Abstract
The goal of this study was to describe predictors and significance of poor exercise tolerance after left ventricular assist device (LVAD) implantation. Despite LVAD therapy some patients continue to exhibit exercise intolerance. The predictors and outcomes of these patients are unknown. A retrospective review of 65 LVAD recipients who performed a 6-minute walk test (6MWT) was conducted. Patients walking <300m were considered having poor exercise tolerance. There were 20 patients who exhibited poor exercise tolerance (221±45m) compared to 45 patients with better exercise tolerance (406±76m). Post-operatively, poor performers were not easily identified by functional symptoms alone since 42% of these patients reported NYHA Class 1 or 2 symptoms. Preoperative NYHA class, inotrope therapy, and intra-aortic balloon pump use were similar between the 2 groups. Multivariable analysis using all adequately powered (n>50) univariate predictors identified diabetes mellitus (OR=10.493, p=0.003) and elevated 1-month right atrial pressure (OR=2.985 for every 5mmHG, P=0.003) as significant predictors of poor performance (<300m, AUC=0.85). The poorly performing group had increased mortality (p=0.011), with 21% increased risk of overall mortality for every 10 meters short of 300m (fitted cox model: HR=1.211, p=0.0001). The distance walked in meters in a post-operative 6MWT was the strongest predictor of late post-LVAD mortality (p=0.0002). In conclusion, despite similar severity of heart failure preoperatively, some LVAD recipients may have persistent exercise intolerance post operatively as assessed by 6MWT that is independently associated with subsequent reduced survival.
Keywords: heart-assist device, exercise intolerance, 6-minute walk test, prognosis
Introduction
The large multicenter left ventricular assist device (LVAD) trials for destination therapy and bridge to transplant have demonstrated that 20% of patients continue to have persistent symptoms of severe heart failure despite LVAD implant1,2. However, the characteristics of these patients, the predictors of poor exercise tolerance after LVAD implant and their outcomes are not clear. The aim of the present study was to describe predictors and the significance of poor exercise tolerance after LVAD implantation defined as those who walk less than 300 meters during a 6-minute walk test (6MWT).
Methods
A retrospective analysis for first available 6MWT performed after recovery from LVAD implant as bridge to transplant or destination was conducted amongst a consecutive cohort of patients with advanced heart failure implanted with the HeartMate II axial flow LVAD (Thoratec, Pleasanton, CA) at our centre between February 2007 and October 2010. Patients were divided into 2 groups dependent on the distance covered on the 6MWT (poor performer (<300 m) or better performer (≥300 m)). All patients underwent a detailed clinical evaluation prior to LVAD implant including history, physical examination, laboratory evaluation, echocardiography and cardiac catheterization. Renal function was evaluated using the modified MDRD equation. To evaluate for complicated peri-operative course we determined the total number of days on initial ventilatory support as well as the need for repeat ventilation. Ventilator support for more than 3 days or need for repeat ventilation after extubation was defined as complex ventilatory course. After implant patients were followed with routine visits usually at one month, three months and 3-6 monthly intervals thereafter. Follow-up echocardiography was performed in the outpatient setting at the first follow-up visit. All cause mortality and causes of death were documented. The study was approved by the Mayo Clinic Institutional Review Board.
