Abstract
Up-regulation of kynurenine (KYN) pathway of tryptophan (TRP) was suggested as one of the mechanisms of aging and aging-associated disorders. Genetic and pharmacological impairment of TRP – KYN metabolism resulted in prolongation of life span in Drosophila models. Minocycline, an antibiotic with anti-inflammatory, antioxidant and neuroprotective properties independent of its antibacterial activity, inhibited KYN formation from TRP. Since minocycline is the only FDA approved for human use medication with inhibitory effect on TRP – KYN metabolism, we were interested to study minocycline effect on life- and health-spans in Drosophila model. Minocycline (0.87mM) prolonged mean, median and maximum life span of wild-type Oregon Drosophila melanogaster of both genders. Minocycline (0.87 mM) stimulated vertical climbing in male flies. Minocycline dose-dependently decreased quantity and survivorship of pupae of filial generation. Minocycline might be a promising candidate drug for anti-aging intervention and treatment of aging-associated medical and psychiatric disorders. The role of TRP – KYN metabolism in the mechanisms of minocycline-effect on life- and health-span might be elucidated by the future assessment of minocycline effects in Drosophila mutants naturally or artificially knockout for genes impacting the key enzymes of KYN pathway of TRP metabolism.
Keywords: Minocycline, life span, Drosophila melanogaster, kynurenine, vertical climbing, health span
Up-regulation of kynurenine (KYN) pathway of tryptophan (TRP) metabolism was suggested to be one of the mechanisms of aging and age-associated medical and psychiatric disorders (AAMPD) [1–4]. Tryptophan (TRP) is an essential amino acid participating in biosynthesis of proteins and methoxyindoles (serotonin and melatonin) [5]. The major non-protein route of TRP metabolism (about 90%) is a production of N-formyl-kynurenine with ensuing formation of kynurenine (KYN). TRP conversion into KYN is catalyzed by indoleamine 2,3-dioxygenase 1 (IDO) (inducible by inflammatory cytokines) [5] or TRP 2,3-dioxygenase 2 (TDO) (inducible by substrate or stress hormones) [6].
KYN serves as a substrate for two post-KYN pathways: formation of kynurenic acid (KYNA), catalyzed by KYN aminotransferase (KAT); and 3-hydroxyKYN (3-HK) catalyzed by KYN 3-monooxygenase (KMO) (Figure 1) [6].
Figure 1.
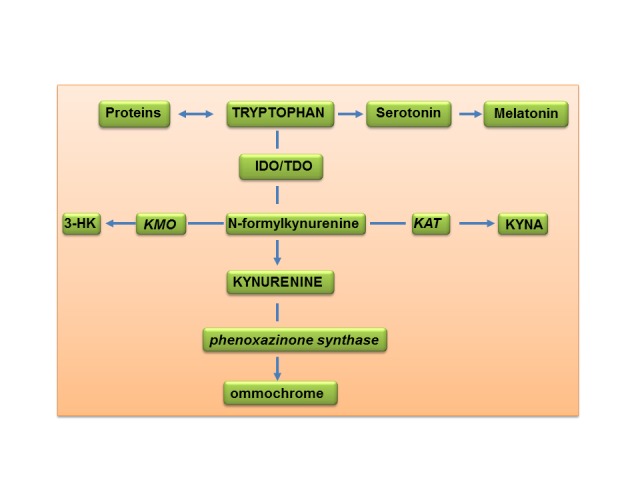
Major pathways of tryptophan metabolism in Drosophila melanogater. Abbreviations: IDO - indoleamine 2,3-dioxygenase; TDO – tryptophan 2,3-dioxygenase; KAT - KYN aminotrasferase I; KMO – KYN monooxygenase; 3-HK-3-hudroxykynurenine; KYNA – kynurenic acid.
