Abstract
Boronates, a group of organic compounds, are emerging as one of the most effective probes for detecting and quantifying peroxynitrite, hypochlorous acid and hydrogen peroxide. Boronates react with peroxynitrite nearly a million times faster than with hydrogen peroxide. Boronate-containing fluorogenic compounds have been used to monitor real time generation of peroxynitrite in cells and for imaging hydrogen peroxide in living animals. This Perspective highlights potential applications of boronates and other fluorescent probes to high-throughput analyses of peroxynitrite and hydroperoxides in toxicological studies.
Keywords: peroxynitrite, boronates, global profiling, reactive oxygen and nitrogen species
Introduction
Several boronic acids and boronic acid derivatives exhibit antimicrobial, antifungal, and other pharmaceutical properties.1–3 Boronates, such as 4-phenylalanine boronic acid, have been used in boron neutron capture therapy for the treatment of brain cancer.2 Boronates are versatile agents for linking functional groups in synthetic organic chemistry and medicinal chemistry.1,2 Some boronic acids have been used as fluorescent sensors for carbohydrates.4 This Perspective is focused on the use of boronates as chemical probes and antioxidants for detection and detoxification of reactive oxygen and nitrogen species (ROS/RNS) generated in biological and cellular systems.
Peroxynitrite (ONOO−) is implicated as a key reactive intermediate in redox biology, toxicology, and in various pathologies including cardiovascular, neurodegenerative, and inflammatory diseases.5–7 Nearly two decades after their renaissance in biological chemistry,8 methodologies to directly detect, quantitate, and detoxify intra- and extracellularly generated ONOO− have not yet been fully refined. The situation has, however, changed during the past few years, with the discovery of a direct, rapid, and stoichiometric reaction between ONOO− and aromatic boronate probes.9 In this Perspective, the use of a global profiling approach to simultaneously detect several oxidants using multiple probes in toxicological studies is also highlighted.
Structure and properties of boronates
Boronates are trivalent boron-containing compounds that have an alkyl or aromatic substituent and two hydroxyl or ester groups (Fig. 1). The boron atom is electron-deficient because of the open shell which confers its acidic character. As a result, boronates are mild organic Lewis acids that can coordinate basic molecules and nucleophilic species. Most arylboronic acids have a pKa in the range of 7–9 depending on the aryl substituents.1,2 Their unique electronic structure and the ability to convert between the sp2 and sp3 forms makes boronate-based compounds good enzyme inhibitors (e.g., proteases).10 Nucleophilic addition of reactive species to electron-deficient boronate probes is a facile reaction. This unique chemical property of boronates has propelled their use as effective traps of ROS and RNS in biological systems.
Figure 1.
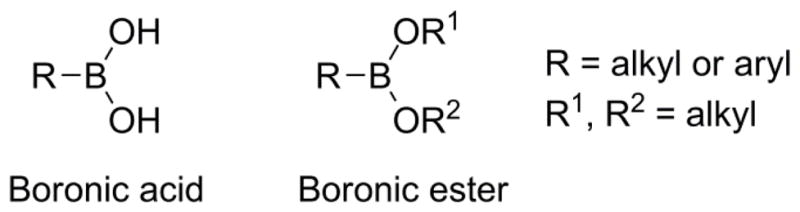
Structures of boronic acid and boronic ester.