Six-MWT was performed after LVAD implantation in an ambulatory setting. There were 65 outpatients who performed the test when first able to do so. Tests performed < 2 months or > 12 months after LVAD implant were excluded from the analysis. Patients walked as far as they could for 6 minutes as previously described 3. Two-dimensional transthoracic echocardiography was performed in a standard manner as previously published 4. Right atrial pressure was estimated by the inferior vena cava diameter and its response to inspiration. Right ventricular function, tricuspid regurgitation and mitral regurgitation severity were qualitatively graded using a 4-point scale (normal, mild, moderate, or severe) using all views. Right ventricular function was assessed using tissue Doppler assessment of lateral tricuspid annular motion, systolic tricuspid regurgitation duration, and the right index of myocardial performance and was characterized as being normal or with mild, moderate and severe dysfunction. We corrected the time intervals of right ventricular ejection time and the time between the onset and the cessation of tricuspid regurgitation flow for heart rate using the correction formula time of tricuspid regurgitation flow corrected for heart rate = TR flow time/√RR
Descriptive analysis was performed by presenting the mean ±SD for numerical data unless markedly non-normal in which case the median and interquartile range (25th and 75th percentile) were used unless specified otherwise. Assessment of normality for numerical variables was performed using the Shapiro-Wilks method. Comparisons between groups were performed using, Fishers exact test, t-test or Wilcoxon’s test as appropriate. To analyze independent determinants of poor exercise tolerance univariate and multivariate analyses based on logistic regression model were performed with 6-minute walk distance <300 meters as dependent variable and the different clinical, hemodynamic and echocardiographic parameters as independent variables. For the multivariate analysis only parameters that were collected in > 50 patients were considered. The multivariable model considered marginally significant univariate variables (p<0.10) with model selection using stepwise combined selection. Survival probabilities were derived using the product-limit estimate where patients first enter the risk set upon classification using the 6 minute walk (left censoring) and (right) censored upon heart transplant or at last follow up if alive at that time. Association between the 6-minute walk assessment and survival was evaluated using Cox proportional hazards regression again allowing for the entry of patients into the risk set at the time of their walk assessment. Both the meters walked (continuous variable) as well as walking more or less than 300m (nominal) were tested. To evaluate the functional form between meters walked and mortality risk, we fit a smoothing spline in the Cox regression model (using cox.zph function of the coxph package in R). We also evaluated Cox models for predictors based upon different cutoff values under which a decreased walking distance would result in increased mortality (for example with values less than 300m as the meters less than 300 and values greater than 300m considered as 0). Comparison between parameters as to their association with mortality was performed by comparing χ2 values for the univariate Cox models and verification using a multivariate model. All p-values were two-sided, and values ≤ 0.05 were considered to indicate statistical significance. All data were analyzed with the JMP System software version 8.0 (SAS Institute, Inc, Cary, NC), SAS version 9.2 (SAS Institute, Inc, Cary, NC) or R (http://www.r-project.org/).
Results
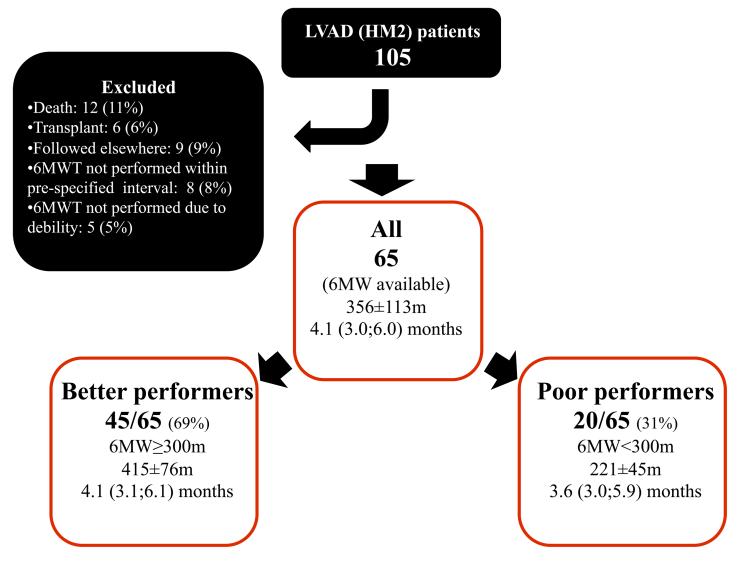
The study groups are outlined in Fig 1. Of 105 potential patients 65 were eligible for analysis. Causes of not performing a 6MWT were death, transplant, debility or inability to perform the test. There were twenty patients (31%) that walked <300 m (poor performers), and 45 patients (69%) that walked ≥300 m (better performers). The time period after LVAD implant when the 6MWT was performed was 3.6 months (3.0;5.9) [median, IQR] for poor performers and 4.1 months (3.1;6.1) [median, IQR] for better performers; not significantly different between the two groups (p=0.326). In the entire cohort, there were 83% males (n=54), and the median age of the group was 65 years. The etiology of heart failure was ischemic in 49% (n=32) of the patients. Twenty-three patients (35%) underwent LVAD implant, as bridge to transplant and the rest were destination. All patients had New-York heart association (NYHA) class 3-4 symptoms before LVAD implant, the majority (60%) had class 4 symptoms. The patients had median N terminal pro brain natriuretic peptide (NT-ProBNP) levels of 4600 (2100;8100) pg/ml and a moderately elevated Leitz-Miller prognostic score (9.3±5.9). Sixteen patients (25%) had an INTERMACS score of 1 or 2. Patients had a low left ventricular ejection fraction (20±7%) and cardiac index (1.9±0.5 lit/min/m2) with increased pulmonary capillary wedge pressure (23±6 mmHg). Preoperative right ventricular dysfunction by echo was characterized as being greater than moderate severity in 36/61 (59%) of patients.