Animal and human studies support the involvement of TRP - KYN metabolism in mechanisms of aging and AAMPD. Up-regulated formation of mitochondrial N-formyl-kynurenine was found among conserved biomarkers of aging in five species [7]. Increased blood KYN/TRP ratio (a marker of IDO activity) was observed in a study of humans of three age groups (34–60, 61–71, and 72–93 years) [8] and of nonagenarians compared with 45-year-old subjects [9]. Increased formation of KYN derivative, KYNA, was reported in human serum [10] and aged rat brain [10–12]. The higher blood KYN/TRP ratio at the entry was predictive of higher mortality in 10-year prospective study of nonagenarians [9]. Increased rate of TRP conversion into KYN was reported in obesity, diabetes, atherosclerosis, menopause and other components of AAMPD [2]. In addition, blood levels of neopterin, an indirect marker of IDO activity [13], was correlated with aging and AAMPD [14, 15]
Drosophila melanogaster mutants with deficiencies of the key enzymes of TRP - KYN metabolism are suitable models to study the age-related aspects of KYN pathway of TRP metabolism [16]. Since the end product of TRP – KYN pathway in flies is brown eye pigment, ommochrome, these mutants have different eye colors [17]. Life span of male vermilion mutant (TDO deficient) was 33% longer than life span of wild-type Canton-S flies [18]. We observed prolonged life span not only in male but in female vermilion mutants in comparison with wild-type Oregon flies [19]. However, the life spans of cinnabar (deficient KMO activity), and cardinal (deficient phenoxazinone synthase) mutants were not different from the wild-type flies [18] suggesting that prolongation of life span in Drosophila melanogaster depends on down-regulation of TRP conversion into KYN rather than on the post-KYN metabolism (Figure 1). The major rate-limiting factors of KYN formation from TRP are activity of TDO/IDO and availability of TRP as a substrate for KYN production [1]. TDO/IDO are intracellular enzymes [20], and inhibition of TRP transport into TDO/IDO containing cells might decrease the availability of TRP as a substrate for KYN formation [1]. We found that white Drosophila mutants (deficient intracellular transport of TRP) [21] live longer than wild-type Oregon flies [19]. Since genetic mutations might affect some other systems besides TRP - KYN metabolism [22], we studied the effects of specific inhibitors of KYN production from TRP on life span of wild-types flies. Administration of TDO inhibitor, alpha-methyl-tryptophan [23] or inhibitor of TRP intracellular transport, 5-methyl-tryptophan [24], prolonged life span of wild-type Oregon flies [25]. Therefore, genetically or pharmacologically-induced impairment of KYN formation from TRP was associated with prolongation of life span. The studied pharmacological agents, alpha- and 5-methyl-tryptophan, are not allowed for human use. We have recently reported that berberine, a natural isoquinoline alkaloid isolated from Berberis aristata, a Chinese and Indian herbal anti-inflammatory medicine, extended life span of wild-type flies without a decline in fertility or physical activity [26]. Berberine possesses a wide range of therapeutic effects related to age-associated diseases and is a powerful inhibitor of IDO (about 40 times stronger than the standard agent, 1-methyltryptophan [27]. However, berberine is not approved by FDA for human use. We were interested to study the life prolongation effect of medication that inhibit KYN formation from TRP and approved by FDA for human use.
Minocycline, a tetracycline derivative, exerted anti-inflammatory, antioxidant and neuroprotective properties independent of its antibacterial activity [28, 29]. Minocycline inhibits lipopolysaccharide-induced up-regulation of KYN formation from TRP [30, 31]. Drosophila melanogaster is a valuable model for preclinical testing of drugs with therapeutic potential for AAMPD because of small genome size, short generation time, and shorter mean life span compared to either mice or humans [32]. Drosophila also exhibits complex behaviors, many of which undergo age-related decline [33]. Therefore, utilization of Drosophila model allows for the estimation of drug effects not only on life span but on health span as well. This feature of Drosophila model is of importance considering that prolongation of life span is not necessarily associated with the positive effect on health span. Thus, caloric restriction and administration of lamotrigine prolonged life- but not health-span in Drosophila model [34, 35]. One of the major markers of health span is locomotor activity. Flies exhibit several forms of locomotor behavior, and the robustness of each behavior declines with age [36].The vertical climbing (or negative geotaxis) assay measures the ability of the organism to climb the walls of a vial when startled), and is an assessment of the animal’s ability to complete a strenuous activity (climbing against gravity, which provides insight into the fly’s level of fitness) [37]. Additional markers of health span assessed in the present study were quantity and survival rate of filial generation of pupae.