Reaction between hydrogen peroxide and boronates
Over 80 years ago, it was reported that arylboronic acids react with alkaline H2O2 to form the corresponding phenols.11 The reaction between boronate and H2O2 was, however, reported to be rather sluggish (typically k~1–2 M−1s−1 at pH 7.4).12,13 More recently, several fluorogenic probes containing a boronate moiety for detecting and imaging intracellular hydrogen peroxide have been developed.14–17 These probes typically are polycyclic aromatic molecules that contain a boronate ester or acid group. Upon reaction with H2O2, these weakly fluorescent boronates are converted to a strongly fluorescent product. It is conceivable that several other deprotonated hydroperoxy intermediates may also react with boronates at physiological pH. For example, it has been reported that peroxymonophosphate reacts with boronates considerably faster than does H2O2.18 Additionally, H2O2 (−OOH) has been shown to react slowly with CO2 to form the monoperoxycarbonate (HOOCO2−).19 Preliminary unpublished data from our laboratory indicates that bicarbonate accelerates H2O2-induced oxidation of boronates, implicating a role for another oxidizing peroxide intermediate. During the course of these investigations, many elegant boronate-containing probes for H2O2 were designed and synthesized.14–17 However, a major drawback is that all of these probes react very slowly with H2O2 (k~1–2 M−1s−1) as compared to other reactive species (e.g., ONOO− and HOCl, with the rate constants at pH 7.4 of 106 M−1s−1 and 104 M−1s−1, respectively). Thus, in systems generating both H2O2 and HOCl, or ONOO−, one has to be careful in the assignment of reactive species. Oxidation of boronates should also be monitored in the presence of selective inhibitors/scavengers of these species.
Reaction between hypohalous acids and boronates
The oxidation of substituted phenylboronic acids by hypochlorite and hypobromite has been reported in 1962 to produce the corresponding phenols, and halogenated phenolic products in the presence of excess hypohalites.20 The hypochlorite anion (OCl−) reacts with aryl boronates ca. 1,000 times faster than H2O2 yielding the same phenolic product.9 However, HOCl reacts rapidly with endogenous amines and thiols.21 As a result, boronates are less likely to compete for HOCl in cellular systems. To test the involvement of HOCl in boronate oxidation, taurine can be used as a specific scavenger of HOCl, as boronates do not react with chloramines.
Reaction between peroxynitrite and boronates
Nearly 40 years ago, it was reported that ONOO− decomposition was accelerated in borate buffer.22 This was attributed to a transperoxidation reaction between ONOO− and borate, forming a peroxyborated intermediate.21 The proposed explanation was that the electrophilic boron was oxidized by ONOO−. We have recently shown that ONOO− reacts stoichiometrically with arylboronic acids and esters to form the corresponding phenols.9,23 The boron atom in boronates is sp2 hybridized and behaves as an electrophile. Thus, its reaction with a powerful nucleophile is energetically favored.9,24 ONOO− reacts with aromatic boronates to form the corresponding phenolic product in 80–85% yield. 24 HPLC analyses and EPR spin-trapping experiments showed that the minor products (~10–15%) were derived from radical intermediates, phenyl and phenoxyl radicals. 9,24
Other reactive nitrogen species such as the nitrogen dioxide radical (•NO2) formed from myeloperoxidase/H2O2-catalyzed oxidation of nitrite anion does not oxidize boronates to phenolic products.9,24 Therefore, boronates can be used to distinctly identify the species (ONOO− or •NO2) involved in the nitration of protein tyrosyl residues during nitrative stress.
Direct reaction between peroxynitrite anion and boronate-based fluorogenic probes
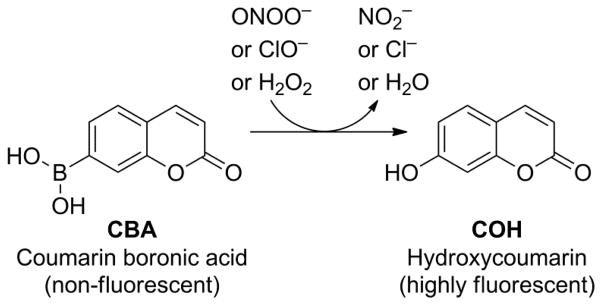
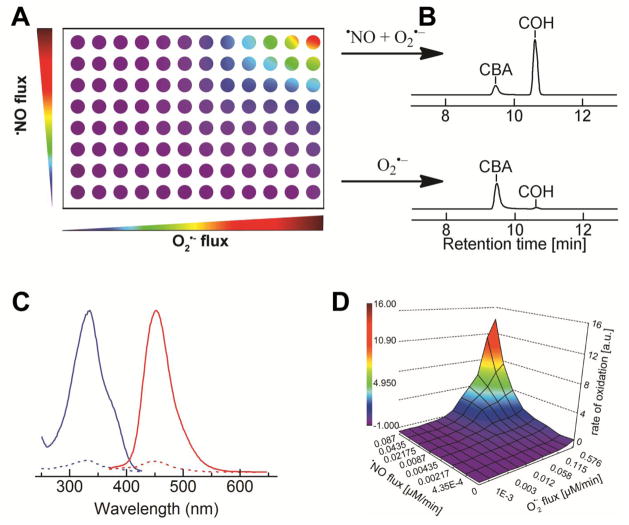
