentage of patients with indication for anticoagulation treatment was 93.8%. Most importantly is that all patients who were 75 years and older were eligible for oral anticoagulation scoring >2 with only a minority (14.4%) scoring 2 based on age alone [35]. Therefore, the refined stroke risk stratification scheme classifies all patients age ≥75 years as high risk. It is also superior to the classic CHADS2 score at identifying truly low risk patients, with no thromboembolic events recorded in this category, whereas thromboembolic events occurred in 1.4% of low risk CHADS 2 subjects. Therefore a risk factor based approach will be easier than the categorization of patients into risk groups with the potential of under treatment in eligible patients [36].
Table 2.
CHA2ADS2-VASc stroke risk stratification scale[36]
| Risk factor | Score |
|---|---|
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age ≥ 75 years | 2 |
| Diabetes mellitus | 1 |
| Stroke/transient ischemic attack or systemic thromboembolism | 1 |
| Vascular disease (myocardial infarction, peripheral arterial disease or aortic plaque) | 1 |
| Age 65–74 years | 1 |
| Sex (female) | 1 |
| Maximum score | 9 |
Score=0 no therapy, score ≥ 1 oral anticoagulation is indicated.
Bleeding risk:
Currently there are a number of bleeding risk stratification schemes incorporating very different characteristics and complex scoring systems, and some of them requiring laboratory parameters or even genetic testing [37]. This limits their practicality in day to day clinical environments. Indeed only a few are actually validated in NVAF population, and not particularly in older people. Furthermore, the definition of major bleeding is inconsistent. Increasing age also exists as a core factor in all bleeding risk stratification schemes [38]. The most recently available scoring scheme is the HAS-BLED {Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile INR, Elderly (>65 years), Drugs/alcohol}. A score of ≥3 indicates high risk of bleeding with the recommendation of careful monitoring and attention to modifiable risk factors, rather than exclusion from anticoagulation therapy. (Table 3) Of note, the labile INR variable cannot be assessed in warfarin naïve patients and falls risk is not included in this risk score. Also there is no current scoring scheme that addresses the risk of ICH, the most feared complication, on its own as it was normally incorporated with other sources of bleeding into an overall category of major bleeding [39].
Table 3.
HAS-BLED bleeding risk stratification scale[39]
| Risk factor* | Score |
|---|---|
| Hypertension | 1 |
| Abnormal renal or liver function (one point each) | 1–2 |
| Stroke | 1 |
| Bleeding history | 1 |
| Labile INR | 1 |
| Elderly (>65 years old) | 1 |
| Drugs or alcohol (one point each) | 1–2 |
| Maximum score | 9 |
Uncontrolled hypertension=systolic >160 mmHg, renal impairment=serum creatinine >200 μmol/L, Abnormal liver function=transaminase or alkaline phosphatase enzymes > 3 times upper limit normal, Drugs=concomitant use of antiplatelets or non steroidal agents. A score of ≥3 is high risk of bleeding and close monitoring is required.
Physicians fear (Table 4)
Table 4:
Physicians fear
|
Beyond conventional contraindications, physicians often avoid anticoagulation in elderly patients for fear of ICH, falls and poor compliance (particularly in patients with cognitive dysfunction) [40]. This has resulted in a bias towards using warfarin more in younger age groups than in the elderly who are likely to be left with a high thromboembolic risk [41]. However, much of the perceived fear by the physicians may not be founded on sound evidence:
Intracranial Hemorrhage:
The risk of ICH, which is the most feared and potentially fatal complication of warfarin therapy, increases by 2.5 fold among older people ≥85 years of age. However, the absolute risk of warfarin associated ICH in NVAF patients is relatively low at 0.2% per year [42]. Also the risk of major hemorrhage is similar among NVAF patients receiving warfarin or aspirin (1.4% vs. 1.6% RR 0.87, 95% CI 0.43 to 1.73) [40]. In a recent analysis of 15 studies of anticoagulation in NVAF, rate of major bleeding, commonly defined as bleeding requiring blood transfusion, was 1.9% (95% CI 1.3 to 2.6), ICH was about 0.45% (0.3 to 0.67) constituting around 23% (17 to 31) of the total major bleeds. The proportion of intracranial bleeds that were fatal was only 35% (24 to 47). Fatal bleeding occurred in 0.26% (0.15 to 0.45) and this constitutes about 13% (8 to 20) of total major bleeding. Quality of anticoagulation control was a more detrimental factor for bleeding than age. For example, the rate of ICH increased by 9% with each year of age (P = 0.0001) but increased by 72% with each 1.0 point increase in target INR (P = 0.0006) and the rate of major bleeding increased by 7% with each year of age (P < 0.0001) and increased by 37% with each 