MATERIALS AND METHODS
Wild-type stock Oregon of Drosophila melanogaster from the collection of V.N. Karazin Kharkiv National University was used in the experiments. The study was carried out between April and June 2011.
Flies were maintained at 23°C in a 12:12 light: dark period on a standard Drosophila medium consisting of sugar, yeast, agar and semolina. Minocycline (Sigma Aldrich Chemical Co, USA) was added to nutrition medium in two doses: 0.87 mM and 8.7 mM. Doses of minocycline were selected based on report of minocycline protective effect against paraquat (a superoxide radical generator) toxicity in Drosophila [38].
Viability was estimated by the quantity and percentage of surviving pupae of filial generation. Three pairs of parent individuals (three males and three females) were transferred into a vial containing 4 ml of standard Drosophila medium or medium with addition of minocycline. Ten vials were set up per treatment (control, minocycline low and minocycline high doses). Number of filial generation pupae and percentage of surviving pupae were calculated per vial.
Life span. One day old adult flies of both genders were collected and then regularly transferred to fresh medium every 3–4 days. The number of dead flies was recorded at the time of transfer as described elsewhere {19, 25, 26].
Locomotor activity. To assess the effect of minocycline on vertical climbing 20 flies were placed at the bottom of a clean 4-inch glass vial over which a second identical vial was inverted. After 30s the two vials were separated. The climbing index was expressed as percentage of the number of flies that climbed to the top vial relative to the total number of flies tested [39]. Four climbing trials (akin to sampling with replacement) were conducted per vial. There were 35 climbing trials for each gender of control group (total number of tested flies = 700/gender); and 25 trials for each gender of minocycline groups (total number of tested flies = 500/gender).
Statistics The data were analyzed using Wilcoxon rank-sum test and two ways ANOVA test.
RESULTS
Viability
Minocycline in a low dose (0.87 mM) decreased the number of pupae of filial generation (by 25%) in comparison with the control group (Wilcoxon rank-sum test, p<0.005).
Minocycline in a high dose (8.7 mM) dramatically decreased the number of pupae of filial generation in comparison with the control (by 95%) (p<0.001) and low dose minocycline group (by 93%) (p<0.001, Wilcoxon rank-sum test) (Figure 2).
Figure 2.
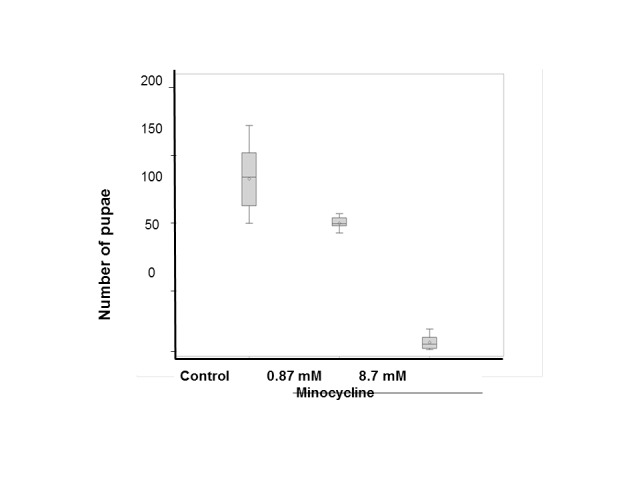
Minocycline effect on quantity of filial generation pupae. Three pairs of parent individuals (three males and three females) were transferred into a vial containing 4 ml of standard medium or medium with addition of minocycline. Ten vials were set up per treatment (control, minocycline 0.87 mM and minocycline 8.7 mM). Number of pupae per vial (mean ± standard deviation). Control group in comparison with minocycline 0.87 mM and minocycline 8.7 mM, p<0.001, Wilcoxon rank-sum test). Minocycline 0.87 mM in comparison with minocycline 8.7 mM, p<0.001, Wilcoxon rank-sum test.