In our experience, the reaction between ONOO− and boronates is quite general, and is not dependent on the R-substituent (shown in Fig.1). Thus, conjugation to a fluorophore yields a boronate which is a viable candidate in user-friendly, routine assays. Coumarin boronate is one such example (Fig. 2). Using coumarin-7-boronic acid (CBA), which reacts stoichiometrically and rapidly with ONOO− to form a product exhibiting blue fluorescence, we showed that ONOO− is formed as a primary and only intermediate during the reaction between •NO and O2•¯ co-generated at different fluxes.23,24 Global profiling of fluorescent products formed from oxidation of CBA under varying fluxes of co-generated •NO and O2•¯ in a 96-well fluorescence plate containing CBA in phosphate buffer (50 mM, pH 7.4) is shown (Fig. 3). HPLC analyses of incubations with CBA from the two corner wells on the right side (top and bottom well) obtained under the same conditions as in the plate reader are shown. It is clear that in the absence of •NO, little or no fluorescent product (COH) is formed, while cogeneration of •NO and O2 •¯ leads to significantly higher levels of COH. These findings suggest that it is feasible to monitor in real time the formation of ONOO− in cellular systems using the fluorogenic boronate probe. Other boronate-based fluorescent dyes [e.g., fluorescein dimethylamide boronate (FlAmBE)] which give rise to a product exhibiting fluorescence in different spectral regions can also be used for monitoring in situ ONOO− formation.25
Figure 2.
Peroxynitrite-dependent oxidation of coumarin boronic acid (CBA) to a highly fluorescent hydroxycoumarin (COH).
Figure 3. Diagrammatic representation of global profiling of products.
(A) Varying fluxes of co-generated •NO and O2 •¯ in a 96-well fluorescence plate containing CBA in phosphate buffer (50 mM, pH 7.4) and DTPA (120 μM). (B) HPLC analyses of incubations with CBA from the two corner wells on the right side is shown. (C) Fluorescence excitation (blue lines) and emission (red lines) spectra of CBA (dashed lines) and COH (solid lines). (D) Rate of increase in fluorescence intensity during CBA oxidation by co-generated fluxes of •NO and O2•¯, as detected using a 96-well fluorescence plate reader.
Kinetics of boronate reactions
As discussed above, kinetics of the reaction is one of the key factors enabling the use of fluorescent probes for intracellular monitoring of ROS/RNS.26 To put things in perspective, at equimolar concentrations (0.1 mM) of phenylboronates and the oxidants, reaction time scales ranged from milliseconds (for ONOO−) to seconds (for HOCl), and hours (for hydrogen peroxide, H2O2).9 In other words, ONOO− reacts with aromatic boronates nearly a million times faster than H2O2 (Table 1) and two hundred times faster than HOCl. At physiological pH (=7.4), the percentage of HOO− during this reaction is 0.005%, compared to ONOO− (83%) and OCl− (46%). This is based on the pKa’s of H2O2 (11.7), ONOOH (6.7), and HOCl (7.47). The differences in the observed second-order rate constants for these species with boronates may, in part, be attributed to differing pKa’s. The difference in the rate constants for the reaction between boronates and ONOO− or H2O2 has important implications in real-time monitoring of these oxidants (Fig. 4). While boronates can be used at low micromolar concentrations to monitor real-time ONOO− formation, these probes should be present at millimolar concentrations to provide reliable data on the rate of H2O2 production. As shown in Figure 4, even at 1 mM concentration of boronate, the steady-state conditions are reached only after ca. 30 min. Stated differently, the rate of boronate oxidation equaled the rate of H2O2 generation only after 30 min. Despite lower reactivity, H2O2 and HOCl in buffered aqueous solution (pH 7.4) are relatively stable as compared to ONOO− in aqueous buffers (pH 7.4). Therefore, if allowed to proceed to completion, H2O2 can quantitatively oxidize boronate probes. On the other hand, in cellular systems both H2O2 and HOCl are rapidly removed/scavenged by endogenous constituents. Therefore slowly-reacting boronate probe need to be present at high millimolar concentration to be able to compete for H2O2. Under physiological conditions, boronates can efficiently compete for ONOO− in the presence of CO2.9
Table 1.