1.0 point increase in target INR (P = 0.003) [43]. In a large prospective observational study of 4093 very old patients (≥80 year), median (interquartile range) age 84 (80 to 102) years on warfarin for NVAF (73.7% of patients) or venous thromboembolism (26.3% of patients), the rate of bleedings was reasonably low after a mean (SD) follow up of 2.35 (2.1) years. Annual major bleeding rate was 1.87% however, the bleeding rate was lower in NVAF patients (1.73%) in comparison to venous thromboembolism (VTE) patients (2.4%), relative risk 1.4, 95% CI 1.02 to 1.85, p <0.03 which is likely due to the fact that NVAF patients spent more time in the therapeutic range (63% vs 60%, p<0.0001) and had lower prevalence of cancer (5.3% vs 11%, p<0.0001) than VTE patients. ICH rate was 0.55% and fatal bleeding rate was 0.27%. The rate of bleeding events was higher in patients ≥85 years old compared to patients <85 years (relative risk, 1.3, 95% CI 1.0 to 1.65, p <0.048) however, age ≥85 was not identified as an independent risk factor for bleeding (HR 1.02, 95% CI 0.71 to 1.47, p=0.88). History of bleeding (HR 5.46, 95% CI 3.29 to 9.05, p<0.0001), active cancer (2.41, 1.47 to 3.95, p<0.0001), and history of falls (3.06, 1.77 to 5.27, p<0.0001) were independently associated with bleeding risk in cox regression analysis while multiple medications (≥3 drugs), history of hypertension or renal failure were not [44]. Although the bleeding risk with oral anticoagulation in older people does not seem to be much higher than with antiplatelets, physician’s attitude and decision for anticoagulation use seems to be affected more by observing a bleeding rather than a thromboembolic event. It has been shown that the occurrence of anticoagulation related bleeding event has a negative impact on the future prescription of warfarin. Patients with NVAF, who were treated by physicians in the three months after a major bleeding event was observed in other patients, had a 21% reduced odds of receiving warfarin compared with patients treated by the same physicians before the bleeding event occurred. In contrast, the occurrence of a thromboembolic stroke in a patient with NVAF not on anticoagulation did not influence the odds that a physician would use anticoagulation in subsequent patients with NVAF [45]. Older people with NVAF are likely to have a greater net clinical benefit from oral anticoagulation therapy than younger people as they have a greater stroke risk. The net clinical benefit of oral anticoagulation therapy in NVAF patients defined as the annual rate of thromboembolic events minus the annual rate of ICH is 0.68% per year (95% CI 0.34% to 0.87%). However, the net benefit of anticoagulation was shown to be greater in those ≥ 85 years old (2.34% per year, 95% C I 1.29% to 3.30%) [17].
Falls:
Physicians seem to be very conservative in selecting patients for warfarin therapy, choosing only those who are sufficiently healthy to be at much lower risk of fall. In a retrospective analysis of consecutive patients who fell during admission to a teaching hospital, a total of 2635 falls in 1861 patients occurred. Patients taking warfarin were less likely to suffer a fall related major hemorrhagic injury compared with persons not taking antithrombotic therapy (warfarin, 6%; no therapy, 11%, p = 0.01). Logistic regression analysis showed that fall related major hemorrhagic injury was not associated with the use of warfarin or the intensity of anticoagulation. The absolute rate of the development of fall related intracranial hemorrhagic injury such as subdural hematomas was low (only one case), even in persons taking warfarin. This suggests that physicians may be overestimating the potential for fall related major hemorrhagic injury in persons taking warfarin with the possible denial of warfarin therapy to many of those who would benefit [46]. Although falls are among the most common causes often cited by physician as the reason for not using anticoagulation in older people with NVAF, it appears that warfarin benefits outweigh its risks even in patients who fall [47]. A meta-analysis of antithrombotic therapy in older patients with NVAF and at risk for falls found that the propensity for falling should not be an important factor when deciding whether or not to use oral anticoagulation. The quality adjusted life expectancy was best for warfarin then aspirin followed by no therapy [48]. In another study of 19,506 patients with NVAF, warfarin was not associated with risk of ICH (HR 1.0, 95% CI 0.8 to 1.4) after adjusting for baseline risk factors. However, ischemic stroke rates were 13.7 in patients at high risk for falls and 6.9 in less risk patients (HR 1.3, 95% CI 1.1 to 1.6, P = .002). This suggests that warfarin prescription in NVAF patients who are prone to falls and have multiple additional stroke risk factors appears to have an overall net clinical benefit in spite of the falls risk [49].