Low concentration of minocycline (0.87 mM) did not affect the percentage of surviving pupae in comparison with the control group. High concentration of minocycline (8.7 mM) drastically decreased the percentage of surviving pupae in comparison with control and low concentration of minocycline (p<0.001, Wilcoxon rank-sum test) (Figure 3).
Figure 3.
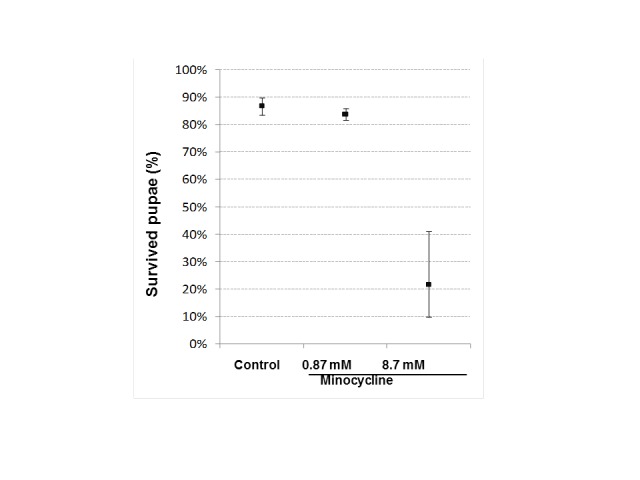
Minocycline effect on percentage on surviving of filial generation pupae. Three pairs of parent individuals (three males and three females) were transferred into a vial containing 4 ml of standard medium or medium with addition of minocycline. Ten vials were set up per treatment (control, minocycline 0.87 mM and minocycline 8.7 mM). Percentage of surviving pupae was calculated per vial (mean ± standard deviation). Control in comparison with minocycline 8.7 mM, p<0.001, Wilcoxon rank-sum test.
Locomotor activity and life span. Considering the toxic effects of high (8.7 mM) dose of minocycline on the viability of pupae, we used only low dose (0.87 mM) to study the effect of minocycline on locomotor activity and life span of flies.
Locomotor activity. Minocycline (0.87 mM) stimulated vertical climbing of male (p<0.01: minocycline VS control group, two-way ANOVA, Bonferroni adjusted) (Figure 4) but not of females flies (data not shown).
Figure 4.
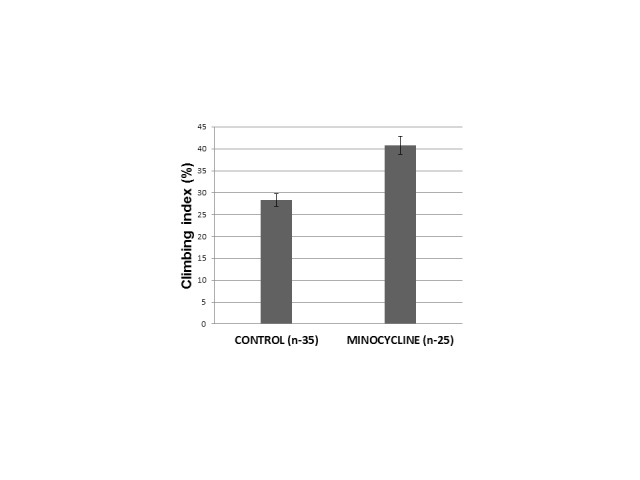
Minocycline effect on vertical climbing in male Drosophila melanogaster. Climbing index = % of flies moved to the top vial. Twenty flies were placed at the bottom of a clean 4-inch glass vial over which a second identical vial was inverted. After 30 s the two vials were separated. The climbing index was expressed as the percentage of the flies that climbed to the top vial relative to the total number of flies tested. Four climbing trials (akin to sampling with replacement) were conducted per vial. There were 35 climbing trials for Control group (total number of tested flies = 700); and 25 trials for minocycline group (total number of tested flies= 500). Mean ± SEM; p < 0.01: minocycline VS control group, two-way ANOVA, Bonferroni adjusted.
Life span
Female flies. Minocycline (0.87mM) increased mean, median and maximum life span of female flies in comparison with control group (Figure 4) (p<0.0001, Wilcoxon test adjusted for multiple comparisons) (Figure 5).
Figure 5.