Figure 1. study groups.
6 minute walk test (6MWT) distance and timing for the total study group (all) as well as for the pre-defined subgroups. Variables are presented as mean±SD if normally distributed or as median (25th; 75th percentile).
A detailed comparison between the poor and better performers on 6MWT is shown in tables 1 and 2. Patients in the poor performance group were older, had a higher prevalence of co-morbidities such as diabetes, hypertension and a lower pre-operative (but not admission) glomerular filtration rate. Patients in the low performance group had a lower right ventricular index of myocardial performance. Peri-operatively, the poor performers required prolonged inotropic support (median 141 hours) as compared to the better performing group (median 72 hours, p=0.008), and a more complex ventilator support (p=0.007) translating to a significantly increased length of hospital stay (median 33 days) as compared to the better performing group (median 22 days, p=0.003). There were no significant differences in pump settings and other pump parameters between the 2 groups that could account for the difference in exercise capacity. Postoperative echocardiography one month after implantation identified poor 6MWT performers to have significantly higher right atrial pressures, shorter tricuspid regurgitation time and shorter mitral E wave deceleration time. The results of univariate analysis for prediction of poor versus better performance on the 6MWT are presented in table 3 and are similar to variables described above. Multivariate model for early predictors identified diabetes mellitus (OR=5.230, 95% CI 1.515-19.540, p=0.010) and low pre-operative glomerular filtration rate (OR=0.962, 95% CI 0.927-0.992 p=0.021) as significant predictors (AUC=0.79, p<0.001). Multivariate model considering all adequately powered (n>50) univariate predictors in table 3 identified diabetes mellitus (OR=10.493, 95% CI 2.463-57.770, p=0.003) and elevated 1 month right atrial pressure (for every 5mmHG OR = 2.985, 95% CI 1.528-6.773, P=0.003) as significant predictors of poor performance in 6MWT (<300m, AUC=0.85).
Table 1.