Cognitive dysfunction:
Cognitive dysfunction may be one of the factors limiting the use of anticoagulation in older people due to the fear of bleeding. Anticoagulants in these patients may present a challenge considering the variability in warfarin dose from time to time, patients’ compliance and lack of awareness of food and drug interactions. It has been previously shown that older patients with NVAF and history of dementia may be at a higher risk of bleeding events (OR 1.56, 95% CI 0.95 to 2.57) but this did not reach statistical significance [50]. However, in an analysis of the time spent in the therapeutic range (TTR) associated with cognitive function measured by Mini Mental State Examination (MMSE) in the ACTIVE-W trial, low MMSE scores were correlated with a low TTR. For every 1-point decline in the MMSE score between 30 and 25, there was a 1 point reduction in TTR. Patients with an MMSE score <26 had more vascular events (6.7% versus 3.6%, P = 0.002) as well as more bleeding (9.6% versus 7% P = 0.04) events. After controlling for TTR, the MMSE no longer conferred increased risk, suggesting that cognitive dysfunction has no direct relation with bleeding or thrombotic events and improvement in the quality of anticoagulation control may improve outcome [51]. In another study of 323 patients ≥80 years old with NVAF discharged from hospital on oral anticoagulation therapy and followed up for a mean of 28.8 months, presence of dementia, functional, visual, or hearing impairments were not associated with increased bleeding events [52]. However, in the same study patient education about oral anticoagulation therapy was associated with lower rate of bleeding and better quality of INR control. In multivariate analysis, insufficient education on oral anticoagulation as perceived by the patient or caregiver was a significant predictor (OR 8.83) for bleeding complications. In addition, the percentage of INR values in the therapeutic range was higher among patients who received a satisfactory explanation about oral anticoagulation (45.1% of INR values) vs an insufficient explanation (34.9%), and vs patients who did not receive any explanation at all (20.0%) (P<0.001) [52].
Discussion
It appears that the incidence of both stroke and NVAF increases with age. As a result, older people with NVAF are at the highest risk for stroke in comparison to younger people in sinus rhythm. As anticoagulation is an effective stroke preventive therapy, older people with NVAF are likely to get the most benefit due to their high base line stroke risk.
Eligibility for anticoagulation:
All older people (≥75 years) with NVAF should be offered anticoagulation therapy if there are no contraindications. It has been shown that older people will benefit from oral anticoagulation regardless of their risk stratification score. In an observational study of 11256 patients with NVAF (mean age 71 years), 77% had at least one stroke risk factor with a CHADS2 score of ≥1. The benefit of warfarin was observed even in patients with minimal stroke risk. For example, for patients with CHADS2 score 0 (no risk factors) thromboembolic event rate was 0.25% (95% CI 0.11 to 0.55) for patients on warfarin and 0.49% (0.3 to 0.78) for those not on warfarin (OR 0.5, 95% CI 0.2 to 1.28) and those with CHADS2 score 1 (one risk factor), 0.72 (0.5 to 1.03) vs 1.52 (1.19 to 1.94) respectively (OR 0.47, 0.3 to 0.73) [23]. Results from the ACTIVE-W study showed that even dual antiplatelet therapy was inferior to oral anticoagulation in the lower stroke risk patients (CHADS2 = 1). Annual stroke rates for those with a CHADS2=1 were 1.25% on clopidogril and aspirin compared to 0.43% on warfarin (RR 2.96, 95% CI 1.26 to 6.98, p = 0.01). The net annual risk (vascular events plus major bleeding) was significantly higher in patients receiving clopidogril and aspirin compared to warfarin regardless of the CHADS2 score (5.25% vs 2.97%, RR 1.79, p = 0.001 for CHADS2 = 1 and 9.10% vs 7.0%, RR 1.32, p = 0.005 for CHADS2>1). Also the absolute benefit of warfarin was similar regardless of the CHADS2 score (2.28% per year risk reduction for CHADS2<1 and 2.10% per year in CHADS2>1, p = 0.18). This analysis suggests that combination of antiplatelet therapy is not equivalent or alternative to warfarin in patients across the CHADS2 score (≥1) in whom warfarin therapy was superior and also associated with lower risk of major bleeding [53] while antiplatelet agents, the only other alternative, lose efficacy in older age. In an analysis of the Atrial Fibrillation Investigators database which included 8932 patients and 17,685 years of observation from 12 trials, the relative benefit of antiplatelets for preventing ischemic stroke decreased significantly in comparison to placebo as patients aged (P<0.01) whereas it does not change for oral anticoagulation. At age 77, the hazard ratio of antiplatelet treatment no longer excluded unity and at age 82, the hazard ratio exceeded 1. Because stroke risk increases with age, the absolute benefit of oral anticoagulation increases as patients get older [40]. The explanation of these findings are likely due to the fact that the aetiology of most ischemic strokes in patients with NVAF are cardioembolic in 75% of cases and atherosclerotic in 25% of cases due to intrinsic cerebrovascular disease which may be associated with widespread atherosclerosis. Warfarin is superior to aspirin for preventing cardioembolic strokes, whereas aspirin has its major effect only on non cardioembolic events. Advanced age is associated with an increased likelihood that NVAF-associated stroke is cardioembolic in nature while younger patients with atherosclerotic cerebrovascular disease may succumb to associated diseases, including coronary artery disease, diabetes, and hypertension. In summary, in those patients ≥75 years with NVAF the stroke risk is high and the only effective therapy is oral anticoagulation which should be offered to all of them if they have no contraindications.