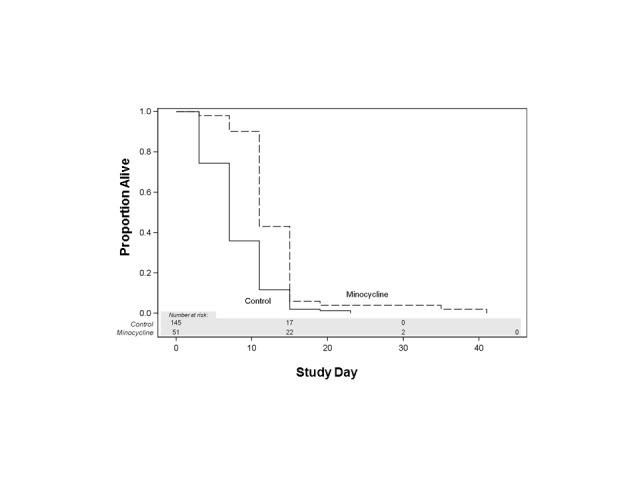
Minocycline effect on life span of female Drosophila melanogaster. Minocyline group in comparison with control group: p<0.0001, Wilcoxon test adjusted for multiple comparisons.
Males flies. Minocycline (0.87mM) increased mean, median and maximum life span of male flies in comparison with control group (p<0.0002, Wilcoxon test adjusted for multiple comparisons) (Figure 6).
Figure 6.
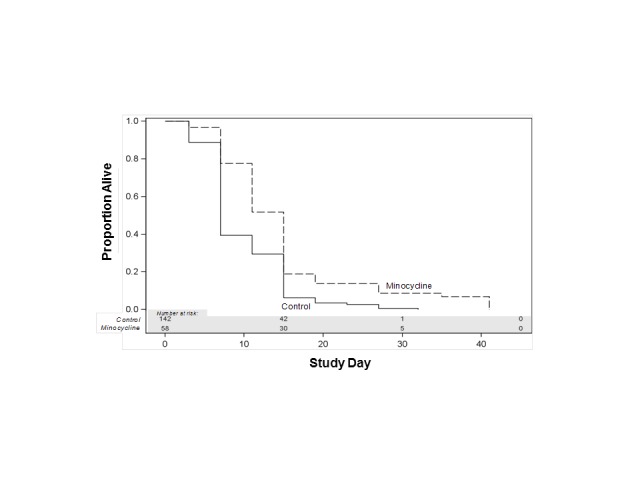
Minocycline effect on life span of male Drosophila melanogaster. Minocyline group in comparison with control group: p<0.0002, Wilcoxon test adjusted for multiple comparisons.
DISCUSSION
The results of our study indicate that minocycline (0.87 mM) prolongs mean, median and maximum life spans of female and male wild-type flies. Although we did not directly assess the effect of minocycline on fecundity, minocycline in our experiments decreased (dose-dependently) the quantity and percentage of surviving pupae of the filial generation. These results might suggest that minocycline depresses fecundity. It is known that fecundity suppression can result in lifespan extension [40]. Further experiments might clarify whether lower doses of minocycline would not affect viability of pupae but prolong life span of flies.
The other possible action mechanisms of minocycline effects might include inhibition of nitric oxide production, modulation of microglial activation, and inhibition of release of inflammatory cytokines [28, 41].