Rate constants for reaction between selected boronates, H2O2 and ONOO− at pH 7.4.
| Compound | k(ONOO−) [M−1s−1] | k(H2O2) [M−1s−1] |
|---|---|---|
| Aliphatic boronates | ||
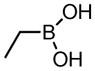 EtBA |
2.8 × 105 | n.d. |
| Substituted phenylboronates | ||
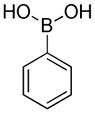 PBA |
1.2 × 106 | 2.2 |
 FBA |
1.4 × 106 | 2.5 |
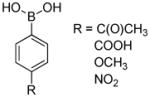 Other phenylboronates |
ca. 1.4 × 106 | |
| Fluorogenic boronates | ||
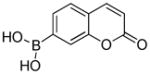 CBA |
1.1 × 106 | 1.5 |
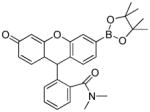 FIAmBE |
> 5 × 105 | n.d. |
n.d. – not determined
Figure 4. Real-time monitoring of H2O2 and ONOO− using boronate probes: a computer simulation.
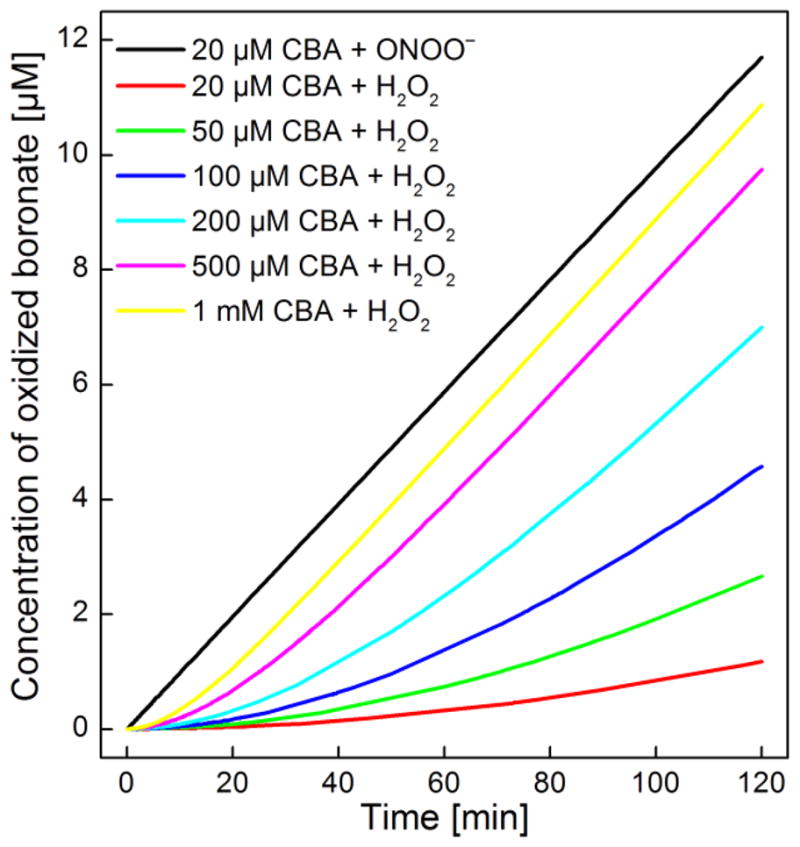
The amounts of probe reacted were simulated using a freely-available Kintecus software, assuming rate constants of the reaction of boronates with H2O2 and ONOO−, 1.5 M−1s−1 and 1.1 × 106 M−1s−1, respectively. The fluxes of H2O2 and ONOO− were set at 0.1 \M/min and the reaction pH was set at 7.4.