Non eligibility for anticoagulation:
There is no reliable tool to predict non eligibility for oral anticoagulation therapy in older people with NVAF. Therefore, known risk factors for major bleeding should be taken into account on an individual basis when starting anticoagulation therapy. Age in itself should not be considered a reason for non eligibility or contraindication to anticoagulation. The evidence for age as an independent risk factor for major bleeding is contradictory [54]. In a large observational study of 4093 very old (≥80 years) patients, the annual major bleeding rate was low (1.87%) and quality of anticoagulation control was good (time in therapeutic range was 62%) suggesting that careful monitoring of anticoagulation allows very old patients to benefit from warfarin therapy. Independent predictors of bleeding were history of bleeding, active cancer and history of falls [44]. Other risk factors for bleeding such as uncontrolled hypertension, anemia, a history of prior bleeding, hepatic or renal impairment, and concomitant use of antiplatelet agents should be considered as a caution when starting anticoagulation therapy [55]. The recently introduced HASBLED scoring tool for bleeding risk was not designed to be used as a tool to justify non eligibility for anticoagulation, but rather to identify high-risk patients so that they could be more closely monitored. Patients and carers preference is a major factor in the decision making for anticoagulation therapy. Therefore clinicians should use their clinical judgment, weighing the bleeding risk in each case individually, as well as considering patients views in clinical decision making [56]. Physicians may consider the task of anticoagulation therapy a burden on older patients, but it has been shown that patients’ ≥75 years old were not much troubled by the task [57] and anticoagulation did not affect physical or mental quality of life measurements [58]. Furthermore, with the increasing availability of the newly developed oral anticoagulants not requiring INR monitoring will make the task of such therapy easier and increase eligibility of patients for anticoagulation.
Pragmatic approach:
The plethora of the stroke risk and bleeding risk stratification schemes developed over the last two decades proves that none of them is ideal in precisely identifying the suitable patient for oral anticoagulation. Furthermore the under use of warfarin in older people of every day clinical practice reflects the difficulty of the clinical application of these schemes. The current scoring schemes include similar and overlapping risk factors for both thromboembolic and bleeding events which may lead to confusion in clinical decision making to balance the risks of bleeding against the risks of stroke, thereby limiting the applicability of such schemes. Furthermore, as these schemes have been derived from studies on populations mostly in clinical trials, their applicability on individual patient in real life situation of clinical practice may be difficult. In a recent analysis of the main existing schemes, including CHA2DS2-VASc, for stratification of risk of stroke in older patients (≥75 years) with NVAF in the BAFTA trial showed only limited ability to predict risk of stroke [59]. Although the newly introduced CHA2DS2-VASc allocates more patients into a high risk category with expected increase in anticoagulation use, in a Canadian population based cohort study of 42,834 NVAF patients, warfarin use did not differ across risk strata using either the CHADS2 (p for trend = 0.85) or CHA2DS2-VASC (p = 0.35). This suggests that warfarin is over utilised in low risk patients and underutilised in high risk patients [60]. The shift to a ‘yes/no’ approach based on presence or absence of risk factors rather than a scoring system would simplify and improve oral anticoagulation use in patients with NVAF. Artificial categorization into high, intermediate and low risk or scaling into different scales may be less helpful. The current recommendation of anticoagulation for high risk patients, aspirin for low risk patients and either aspirin or warfarin for intermediate risk patients may add to the uncertainties for the clinicians and the either aspirin or warfarin choice may be taken as an excuse not to prescribe anticoagulation for patients who would benefit from such treatment. Ideally aspirin should be removed from the guidelines as an alternative antithrombotic treatment for older patients (≥75 years) with NVAF due to lack or diminishing efficacy in this age group. This will greatly simplify the treatment options available to clinicians as either a yes or no decision for choosing anticoagulation therapy. Even in low risk patients with NVAF, aspirin is no better than control for reducing thromboembolic events but have the potential of increasing bleeding risk [61]. In fact, there was no significant difference in major bleeding events between warfarin and aspirin in the BAFTA trial of a community based elderly NVAF population therefore, aspirin cannot be considered as a safer alternative to warfarin. Equally the use of a scoring scale for bleeding risk is likely to be less practical and less helpful in the elderly with NVAF. Bleeding risk stratification schemes such as HASBLED can only be used as a general guidance for