One of the mechanisms of minocycline-induced prolongation of life span might be related to inhibition of KYN production from TRP. KYN is a common substrate for the biosynthesis of neuroprotective KYNA and neurotoxic 3-HK. Impairment of TRP conversion into KYN in the vermilion mutant (TDO deficient) was associated with the increase of KYNA and decreased of 3-HK content in adult head tissues [42]. Authors suggested that the vermilion mutation might not completely eliminate KYN production [43]. The alternative explanation might be that KAT (the key enzyme of the biosynthesizes of KYNA) has higher affinity for KYN than KMO (the key enzyme of the biosynthesis of 3-HK), and, therefore, deficiency of KYN might limit formation of 3-HK to a greater degree than the production of KYNA. Therefore, minocycline-induced down-regulation of KYN production might lead to a shift of post-KYN metabolism from formation of neurotoxic 3-HK towards production of neuroprotective KYNA [5]. In addition, inhibition of TRY – KYN metabolism could “save” some tryptophan from degradation and make it available for biosynthesis of serotonin (and, consequently, N-acetylserotonin, BDNF TrkB receptor agonist [44,45]. Our results warrant the further studies of minocycline effect on life span and content of KYNA and 3-HK and serotonin and N-acetylserotonin in flies head tissues. Prolongation of life span not always associates with improvement of health span [34, 35]. While evaluation of spontaneous or explorative locomotor activity is a measure of a non-strenuous activity [36], the vertical climbing (or negative geotaxis) assay measures the ability of the organism to climb the walls of a vial when startled), and is an assessment of the animal’s ability to complete a strenuous activity (climbing against gravity), which provides insight into the fly’s level of fitness [33]. An age-dependent decline in negative geotaxis reflects the physiological age of flies (strictly dependent on aging) versus changes that are due to chronological age (strictly dependent on time) [37]. We observed the stimulating effect of minocycline on vertical climbing in male but not in female flies. These results are in line with observation that KYN decreases locomotor activity in a rat model [46]. Further studies are needed to assess the effect of minocycline on negative geotaxis in middle- and old–aged flies.
Additional measure of the health span might be the evaluation of the effect of minocycline on circadian locomotor activity. Aging effects on circadian locomotor activity have been reported in numerous studies in different animal species [47], and, importantly, circadian mechanisms are conserved between fruit flies and mammals [48]. In addition, glial cell modulate clock neurons and circadian locomotor activity [49], and minocycline, as a modulator of glial activity [41] might affect circadian locomotor activity.
Minocycline-induced prolongation of life span suggests that minocycline is a promising candidate drug for anti-aging intervention and treatment of AAMPD. The role of KP in the mechanisms of minocycline-effect on life- and health-span might be elucidated by the future assessment of minocycline effects in Drosophila mutants naturally or artificially knockout for genes impacting the key enzymes of KYN pathway of TRP metabolism..
Acknowledgments
Authors greatly appreciate invaluable statistical expertise of Robin Ruthazer. GF Oxenkrug is a recipient of NIH MH083225
Footnotes
Conflict of Interest Disclosure
Pual Summergrad is a non-promotional speaker for CME outfitters, Inc., and consultant and non-promotional speaker for Pri-med, Inc.
References
- [1].Oxenkrug GF. Genetic and hormonal regulation of the kynurenine pathway of tryptophan metabolism: new target for clinical intervention in vascular dementia, depression and aging. Ann N Y Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- [2].Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders and dysregulation of tryptophan-kynurenine pathway metabolism. Ann NY Acad Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- [3].Oxenkrug G. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated medical and psychiatric disorders”. J Neural Transm. 2011;118:75–85. doi: 10.1007/s00702-010-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oxenkrug GF. Interferon-gamma – Inducible Inflammation: Contribution to Aging and Aging-Associated Psychiatric Disorders”. Aging and Disease. 2011;2:474–486. [PMC free article] [PubMed] [Google Scholar]