Pre-operative characteristics of post-left ventricular assist devise exercise groups based on six-minute walk testing
| Variable | <300m (n=20) |
≥300m (n=45) |
p |
|---|---|---|---|
| Age (years) | 68(59:74) | 65(53:69) | 0.046* |
| Men | 15(75%) | 39(87%) | 0.292 |
| Hypertension | 11(55%) | 14(31%) | 0.098 |
| Diabetes mellitus | 11(55%) | 8(18%) | 0.006* |
| Chronic kidney disease | 13(65%) | 23(51%) | 0.418 |
| Bridge to transplant | 5 (25%) | 18(40%) | 0.276 |
| Ischemic etiology | 12(60%) | 20(44%) | 0.290 |
| Weight (Kg) | 89±21 | 85±17 | 0.487 |
| Atrial fibrillation | 4(20%) | 10(22%) | 1.000 |
| Glomerular filtration rate on admission (ml/min/1.73m2) |
47±20 | 57±20 | 0.065 |
| Glomerular filtration rate pre-op (ml/min/1.73m2) |
55±15 | 72±24 | 0.001* |
| New-York heart association class IV |
12 (60%) | 27 (61%) | 1.000 |
| Six-minute walk test | 180±106 (n=4) | 333±85 (n=19) | 0.056 |
| peakVO2 (mL/kg/min) pre-op | 9±4 (n=11) | 11±2 (n=22) | 0.128 |
| Intermacs score | 4(2.5:5.5) | 4(3:5) | 1.000 |
| Lietz Miller score | 10±6 | 9±6 | 0.372 |
| Intra-aortic balloon pump | 8(40%) | 16(36%) | 0.788 |
| Inotropic support | 11(55%) | 30(68%) | 0.401 |
| Angiotensin inhibitors | 14(70%) | 29(66%) | 1.000 |
| Beta blockers | 19(95%) | 36(82%) | 0.252 |
| Diuretic | 18(95%) | 39(89%) | 0.658 |
| Intracardiac defibrillator /resynchronization |
11(58%) | 27(61%) | 1.000 |
| Hemoglobin (g/dL) | 11.6±2.0 | 12.4±1.8 | 0.151 |
| Bilirubin (mg/dL) | 0.9(0.7;1.2) | 1.3(0.8;1.7) | 0.087 |
| Aspartate aminotransferase (u/L) |
28(20;40) | 36(29;50) | 0.058 |
| NTproBNP/100 (pg/ml) | 37(20;63) n=12 | 48(24;84) n=32 | 0.452 |
| Platelets/1000 | 157(129;231) | 153(130;222) | 0.759 |
| Albumin (g/dL) | 3.7±0.5 | 3.8±0.5 n=42 | 0.226 |
|
| |||
| Echocardiography | |||
|
| |||
| Right index of myocardial performance |
0.5±0.2 n=17 | 0.6±0.3 n=38 | 0.049* |
| Tricuspid regurgitation time (msec, corrected) |
457±53 | 474±71 n=41 | 0.287 |
| Right ventricular dysfunction (>moderate) |
11/18 (61%) | 25/43 (58%) | 1.000 |
| Right atrial pressure (mmHg) | 20(10;20) n=18 | 14(10;20) n=41 | 0.174 |
| Left ventricular end diastolic diameter (mm) |
67±8 | 68±9 n=43 | 0.674 |
| Left atrial volume index | 61(47;77) | 63(51;78) n=38 | 0.572 |
| Mitral valve E (m/sec) | 0.9(0.8;1.2) n=15 | 1(0.8;1.1) n=37 | 0.799 |
| E wave deceleration time (msec) |
136±31 n=14 | 137±29 n=35 | 0.961 |
| Ejection fraction (%) | 20(16;20) | 17(15;23) | 0.457 |
| Tricuspid regurgitation>moderate |
7(35%) | 12(27%) | 0.560 |
| Mitral regurgitation>moderate | 4(20%) | 18(40%) | 0.159 |
|
| |||
| Catheterization | |||
|
| |||
| Right atrial mean (mmHg) | 18±8 n=18 | 14±6 n=41 | 0.063 |
| Right ventricular stroke work index (g/m) |
6.5±4.1 n=18 | 7.7±3.7 n=40 | 0.313 |
| Stroke index (ml/beat/ m2) | 26(19;34) n=19 | 24(19;29) n=40 | 0.501 |
| Cardiac index (l/min/m2) | 1.9±0.6 n=18 | 1.9±0.5 n=41 | 0.688 |
| Systemic vascular resistance (wood units) |
18(12;22) n=15 | 20(10;23) n=28 | 0.868 |
| Pulmonary vascular resistance (wood units) |
3.1(2.2;4.7) n=17 | 4.9(2.8;8.6) n=39 | 0.084 |
| Wedge pressure (mmHg) | 24±6 n=18 | 23±6 n=39 | 0.710 |
| Pulmonary artery mean (mmHg) |
36±8 n=19 | 37±9 n=41 | 0.706 |
Continuous variables are represented as mean±SD or median and interquartile range for markedly non-normal data. Categorical variables are represented as total number and proportion within the subgroup. N is specified if results were missing. Means are compared using t-test, medians the Wilcoxon test and counts the Fisher exact chi-square test.