clinicians rather than a strict scoring system. Patients in every day clinical practice may be a more heterogeneous group of individuals as compared to clinical trial patients and the decision to prescribe oral anticoagulation, which is never straightforward, should take this into consideration. Using a tool of two stratification schemes (stroke and bleeding risk scheme) is unlikely to have a real meaning in older people in clinical practice. This kind of tool focuses on quantitative comparisons of two risks (stroke or bleeding) but ignores any qualitative comparison. In other words it compares two incomparable issues. Physicians may be more concerned with the greater risk of bleeding of oral anticoagulation therapy whereas patients may be more concerned with the risk of thromboembolic stroke. Therefore, a patient who has a marginally higher risk of bleeding than stroke risk does not necessarily mean that he will not be eligible for anticoagulation. He may prefer to have a slightly elevated risk of developing a gastrointestinal bleeding episode, for example, rather than suffering a disabling stroke for life. In addition, the lack of a specific scoring scale to predict ICH, the most fearful bleeding risk, makes these scoring schemes less practical. It has been suggested that keeping INR <3.0, hypertension control and avoiding concomitant antiplatelet agents prescription with oral anticoagulation may reduce risk of major bleeding [62]. Education is also a key factor in further reducing the risk of bleeding as oral anticoagulation therapy may be safer if patients are well informed [52]. This pragmatic approach may help to increase the use of oral anticoagulation for patients who are likely to benefit from such therapy and may help keeping bleeding risk reasonably low even in the very old. (Figure 1)
Figure 1:
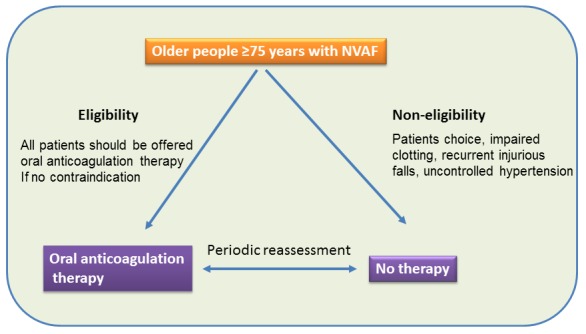
A pragmatic approach of oral anticoagulation for older people ≥75 years old with NVAF. All should be considered for oral anticoagulation if no contraindications. Periodic reassessment is recommended for further eligibility, and vice versa, of non eligible patients if their modified risk of bleeding e g uncontrolled hypertension is treated.
Future perspectives
The inconvenience of the INR monitoring and frequent blood testing could be a holding factor for patients not to choose warfarin therapy. On the other hand narrow therapeutic range of INR, drug or food interactions and risk of bleeding could be another holding factor for physicians. These factors may have contributed to under utilisation of warfarin in many eligible older patients with NVAF. Newer anticoagulants such as dabigatran (direct thrombin inhibitor) have been shown to be as effective as warfarin at stroke prevention, incurring similar or lower bleeding complications without imparting the inconveniences of INR monitoring and dose adjustment [63]. The most important advantage of dabigatran was the lower rate of intracranial bleeding than warfarin. These results are likely to revolutionize our approach to oral anticoagulation therapy. With potentially less intracranial bleeding risk, physicians may be encouraged to a wider use of anticoagulation especially with the availability of simple, fixed dose, unmonitored therapy which will appeal to patients as well. These new developments will further support the suggestion of a pragmatic approach and hopefully lead to a wider use of anticoagulation and taking antiplatelets out of the guidelines. It is likely that the transition from warfarin to the new oral anticoagulants to be a gradual process considering the current high cost and the short duration of clinical practice experience with the new anticoagulants.
Conclusion
The risk of both stroke and NVAF are age dependent with increasing prevalence as we get older. With aging of the population and increased life expectancy the prevalence of NVAF is bound to increase with expected increase in the risk of stroke. NVAF related stroke tends to be more disabling with associated poor outcomes and huge cost burden on health care systems. Oral anticoagulation therapy is effective in stroke prevention and likely to be more cost effective in older people with NVAF due to their high base line risk. The current stroke and bleeding risk stratification schemes, developed over the last two decades, have been based on complex scoring systems that are difficult to apply in clinical practice. They include similar and overlapping risk factors for both thromboembolic and bleeding events which may lead to confusion in clinical decision making to balance the risks of bleeding against the risks of stroke, thereby limiting the applicability of such schemes. Considering this confusion we are suggesting a pragmatic approach based on a yes/no decision rather than risk scoring. All older people (≥75 years) will benefit from and should be offered oral anticoagulation without a scoring scheme if there is no contraindication. Antiplatelet agents should be removed from the guidelines as an alternative option for antithrombotic treatment for older people with NVAF due to lack of efficacy and the potential of being used as an excuse of not using anticoagulation. Bleeding risk should be assessed on an individual basis and the decision to anticoagulate should include patients’ views.