Both H2O2 and HOCl react with aryl boronates to form the corresponding phenolic product in 100% yield. In contrast, ONOO− reacts with boronates to form the same product with an 80–85% yield. The lower yield is not, however, due to less efficient trapping, as even at low micromolar concentration, boronates can effectively compete with self-decomposition of peroxynitrite. The peroxide bond in the adduct of ONOO− to boronates can undergo a heterolytic (ca. 90%) as well as a homolytic (ca. 10%) cleavage, leading to a lower yield of the phenolic product and formation of other phenyl radical-derived products.9,24 In fact, it was possible to detect the MNP-phenyl and DEPMPO-phenyl radical spin adducts, using the EPR spin trapping technique.24 This minor free-radical pathway is observed only with ONOO− but not with H2O2 or HOCl, and therefore may be used to further support the identity of species being detected.
Real-time monitoring of peroxynitrite from activated macrophages
Boronate-based fluorogenic probes have been used to selectively monitor ONOO− formed from activated macrophages.25 RAW 264.7 macrophages were treated with phorbol myristate acetate (PMA) to induce superoxide O2 •¯ generation, and LPS and IFN-γ to stimulate •NO production. In the presence of either CBA or another fluorogenic boronate probe, there was a steady increase in fluorescence intensity. Concomitant HPLC analysis showed that the fluorescence intensity was due to formation of the corresponding phenolic product. The nitric oxide synthase inhibitor L-NAME attenuated PMA, LPS, and IFN-γ-induced fluorescence and phenolic product formation. Addition of superoxide dismutase but not catalase significantly inhibited CBA-derived fluorescence. These results point to formation of ONOO− and its rapid trapping with CBA to form the fluorescent product. Thus, under conditions generating both H2O2 and ONOO−, boronates preferentially react with ONOO−.
Global profiling of oxidants
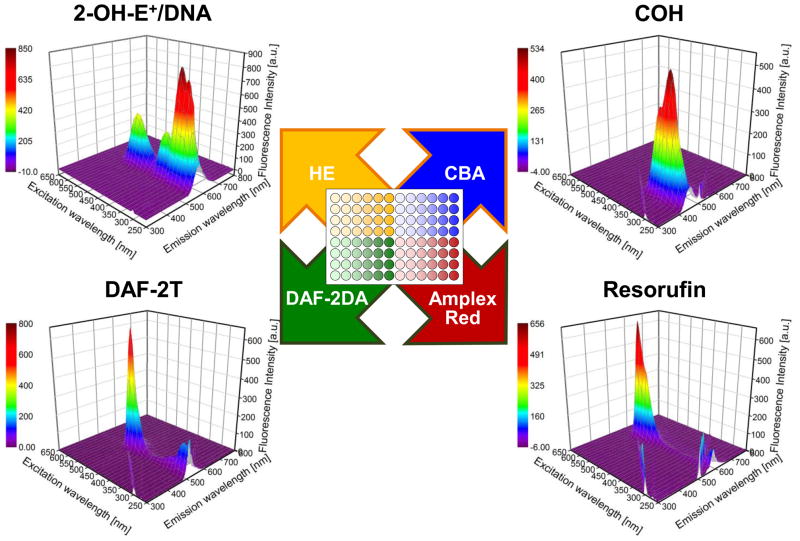
As we recently suggested, a global profiling approach with real-time monitoring of multiple oxidants is key to accurate characterization of oxidant species produced in an investigator’s system of choice.25 This approach involves treating cells (e.g., macrophages) in a 96-well plate with hydroethidine (HE) for tracking O2 •¯ and/or other oxidants, CBA for monitoring ONOO− and H2O2, Amplex Red and horseradish peroxidase (HRP) for measuring H2O2, and DAF-2DA for detecting •NO-derived nitrosating species (Fig. 5, and TOC Graphic). When coupled with enzymatic and chemical inhibitors of ROS/RNS (e.g., SOD and catalase alone and in combination and L-NAME), these experiments will empower and enhance the confidence of the investigator during simultaneous characterization of oxidants by high-throughput plate reader analysis. These fluorescence-based assays should be accompanied by HPLC-based verification of the identity(ies) of the fluorescent species being monitored. We anticipate that with the progress in ultra-high pressure liquid chromatography/mass spectrocmetry (UHPLC/MS) technology, the high-throughput analyses of ROS/RNS and screening of small molecule inhibitors in 96- and 384-well plates will soon be feasible in various cell-based systems. The above-mentioned dyes for global analysis of ROS/RNS were initially chosen as their free radical chemistry is relatively well characterized. These dyes can be substituted with additional ROS/RNS-specific probes once their free radical chemistry is well understood.