P<0.05 (*) was considered significant.
Table 2.
Post-operative characteristics of post-left ventricular assist device exercise groups based on six-minute walk testing
| Variable | <300m (n=20) |
≥300m (n=45) |
p |
|---|---|---|---|
|
| |||
| Surgery and post-op | |||
|
| |||
| Bypass time (min) | 91(77;118) n=19 | 87(67;114) n=39 | 0.619 |
| Complex ventilatory course | 11/18 (61%) | 10/44 (23%) | 0.007* |
| Inotropic support (hours) | 141(84;205) | 72(48;121) | 0.008* |
| Inotropes>168hr | 6(30%) | 5(11%) | 0.083 |
| Hospital stay (day) | 33(27;41) | 22(14;33) | 0.003* |
| Beta blocker † | 7(35%) | 18(40%) | 0.787 |
| Angiotensin inhibitors † | 7(35%) | 15(33%) | 1.000 |
| Loop diuretic (furosemide equivalent mg/day)† |
40(0;80) | 40(20;80) | 0.268 |
| Spironolactone † | 6(30%) | 10(22%) | 0.542 |
| Discharge pump speed>9200 RPM |
15(75%) | 31(72%) | 1.000 |
| discharge pump flow (L/min) |
5.2±0.6 | 5.3±0.7 | 0.473 |
| discharge pump pulsatility index |
5.0±0.6 n=19 | 5.0±0.7 n=42 | 0.991 |
|
| |||
| Early Follow-up | |||
|
| |||
| New-York heart association class >II |
11(58%) | 4(9%) | <0.001* |
| Hemoglobin (g/dL) | 10.4±1.5 n=18 | 10.6±2.0 n=36 | 0.661 |
| Platelets/1,000 | 248(226;296) n=18 | 276(228;370) n=36 | 0.209 |
| Albumin (g/dL) | 3.6±0.5 n=13 | 3.6±0.5 n=31 | 0.952 |
| Creatinine (mg/dL) | 0.9(0.8;1.4) n=17 | 0.9(0.8;1.1) n=39 | 0.495 |
| Tricuspid regurgitation time (corrected) |
399±43 n=16 | 430±41 n=34 | 0.025* |
| Right index of myocardial performance |
0.2(0.1;0.3) n=12 | 0.3(0.2;0.4) n=24 | 0.274 |
| Right atrial pressure (mmHg) |
10(10;20) n=18 | 5(5;10) n=38 | 0.001* |
| E wave dec. time (msec) | 142±27 n=11 | 205±51 n=21 | <0.001* |
| Left ventricular end diastolic diameter (mm) |
54±10 n=17 | 57±9 n=26 | 0.381 |
| Ejection fraction (%) | 20(20;27) n=17 | 25(20;31) n=26 | 0.363 |
| Aortic valve not opening | 10/19 (53%) | 23/39 (59%) | 0.779 |
| Heart rate at peak 6MWT | 98±14 n=16 | 103±18 n=40 | 0.223 |
Complex ventilatory course: ventilator support for more than 3 days or need for repeat ventilation after extubation.
discharge medications.
Continuous variables are represented as mean±SD or median and interquartile range for markedly non-normal data. Categorical variables are represented as total number and proportion within the subgroup. N is specified if results were missing. Means are compared using t-test, medians the Wilcoxon test and counts the Fisher exact chi-square test.
P<0.05 (*) was considered significant.
Table 3.