Key points
All older people (≥75 years) with NVAF should be offered oral anticoagulation therapy, without stroke risk scoring, if they have no contraindication.
Antiplatelet agents should be removed from guidelines as an alternative therapy to oral anticoagulation due to lack of efficacy.
Bleeding risk stratification schemes can only be used as a general guidance for clinicians rather than a strict scoring system.
The decision to use oral anticoagulation in older people with NVAF should be individualised and considers patient views.
References
- [1].Singer DE, Albers GW, Dalen JE, Go AS, Halperin JL. Antithrombotic therapy in atrial fibrillation: the seventh ACCP Conference onantithrombotic and thrombolytic therapy. Chest. 2004;126(Suppl):429s–456s. doi: 10.1378/chest.126.3_suppl.429S. [DOI] [PubMed] [Google Scholar]
- [2].Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- [3].Risk factors for stroke and efficacy of antithrombotic therapy in atria1 fibrillation Analysis of pooled data from five randomized controlled trials. Arch lntern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- [4].Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA. 1999;281:1830–1835. doi: 10.1001/jama.281.19.1830. [DOI] [PubMed] [Google Scholar]
- [5].Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Mark Estes NA, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, Stevenson WG, Tracy CM. 2011 ACCF/AHA/HRS Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline) A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223–242. doi: 10.1016/j.jacc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- [6].Tulner LR, Van Campen JP, Kuper IM, Gijsen GJ, Koks CH, Mac Gillavry M R, van Tinteren H, Beijnen J H, Brandjes D P. Reasons for under treatment with oral anticoagulants in frail geriatric outpatients with atrial fibrillation: a prospective, descriptive study. Drugs Aging. 2010;27:39–50. doi: 10.2165/11319540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [7].Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17–40. ix. doi: 10.1016/j.mcna.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol. 2008;31:55–62. doi: 10.1002/clc.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Go AS, Hylek EM, Philips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- [10].Fitzmaurice DA, Hobbs FDR, Jowett SM, Mant J, Murray ET, Holder RL, Raftery J, Bryan S, Davies M, Lip GYH, Allan T. Screening versus routine practice for detection of atrial fibrillation in people aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:383–386. doi: 10.1136/bmj.39280.660567.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary Prevention of Ischemic Stroke. A Guideline From the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- [12].Lip GYH, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with Nonvalvular atrial fibrillation: a systematic review and meta-analysis. Thrombosis Research. 2006;118:321–333. doi: 10.1016/j.thromres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- [13].Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- [14].Kammersgaard LP, Jorgensen HS, Reith J, Nakayama H, Pederson PM, Olsen TS, Copenhagen Stroke Study Short- and long-term prognosis for very old stroke patients. The Copenhagen Stroke Study. Age Ageing. 2004;33:149–154. doi: 10.1093/ageing/afh052. [DOI] [PubMed] [Google Scholar]
- [15].Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic Therapy to Prevent Stroke in Patients Who Have Nonvalvular Atrial Fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- [16].Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- [17].Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The Net Clinical Benefit of Warfarin Anticoagulation in Atrial Fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rash A, Downes T, Portner R, Yeo WW, Morgan N, Channer KS. A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO) Age Ageing. 2007;36:151–156. doi: 10.1093/ageing/afl129. [DOI] [PubMed] [Google Scholar]
- [19].Hylek E, Go A, Chang Y. Increasing age is not associated with poorer anticoagulation control in outpatients with nonvalvular atrial fibrillation. J Am Geriatr Soc. 2000;48:S58. [Google Scholar]
- [20].The ACTIVE Writing Group on behalf of the ACTIVE Investigators Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- [21].Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunso J, Gadsboll N, Gislasson GH, Folke F, Andersen SS, Schramm TK, Abildstrom SZ, Poulsen HE, Kober L, Torp-Pedersen C. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–1441. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- [22].Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation how well do randomized trials translate into clinical practice. JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- [23].Mercaldi CJ, Ciarametaro M, Hahn B, Chalissery G, Reynolds MW, Sander SD, Samsa GP, Matcha DB. Cost Efficiency of Anticoagulation With Warfarin to Prevent Stroke in Medicare Beneficiaries With Nonvalvular Atrial Fibrillation. Stroke. 2011;42:112–118. doi: 10.1161/STROKEAHA.110.592907. [DOI] [PubMed] [Google Scholar]