Figure 5. High-throughput analyses of ROS/RNS.
The 96-well plate consists of four dyes in four quadrants, as indicated. The four dyes in these fluorescence probes will react with O2•¯, H2O2, ONOO− and •NO-derived nitrosating species. Next to each quadrant are shown the fluorescence excitation-emission matrix (FEEM) spectra of the fluorescent products detected.
Monitoring IN VIVO generation of oxidants
A boronate-based bioluminescent probe was recently used for in vivo imaging of hydrogen peroxide in mice.27 This approach was also employed to detect endogenous H2O2 in a prostate cancer xenograft model.27 The experimental strategy involved the use of a Peroxy Caged Luciferin-1 (PCL-1) molecule formed by attaching an arylboronic acid to the phenolic position of firefly luciferin. Upon reaction with H2O2, luciferin is released, triggering a bioluminescence signal in the presence of ATP and luciferase.27 Again, one can predict that ONOO− will react with the PCL-1 bioluminogenic probe nearly a million times faster than with H2O2. Appropriate inhibitors (i.e., L-NAME) should be used to rule out (or confirm) the reaction between ONOO− and PCL-1 in biological settings. Nevertheless, the development of bioluminescent probes for real time detection of ROS/RNS in living mice is a significant achievement in free radical research.27
Future perspectives and examples in toxicology
Selective targeting of boronates to mitochondria will be useful both as a probe for ONOO− detection and detoxification. Ongoing research suggests that boronates conjugated to triphenylphosphonium cation that are targeted to mitochondria are indeed very effective traps of ONOO− (Sikora, Zielonka and Kalyanaraman, unpublished data). In general, boronate compounds can be used to detect and mitigate nitrative and oxidative stress during inflammatory processes. The recent discoveries highlighted in this Perspective demonstrate that there are many systems which can rapidly benefit from the use of boronate-based ONOO− detection. Below are several examples in toxicology which have a high likelihood of ONOO− involvement. In each case, the ability to confirm or rule out the involvement of ONOO− would be a major advance in our understanding of the drug toxicology and disease model described.
Several xenobiotics and drugs have been reported to induce peroxynitrite formation in biological systems.28 One of the most widely used over-the-counter drugs is acetaminophen. Recent reports suggest that peroxynitrite is formed as a key toxic intermediate during biotransformation of acetaminophen, and the inactivation of hepatic MnSOD has been proposed as a major reason for dose-dependent hepatotoxicity.29 As indicated earlier, many boronates have been used in patients during neutron capture treatment of brain cancer.2 It is likely these boronates could be used therapeutically to inhibit MnSOD nitration and attenuate acetaminophen toxicity.
The environmental herbicide, paraquat dication (PQ++), has been known for years to be a redox-cycling agent, leading to production of superoxide radical anion and depletion of cellular reducing equivalents. While many reports indicate the beneficial effects of inhaling •NO in PQ++ poisoning, other studies implicate •NO in PQ++ toxicity.30 Both protective and toxic effects of •NO are assumed to originate from a direct reaction of •NO with O2 •¯ to form ONOO−. Clearly, the use of boronate-based probes and scavengers should help clarify the role of •NO and ONOO− in PQ++ poisoning.
Chronic ethanol consumption-induced pancreatic beta-cell dysfunction and apoptosis was attributed to nitration of glucokinase, a critical sensor of beta-cell glucose levels and glucose metabolism.31 ONOO− was proposed as a key reactive nitrogen intermediate formed in an iNOS-dependent process. The use of boronate probes and a global profiling approach should corroborate the proposed model.