Univariate logistic regression model for variables predicting poor exercise including significant and borderline predictors
| Variable | n | Odds Ratio | 95% CI | p |
|---|---|---|---|---|
|
| ||||
| Pre-op | ||||
|
| ||||
| Age (per 10 year increase) | 65 | 1.82 | 1.07-3.60 | 0.024 |
| Hypertension | 65 | 2.71 | 0.92-8.21 | 0.070 |
| Diabetes mellitus | 65 | 5.65 | 1.80-18.97 | 0.003 |
| Glomerular filtration rate pre-op (per ml/min/1.73m2) |
64 | 0.96 | 0.92-0.99 | 0.002 |
| Right atrial pressure, mean (mmHg ) | 59 | 1.10 | 1.01-1.22 | 0.029 |
|
| ||||
| Post-op | ||||
|
| ||||
| >168hr inotrope support (Right ventricular failure equivalent) |
64 | 3.34 | 0.88-13.34 | 0.076 |
| Complicated ventilator course | 62 | 5.34 | 1.68-18.28 | 0.004 |
| Hospital stay (per day) | 65 | 1.04 | 1.01-1.07 | 0.010 |
| One month right pressure by echo (per 5mmHg) | 56 | 2.42 | 1.37-4.65 | 0.002 |
| One month tricuspid regurgitation time (corrected) per unit |
50 | 0.98 | 0.96-1.00 | 0.017 |
| One month mitral E wave dec. time (per 10msec) | 32 | 0.67 | 0.46-0.85 | <0.001 |
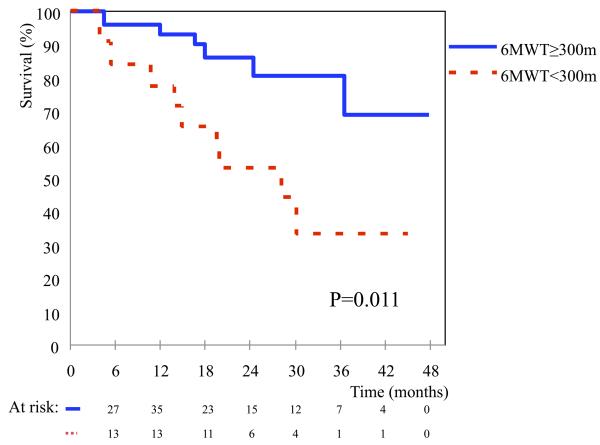
Patients were followed for a median of 592 days after implantation (range 115 to 1453 days). Median follow-up duration was 595 days for the poor exercise group and 587 days for the better performing group. Survival plots for both groups are shown in Fig 2. There was a significant difference in all cause mortality between the two groups (HR=0.26, p=0.011). Causes of death in the poor performing group were sudden unexplained death (3 patients), hemorrhagic stroke (2), sepsis (1), severe right ventricular failure (1), after operation for severe aortic incompetence (1) and withdrawal of support for severely decompensated quality of life (1). Causes of death in the better performing group were cancer related (2), sudden death (1), head trauma (1), intracranial bleed (1) and severe hemolysis (1).
Figure 2. Patient survival after LVAD implantation by 6MWT performance group.
The figure depicts survival curves for the poor performance group (walking <300m on the 6MWT) compared with the better performance group (≥300m). As depicted the poor performance group has worse survival (p=0.011).