- [24].Hannon N, Callaly E, Moore A, Ni Chroinin D, Sheehan O, Marnane M, Merwick A, Kyne L, Duggan J, McCormack PM, Dolan E, Crispino-O’Connell G, Harris D, Horgan G, Williams D, Kelly PJ. Improved late survival and disability after stroke with therapeutic anticoagulation for atrial fibrillation: a population study. Stroke. 2011;42:2503–2508. doi: 10.1161/STROKEAHA.110.602235. [DOI] [PubMed] [Google Scholar]
- [25].Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, Spolveri S, Baruffi MC, Landini G, Ghetti A, Wolfe CD, Inzitari D. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project) Stroke. 2001;32:392–398. doi: 10.1161/01.str.32.2.392. [DOI] [PubMed] [Google Scholar]
- [26].Ghatnekar O, Glader EL. The effect of atrial fibrillation on stroke-related inpatient costs in Sweden: a 3-year analysis of registry incidence data from 2001. Value Health. 2008;11:862–868. doi: 10.1111/j.1524-4733.2008.00359.x. [DOI] [PubMed] [Google Scholar]
- [27].Jowett S, Bryan S, Mant J, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Hobbs FD. Cost effectiveness of warfarin versus aspirin in patients older than 75 years with atrial fibrillation. Stroke. 2011;42:1717–1721. doi: 10.1161/STROKEAHA.110.600767. [DOI] [PubMed] [Google Scholar]
- [28].Fanikos J, Grasso-Correnti N, Shah R, Kucher N, Goldhaber SZ. Major bleeding complications in a specialized anticoagulation service. Am J Cardiol. 2005;96:595–598. doi: 10.1016/j.amjcard.2005.03.104. [DOI] [PubMed] [Google Scholar]
- [29].Abdelhafiz AH, Wheeldon NM. Use of resources and cost implications of stroke prophylaxis with warfarin for patients with nonvalvular atrial fibrillation. Am J Geriatr Pharmacother. 2003;1:53–60. doi: 10.1016/s1543-5946(03)90001-8. [DOI] [PubMed] [Google Scholar]
- [30].McBride D, Mattenklotz A M, Willich S N, Bruggenjurgen B. The costs of care in atrial fibrillation and the effect of treatment modalities in Germany. Value Health. 2009;12:293–301. doi: 10.1111/j.1524-4733.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- [31].Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- [32].Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S. Benefit of Oral Anticoagulant Over Antiplatelet Therapy in Atrial Fibrillation Depends on the Quality of International Normalized Ratio Control Achieved by Centers and Countries as Measured by Time in Therapeutic Range. Circulation. 2008;118:2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- [33].Stroke Risk in Atrial Fibrillation Working Group Independent predictors of stroke in patients with atrial fibrillation: A systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- [34].Stroke Risk in Atrial Fibrillation Working Group Comparison of 12 Risk Stratification Schemes to Predict Stroke in Patients With Nonvalvular Atrial Fibrillation. Stroke. 2008;39:1901–1910. doi: 10.1161/STROKEAHA.107.501825. [DOI] [PubMed] [Google Scholar]
- [35].Rodrıguez-Manero M, Cordero A, Bertomeu-Gonzalez V, Moreno-Arribas J, Bertomeu-Martinez V, Mazon P, Facila L, Cosin J, Lekuona I, Galve E, Gonzalez-Juanatey JR. Impact of New Criteria for Anticoagulant Treatment in Atrial Fibrillation. Rev Esp Cardiol. 2011;64:649–653. doi: 10.1016/j.recesp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- [36].Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. The Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- [37].Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- [38].Tay KH, Lip GY, Lane DA. Bleeding rates in elderly patients on vitamin K antagonist therapy for nonvalvular atrial fibrillation. Blood Coagul Fibrinolysis. 2009;20:392–393. doi: 10.1097/MBC.0b013e32832aec47. [DOI] [PubMed] [Google Scholar]
- [39].Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- [40].van Walraven C, Hart RG, Connell S, Austin PC, Mant J, Hobbs FD, Koudstaal PJ, Petersen P, Perez-Gomez F, Knottnerus J A, Boode B, Ezekowitz MD, Singer DE. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke. 2009;40:1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- [41].Choudhry NK, Soumerai SB, Normand SLT, Ross-Degnan D, Laupacis A, Anderson GM. Warfarin prescribing in atrial fibrillation: the impact of physician, patient, and hospital characteristics. Am J Med. 2006;119:607–615. doi: 10.1016/j.amjmed.2005.09.052. [DOI] [PubMed] [Google Scholar]
- [42].Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Intern Med. 2007;147:590–592. doi: 10.7326/0003-4819-147-8-200710160-00018. [DOI] [PubMed] [Google Scholar]