Pulmonary vascular dysfunction and vascular remodeling that occurs following continued long-term exposure of tobacco smoke in an emphysema mouse model (mimicking COPD) was attributed to oxidative and nitrative stress.32 Boronates should help establish the mechanistic role of ONOO− in this mouse model.
Arsenic-induced environmental vascular pathogenesis was attributed to stimulation of H2O2 and ONOO− .33 Mitochondrial ONOO− stress was shown to occur in a mouse model following administration of antitumor (cis-platin)34, doxorubicin,35,36 and fluoroquinolone antibiotics.37 In conclusion, we believe that boronate-based fluorogenic dyes and boronates targeted to mitochondria will serve as useful tools for detection and detoxification of ONOO− and hydroperoxides in toxicological research.
Acknowledgments
Funding sources: This work was supported by NIH grants R01 CA152810 (BK) and R01 NS039958 (BK). A.S. was supported by a grant from the Foundation for Polish Science (FNP) within the “Homing Plus” program supported by the European Union within European Regional Development Fund, through the Innovative Economy program.
Abbreviations
- ONOO−
Peroxynitrite
- •NO2
nitrogen dioxide radical
- CBA
coumarin-7-boronic acid
- FlAmBE
fluorescein dimethylamide boronate
- HE
hydroethidine
- PCL-1
Peroxy Caged Luciferin-1
- iNOS
inducible nitric oxide synthase
Contributor Information
Jacek Zielonka, Email: jzielonk@mcw.edu.
Adam Sikora, Email: asikora@mitr.p.lodz.pl.
Micael Hardy, Email: micael.hardy@univ-provence.fr.
Joy Joseph, Email: jjoseph@mcw.edu.
Brian P. Dranka, Email: bdranka@mcw.edu.
Balaraman Kalyanaraman, Email: balarama@mcw.edu.
References
- 1.Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Wiley-VCH; 2005. [Google Scholar]
- 2.Soloway AH, Tjarks W, Barnum BA, Rong FG, Barth RF, Codogni IM, Wilson JG. The chemistry of neutron capture therapy. Chem Rev. 1998;98:1515–1562. doi: 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- 3.Trippier PC, McGuigan C. Boronic acids in medicinal chemistry: Anticancer, antibacterial and antiviral applications. Med Chem Commun. 2010;1:183–198. [Google Scholar]
- 4.Fang H, Kaur G, Wang B. Progress in boronic acid-based fluorescent glucose sensors. J Fluoresc. 2004;14:481–489. doi: 10.1023/b:jofl.0000039336.51399.3b. [DOI] [PubMed] [Google Scholar]
- 5.Packer P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radi R. Nitric oxide, oxidants, and proteome tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 8.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung IKH, Brown T, Jr, Schofield CJ, Claridge TDW. An approach to enzyme inhibition employing reversible boronate ester formation. Med Chem Commun. 2011;2:390–395. [Google Scholar]
- 11.Ainley AD, Challenger F. Studies of the boron-carbon linkage. Part I. The oxidation and nitration of phenyl-boric acid. J Chem Soc. 1930:2171–2180. [Google Scholar]
- 12.Kuivila HG. Electrophilic displacement reactions. III Kinetics of the reaction between hydrogen peroxide and benzeneboronic acid. J Am Chem Soc. 1954;76:870–874. [Google Scholar]
- 13.Kuivila HG, Armour AG. Electrophilic displacement reactions. IX Effects of substituents on rates of reactions between hydrogen peroxide and benzeneboronic acid. J Am Chem Soc. 1957;79:5659–5662. [Google Scholar]
- 14.Lo LC, Chu CY. Development of highly selective and sensitive probes for hydrogen peroxide. Chem Comm. 2003;9:2728–2729. doi: 10.1039/b309393j. [DOI] [PubMed] [Google Scholar]
- 15.Lippert AR, Van de Bittner GC, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc Chem Res. 2011;44:793–804. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L, Li M, Zheng S, Wang B. Rational design of a fluorescent hydrogen peroxide probe based on the umbelliferone fluorophore. Tetrahedron Lett. 2008;49:3045–3048. doi: 10.1016/j.tetlet.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson BC, Huynh C, Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc. 2010;132:5906–5915. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaButti JN, Gates KS. Biologically relevant chemical properties of peroxymonophosphate (=O3POOH) Bioorg Med Chem Lett. 2009;19:218–221. doi: 10.1016/j.bmcl.2008.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakhmutova-Albert EV, Yao H, Denevan DE, Richardson DE. Kinetics and mechanism of peroxymonocarbonate formation. Inorg Chem. 2010;49:11287–11296. doi: 10.1021/ic1007389. [DOI] [PubMed] [Google Scholar]