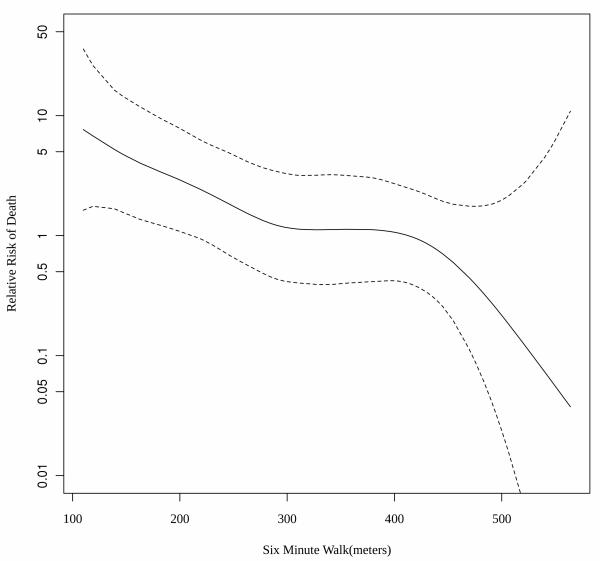
The relation of the distance walked in post-LVAD 6MWT to mortality is illustrated in figure 3. As illustrated there is an overall decreased risk of mortality in patients that were able to walk farther (HR=0.898; 95%CI 0.844-0.956 per 10m walked, p=0.0002). The risk of death is augmented when the walk distance is below the 300m cutoff. A modified Cox model assessing the association of meters walked less than 300 and mortality was indeed stronger than other tested cutoffs corresponding to 21% increased risk of overall mortality for every 10 meters short of 300m (HR=1.211; 95%CI 1.108-1.322 per 10m walked short of 300m, p=0.0001). Compared to all available predictors described in table 2, including predictors for walking less than 300m such as diabetes mellitus and renal dysfunction, the distance walked on post-LVAD 6MWT remained the best predictor of late post-LVAD mortality.
Figure 3. Performance in post LVAD 6MWT and risk of late mortality.
A spline representation of the relative risk for all cause mortality (in logarithmic scale) by the number of meters walked in a 6 minute walk test performed after recovery from LVAD implantation. Risk increases with reduced ability to walk 300 meters with 21% increased risk for every 10 meters short of 300m (HR1.211;95%CI 1.108-1.322, p=0.0001)
Discussion
This is the first study to examine the characteristics of post LVAD patients with poor exercise tolerance and the predictors and causes of exercise intolerance despite LVAD implantation. This is also the first study to demonstrate that poor exercise performance on a 6MWT after LVAD implant is associated with increased mortality. While it may seem obvious, that patients with limited exercise ability may not have a favorable clinical outcome, the reduced exercise tolerance is often not apparent unless specifically investigated for with an objective test. Patients and physicians may be biased by the improved subjective well being reported by patients that is often compared to significant pre-operative morbidity as identified in 42% of patients that walked less than 300m who were assessed as being NYHA Class 1 or 2 after LVAD implant. This issue is becoming increasingly important as the use of LVAD in heart failure patients especially as destination therapy is being considered for functional improvement and a better quality of life rather than survival alone.
Prior LVAD trials have demonstrated that although most patients improve their functional ability after LVAD implantation about 20-30%, similar to this study, fail to improve. In a previous large multicenter destination therapy trial, lack of improvement in functional capacity (NYHA class III-IV symptoms) persisted at 1 year in 24% of patients2. The mean distance covered in 6 MWT was 319±191m at 3 months and remained 318±164m one year after LVAD implant.. However, the survival of persistently symptomatic patients and possible predictors for lack of functional improvement in these trials has not been specifically investigated.
The 6MWT is a valid measurement of functional capacity and outcomes in heart failure but its utility after LVAD implant was unknown. The ability to walk less than 300 meters is representative of poor functional class and increased morbidity and mortality in heart failure5. Similar to prior heart failure trials, this study demonstrates that even in the LVAD population, patients who walked less than 300 m had increased subsequent mortality. Hence identifying patients who continue to have symptoms suggestive of heart failure after LVAD implant is important. Furthermore, searching for causes of poor exercise tolerance in an attempt to better select patients and correct reversible causes may improve functional capacity and outcomes.