- [5].Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murray MF. The human indoleamine 2,3-dioxygenase gene and related human genes. Curr Drug Metab. 2001;8:197–200. doi: 10.2174/138920007780362509. [DOI] [PubMed] [Google Scholar]
- [7].Groebe K, Krause F, Kunstmann B, Unterluggauer H, Reifschneider NH, Scheckhuber CQ, Sastri C, Stegmann W, Wozny W, Schwall GP, Poznanović S, Dencher NA, Jansen-Dürr P, Osiewacz HD, Schrattenholz A. Differential Proteomic Profiling of Mitochondria from Podospora Anserina, Rat and Human Reveals Distinct Patterns of Age-Related Oxidative Changes. Experimental Gerontology. 2007;42:887–898. doi: 10.1016/j.exger.2007.07.001. [DOI] [PubMed] [Google Scholar]
- [8].Frick B, Schroecksnadel K, Neurauter G. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [9].Pertovaara M, Raitala A, Lehtimäki T. Indoleamine-2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- [10].Urbańska EM, Luchowski P, Luchowska E. Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharm Rep. 2006;58:507–511. [PubMed] [Google Scholar]
- [11].Gramsbergen JB, Schmidt W, Turski WA, Schwarcz R. Age related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- [12].Moroni F, Russi P, Carlá V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci Lett. 1988;94:145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- [13].Fuch D, Avanzas P, Arroyo-Espliquero R, Jenny M, Consuegra-Sanchez L, Kaski JC. The role of neopterin in atherosclerosis and cardiovascular risk assesment. Current Medicinal Chemistry. 2009;16:4644–4650. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- [14].Ledochowski M, Murr B, Widner B, Fuchs D. Association between insulin resistance,body mass and neopterin concentrations. Clin Chim Acta. 1999;282:115–118. doi: 10.1016/s0009-8981(99)00019-4. [DOI] [PubMed] [Google Scholar]
- [15].Oxenkrug G, Tucker K, Requintina P, Summergrad P. Pyridoxal-5′-phosphate and insulin resistance correlate with neopterin, a marker of interferon-gamma-inducible inflammation cascade: implications for mechanisms of type 2 diabetes. American J Neuroprotection and Neuroregeneration. 2011;3:48–52. doi: 10.1166/ajnn.2011.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Savvateeva-Popova EV, Popov AV, Heinemann T, Riederer P. Drosophila mutants of the kynurenine pathway as a model for ageing studies. Adv Exp Med Biol. 2003;527:713–722. doi: 10.1007/978-1-4615-0135-0_84. [DOI] [PubMed] [Google Scholar]
- [17].Tearle R. Tissue specific effects of ommochrome pathway mutations in Drosophila melanogaster. Genet Res. 1991;57:257–266. doi: 10.1017/s0016672300029402. [DOI] [PubMed] [Google Scholar]
- [18].Kamyshev MG. Longevity and its relation to the locomotor activity in tryptophan xanthommatin metabolic pathway mutant of Drosophila. Dokl Acad Nauk USSR. 1980;253:1476–1480. [Google Scholar]
- [19].Oxenkrug G. The extended life span of Drosophila melanogaster eye-color (white and vermilion) mutants with impaired formation of kynurenine. J Neural Transm. 2010;117:23–26. doi: 10.1007/s00702-009-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kudo Y, Boyd CA, Sargent IL, Redman CW. Tryptophan degradation by human placental indoleamine 2, 3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001;535(Pt 1):207–215. doi: 10.1111/j.1469-7793.2001.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mackenzie SM, Brooker MR, Gill TR, Cox GB, Howells AJ, Ewart GD. Mutations in the white gene of Drosophila melanogaster affecting ABC transporters that determine eye colouration. Biochim Biophys Acta. 1999;1419:173–185. doi: 10.1016/s0005-2736(99)00064-4. [DOI] [PubMed] [Google Scholar]
- [22].Borycz J, Borycz JA, Kubów A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol. 2008;211:3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- [23].Lancaster GA, Sourkes TL. Effect of alpha-methyl-dl-tryptophan on tryptophan metabolism of Musca Domestica L. Comp. Biochem Physiol. 1969;28:1435–1441. doi: 10.1016/0010-406x(69)90581-7. [DOI] [PubMed] [Google Scholar]
- [24].Sullivan DT, Sullivan MC. Transport defects as the physiological basis for eye color mutants of Drosophila melanogaster. Biochem Genet. 1975;13:603–613. doi: 10.1007/BF00484918. [DOI] [PubMed] [Google Scholar]