- [43].Linkins L, O’Donnell M, Julian J A, Kearon C. Intracranial and fatal bleeding according to indication for long-term oral anticoagulant therapy. J Thromb Haemost. 2010;8:2201–2207. doi: 10.1111/j.1538-7836.2010.04016.x. [DOI] [PubMed] [Google Scholar]
- [44].Poli D, Antonucci E, Testa S, Tosetto A, Ageno W, Palareti G, Italian Federation of Anticoagulation Clinics Bleeding risk in very old patients on vitamin K antagonist treatment: results of a prospective collaborative study on elderly patients followed by Italian centres for anticoagulation. Circulation. 2011;124:824–829. doi: 10.1161/CIRCULATIONAHA.110.007864. [DOI] [PubMed] [Google Scholar]
- [45].Choudhry NK, Anderson GM, Laupacis A, Ross-Degnana D, Normand SL, Soumerai SB. Impact of adverse events on prescribing warfarin in patients with atrial fibrillation: matched pair analysis. Br Med J. 2006;332:141–143. doi: 10.1136/bmj.38698.709572.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bond AJ, Molnar FJ, Li M, Mackey M, Man-Son-Hing M. The risk of hemorrhagic complications in hospital in-patients who fall while receiving antithrombotic therapy. Thrombosis Journal. 2005;3:1. doi: 10.1186/1477-9560-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garwood CL, Corbett TL. Use of anticoagulation in elderly patients with atrial fibrillation who are at risk for falls. Ann Pharmacother. 2008;42:523–532. doi: 10.1345/aph.1K498. [DOI] [PubMed] [Google Scholar]
- [48].Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159:677–685. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- [49].Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118:612–617. doi: 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- [50].Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant-antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke. 2004;35:2362–2367. doi: 10.1161/01.STR.0000141933.75462.c2. [DOI] [PubMed] [Google Scholar]
- [51].Flaker GC, Pogue J, Yusuf S, Pfeffer MA, Goldhaber SZ, Granger CB, Anand IS, Hart R, Connolly SJ. Cognitive Function and Anticoagulation Control in Patients With Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:277–283. doi: 10.1161/CIRCOUTCOMES.109.884171. [DOI] [PubMed] [Google Scholar]
- [52].Kagansky N, Knobler H, Rimon E, Ozer Z, Levy S. Safety of Anticoagulation Therapy in Well-informed Older Patients. Arch Intern Med. 2004;164:2044–2050. doi: 10.1001/archinte.164.18.2044. [DOI] [PubMed] [Google Scholar]
- [53].Healey JS, Hart RG, Pogue J, Pfeffer MA, Hohnloser SH, De Caterina R, Flaker G, Yusuf S, Connolly SJ. Risks and Benefits of Oral Anticoagulation Compared With Clopidogrel Plus Aspirin in Patients With Atrial Fibrillation According to Stroke Risk. The Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE-W) Stroke. 2008;39:1482–1486. doi: 10.1161/STROKEAHA.107.500199. [DOI] [PubMed] [Google Scholar]
- [54].Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. The pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- [55].Hughes M, Lip GYH. Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. Quart J Med. 2007;100:599–607. doi: 10.1093/qjmed/hcm076. [DOI] [PubMed] [Google Scholar]
- [56].Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J. 2011;161:241–246. doi: 10.1016/j.ahj.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [57].Abdelhafiz AH, Wheeldon NM. Results of an open-label, prospective study on anticoagulant therapy for atrial fibrillation in an outpatient anticoagulant clinic. Clinical Therapeutics. 2004;26:1470–1478. doi: 10.1016/j.clinthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [58].Das AK, Ahmed A, Corrado OJ. Quality of life of elderly people on warfarin for atrial fibrillation. Age Ageing. 2009;158:751–754. doi: 10.1093/ageing/afp158. [DOI] [PubMed] [Google Scholar]
- [59].Hobbs FDR, Roalfe AK, Lip GYH, Fletcher K, Fitzmaurice DA, Mant J. Performance of stroke risk scores in older people with atrial fibrillation not taking warfarin: comparative cohort study from BAFTA trial. BMJ. 2011;342:d3653. doi: 10.1136/bmj.d3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risketreatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97:2046–2050. doi: 10.1136/heartjnl-2011-300901. [DOI] [PubMed] [Google Scholar]
- [61].Fauchier L, Lip GYH. Guidelines for antithrombotic therapy in atrial fibrillation: understanding the reasons for nonadherence and moving forwards with simplifying risk stratification for stroke and bleeding. Europace. 2010;12:761–763. doi: 10.1093/europace/euq102. [DOI] [PubMed] [Google Scholar]
- [62].Hart RG, Tonarelli SB, Pearce LA. Avoiding Central Nervous System Bleeding During Antithrombotic Therapy. Recent Data and Ideas Stroke. 2005;36:1588–1593. doi: 10.1161/01.STR.0000170642.39876.f2. [DOI] [PubMed] [Google Scholar]
- [63].Connnolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, The RE-LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]