- 20.Kuivila HG, Benjamin LE, Murphy CJ, Price AD, Polevy JH. Electrophilic displacement reactions. XIV Two novel reactions involving areneboronic acids and halogens. J Org Chem. 1962;27:825–829. [Google Scholar]
- 21.Folkes LK, Candeias LP, Wardman P. Kinetics and mechanisms of hypochlorous acid reactions. Arch Biochem Biophys. 1995;323:120–126. doi: 10.1006/abbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 22.Keith WG, Powell RE. Kinetics of decomposition of peroxynitrous acid. J Chem Soc A. 1969:90. [Google Scholar]
- 23.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: Direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–14216. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikora A, Zielonka J, Lopez M, Dybala-Defratyka A, Joseph J, Marcinek A, Kalyanaraman B. Reaction between peroxynitrite and boronates: EPR spin-trapping, HPLC analyses, and quantum mechanical study of the free radical pathway. Chem Res Toxicol. 2011;24:687–697. doi: 10.1021/tx100439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zielonka J, Zielonka M, Sikora A, Adamus J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J Biol Chem. 2011;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Van de Bittner GC, Dubikovskay EA, Bertozzi CR, Chang CJ. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc Natl Acad Sci USA. 2010;107:21316–21321. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denicola A, Radi R. Peroxynitrite and drug-dependent toxicity. Toxicology. 2005;208:273–288. doi: 10.1016/j.tox.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, MacMillan-Crow LA, Hinson JA. Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Therapeut. 2012;340:134–142. doi: 10.1124/jpet.111.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran JM, Ortiz-Ortiz MA, Ruiz-Mesa LM, Fuentes JM. Nitric oxide in paraquat-mediated toxicity: A review. J Biochem Mole Toxicol. 2010;24:402–409. doi: 10.1002/jbt.20348. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Song EH, Lee HJ, Oh YK, Park YS, Park JW, Kim BJ, Kim DJ, Lee I, Song J, Kim WH. Chronic ethanol consumption-induced pancreatic beta-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J Biol Chem. 2010;285:37251–37262. doi: 10.1074/jbc.M110.142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, Nikam S, Roth M, Sydykov A, Medebach T, Klepetko W, Jaksch P, Dumitrascu R, Garn H, Voswinckel R, Kostin S, Seeger W, Schermuly RT, Grimminger F, Ghofrani HA, Weissmann N. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 33.States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107:312–323. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay P, Horvath B, Zsengeller Z, Zielonka J, Tanchian G, Holovac E, Kechrid M, Patel V, Stillman IE, Parikh SM, Joseph J, Kalyanaraman B, Pacher P. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med. 2012;52:497–506. doi: 10.1016/j.freeradbiomed.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukhopadhyay P, Rajesh M, Bátkai S, Kashiwaya Y, Haskó G, Liaudet L, Szabó C, Pacher P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–H1483. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabo E, Ungvári Z, Wolin MS, Groves JT, Szabó C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 37.Hsiao CJ, Younis H, Boelsterli UA. Trovafloxacin, a fluoroquinolone antibiotic with hepatotoxic potential, causes mitochondrial peroxynitrite stress in a mouse model of underlying mitochondrial dysfunction. Chem Biol Interact. 2010;188:204–213. doi: 10.1016/j.cbi.2010.07.017. [DOI] [PubMed] [Google Scholar]