Our analysis of clinical factors associated with poor exercise capacity after LVAD reveals 3 types of variables. The first group includes pre-operative patient related variables such as older age, comorbidities such as diabetes mellitus, impaired renal function and right heart failure (increased right atrial pressure). A second group of variables is related to the early postoperative course. Patients with post-implant right ventricular dysfunction and prolonged length of hospital stay were more inclined to have functional limitations later in their clinical course. The third group of variables associated with poor exercise tolerance includes markers of non-optimal LVAD physiology as determined by echocardiography 1 month after implant. Postoperative high right atrial pressures, short tricuspid regurgitation time and decreased systolic right ventricular pressures are suggestive of right heart failure and volume overload. The significance of a short tricuspid regurgitation time as a predictor of early adverse outcomes after LVAD implant was previously demonstrated by our group 4 and is shown in this study to be associated with poor exercise performance. A short E wave deceleration time is associated with rapid equalization of pressure during diastole, high left ventricular (LV) end diastolic pressure, restrictive LV physiology and inefficient LV unloading and has also been associated with increased mortality in patients with LV dysfunction without LVAD 6,7. Recently we described an association between insufficient LV unloading determined by echocardiography 1 month after LVAD implant and adverse outcomes 7. The presence of these clinical variables and their association with poor exercise capacity may imply the potential for modification of symptoms by altering pump settings and optimizing medical treatment such as the use of sildenafil in post LVAD pulmonary hypertension 8. We have now implemented these results in our daily clinical practice by risk stratifying patients based on their performance on the 6MWT and perform optimization of pump speed under echocardiographic and hemodynamic monitoring for select patients with poor exercise performance resulting in improvement in post-optimization functional performance.
Our study has the limitations of a retrospective analysis. The limited availability of 6MWT before LVAD implant due to patients being unable to perform the test could introduce a selection bias. However, in this sick population obtaining routine preoperative exercise testing is often not possible and reflects current clinical practice, limiting its utility to risk stratify these patients. Patients that did not perform the 6MWT after LVAD implant were not included. These included patients too sick to perform the test and hence may have actually resulted in an underestimation of the proportion of patients with limited function and their inclusion may have strengthened the findings of this study. To address the question of the influence of the exclusion on population characteristics we performed a sensitivity analysis including patients that died or could not perform the test due to debility in the low performance group. Their characteristics were comparable to the original poor performing group but with lower baseline hemoglobin and albumin and a longer bypass time during operation.
Acknowledgments
Funding Sources: Supported in part by HL 84904 (Heart Failure Clinical Research Network) (NLP), KL2RR024151 (NLP), a Marie Ingalls Cardiovascular Career Development Award (NLP), UL1RR24150 (NLP)
Footnotes
Disclosures: Authors T.H, S.K and S.P declare accepting a non-restricted clinical research grant from Thoratec to study endothelial function post-LVAD. Author L.J declares accepting a clinical research grant from Thoratec to study GI bleeding post-LVAD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Pugsley SO, Sullivan MJ, Thompson PJ, Berman L, Jones NL, Fallen EL, Taylor DW. Effect of encouragement on walking test performance. Thorax. 1984;39:818–822. doi: 10.1136/thx.39.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topilsky Y, Oh JK, Shah DK, Boilson BA, Schirger JA, Kushwaha SS, Pereira NL, Park SJ. Echocardiographic predictors of adverse outcomes after continuous left ventricular assist device implantation. JACC Cardiovascular imaging. 2011;4:211–222. doi: 10.1016/j.jcmg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 6.Peltier M, Leborgne L, Zoubidi M, Slama M, Tribouilloy CM. Prognostic value of short-deceleration time of mitral inflow E velocity: implications in patients with atrial fibrillation and left-ventricular systolic dysfunction. Arch Cardiovasc Dis. 2008;101:317–325. doi: 10.1016/j.acvd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Topilsky Y, Hasin T, Oh JK, Borgeson DD, Boilson BA, Schirger JA, Clavell AL, Frantz RP, Tsutsui R, Liu M, Maltais S, Kushwaha SS, Pereira NL, Park SJ. Echocardiographic variables after left ventricular assist device implantation associated with adverse outcome. Circ Cardiovasc Imaging. 2011;4:648–661. doi: 10.1161/CIRCIMAGING.111.965335. [DOI] [PubMed] [Google Scholar]
- 8.Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, Mathai SC, Thiemann DR, Hassoun PM, Girgis RE, Orens JB, Shah AS, Yuh D, Conte JV, Champion HC. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circulation Heart failure. 2008;1:213–219. doi: 10.1161/CIRCHEARTFAILURE.108.796789. [DOI] [PMC free article] [PubMed] [Google Scholar]