- [25].Oxenkrug G, Navrotskaya V, Vorobyova L, Summergrad P. Extension of life span of Drosophila melanogaster by the inhibitors of tryptophan – kynurenine metabolism. Fly (Austin) 2011;5:1–3. doi: 10.4161/fly.5.4.18414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Navrotskaya V, Oxenkrug G, Vorobyova L, Summergrad P. Berberine prolongs life span and stimulates locomotor activity of Drosophila melanogaster. Amer J Plant Sci. 2012;3:1037–1040. doi: 10.4236/ajps.2012.327123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yu CJ, Zheng MF, Kuang CX, Huang WD, Yang Q. Oren-gedoku-to and its constituents with therapeutic potential in Alzheimer’s disease inhibit indoleamine 2, 3-dioxygenase activity in vitro. J Alzheimers Dis. 22:257–266. doi: 10.3233/JAD-2010-100684. [DOI] [PubMed] [Google Scholar]
- [28].Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004;17:359–366. doi: 10.1016/j.nbd.2004.07.012. [DOI] [PubMed] [Google Scholar]
- [29].Levkovitz Y, Mendlovich S, Issaki S, Braw Y, Levkovitz-Verbin H, Gal G, Fenning S, Treves I, Kron S. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- [30].O’Connor JC, Lawson MA, André C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15–19. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Partridge L, Tower J. Yeast, a feast: the fruit fly Drosophila as a model organism for research into aging. In: Guarente LP, Partridge L, Wallace DC, editors. Molecular Biology of Aging. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2008. pp. 267–308. [Google Scholar]
- [33].Jones MA, Grotewiel M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp Gerontol. 2011;46:320–325. doi: 10.1016/j.exger.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Avanesian A, Khodayari B, Felgner JS, Jafari M. Lamotrigine extends lifespan but compromises health span in Drosophila melanogaster. Biogerontology. 2010;11:45–52. doi: 10.1007/s10522-009-9227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bhandari P, Jones MA, Martin I, Grotewiel MS. Dietary restriction alters demographic but not behavioral aging in Drosophila. Aging Cell. 2007;6:631–637. doi: 10.1111/j.1474-9726.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- [36].Le Bourg E. The rate of living theory. Spontaneous locomotor activity, aging and longevity in Drosophila melanogaster. Exp Gerontol. 1987;22:359–369. doi: 10.1016/0531-5565(87)90034-9. [DOI] [PubMed] [Google Scholar]
- [37].Martinez VG, et al. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol. 2001;67:778–791. doi: 10.1002/dneu.20388. [DOI] [PubMed] [Google Scholar]
- [38].Bonilla E, Medina-Leendertz S, Villalobos V, Molero L, Bohorquez A. Paraquat-induced oxidative stress in Drosophila melanogaster: effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem Res. 2006;31:1425–1432. doi: 10.1007/s11064-006-9194-8. [DOI] [PubMed] [Google Scholar]
- [39].Chandrashekara KT, Shakarad MN. Aloe vera or Resveratrol Supplementation in Larval Diet Delays Adult Aging in the Fruit Fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011;66:965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- [40].Jafari M, Rose M. Rules for the use of model organisms in antiaging pharmacology. Aging Cell. 2006;5:17–22. doi: 10.1111/j.1474-9726.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- [41].Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]
- [42].Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, Kyriacou CP. The kynurenine pathway modulates neurodegeneration in a drosophila model of Huntington’s disease. Curr Biol. 2011;21:961–966. doi: 10.1016/j.cub.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Green EW, Campesan S, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, Kyriacou CP, Giorgini F. Drosophila eye color mutants as therapeutic tools for Huntington disease. Fly (Austin) 2012;6:117–20. doi: 10.4161/fly.19999. [DOI] [PubMed] [Google Scholar]
- [44].Oxenkrug G, Ratner R. N-Acetylserotonin and Aging-Associated Cognitive Impairment and Depression. Aging and Disease. 2012 Aug;3(4) June 19 (Epub ahead of print) [PMC free article] [PubMed] [Google Scholar]
- [45].Tosini G, Ye K, Iuvone PM. N-Acetylserotonin: Neuroprotection, Neurogenesis, and the Sleepy Brain. 2012 May 14; doi: 10.1177/1073858412446634. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J Neurochem. 1995;65:1176–1183. doi: 10.1046/j.1471-4159.1995.65031176.x. [DOI] [PubMed] [Google Scholar]
- [47].Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010;2:936–944. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang GK, Sehgal A. Signaling components that drive circadian rhythms. Curr Opin Neurobiol. 2002;12:331–338. doi: 10.1016/s0959-4388(02)00324-0. [DOI] [PubMed] [Google Scholar]
- [49].Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol. 2011;21:625–634. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


