Abstract
Cerebrovascular disease remains a significant public health burden with its greatest impact on the elderly population. Advances in neuroimaging techniques allow detailed and sophisticated evaluation of many manifestations of cerebrovascular disease in the brain parenchyma as well as in the intracranial and extracranial vasculature. These tools continue to contribute to our understanding of the multifactorial processes that occur in the age-dependent development of cerebrovascular disease. Structural abnormalities related to vascular disease in the brain and vessels have been well characterized with CT and MRI based techniques. We review some of the pathophysiologic mechanisms in the aging brain and cerebral vasculature and the related structural abnormalities detectable on neuroimaging, including evaluation of age-related white matter changes, atherosclerosis of the cerebral vasculature, and cerebral infarction. In addition, newer neuroimaging techniques, such as diffusion tensor imaging, perfusion techniques, and assessment of cerebrovascular reserve, are also reviewed, as these techniques can detect physiologic alterations which complement the morphologic changes that cause cerebrovascular disease in the aging brain.Further investigation of these advanced imaging techniques has potential application to the understanding and diagnosis of cerebrovascular disease in the elderly.
Keywords: Neuroradiology, neuroimaging, aging, cerebrovascular disease, white matter, cerebral infarction
Cerebrovascular disease remains a significant public health issue worldwide. In the United States, it is estimated that 795,000 Americans have a new or recurrent stroke each year and there are 7,000,000 Americans who have previously had a stroke [1]. Cerebrovascular disease is the 3rd leading cause of death in the United States, with a cerebrovascular disease-related death occurring once every 4 minutes [2]. End-organ damage to the brain from cerebrovascular disease occurs in the form of a stroke or cerebrovascular accident (CVA) and is defined by the World Health Organization as a neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours. Though many categorization schemes exist for CVAs, one such distinction is the division of CVAs into underlying ischemic or hemorrhagic etiologies. Approximately 80% of strokes are related to ischemic events that can be precipitated by thrombosis, embolism, or hypoperfusion in a vessel supplying the brain parenchyma. The other 20% of strokes are hemorrhagic in etiology and can be due to hypertension or due to an underlying vascular lesion such as a ruptured aneurysm or arteriovenous malformation.
There are many modifiable and non-modifiable risk factors associated with the development of cerebrovascular disease, many of which are similar to risk factors for cardiovascular disease and myocardial infarction. The etiology of cerebrovascular disease is complex and multifactorial, with hypertension, diabetes, smoking, and atrial fibrillation identified as well known potentially modifiable risk factors [3]. While these predisposing risk factors play a critical role in the etiology of cerebrovascular disease, several large population based cohorts have identified age to be the single most important risk factor for stroke, as the stroke rate more than doubles for men and women for every 10 years after the age of 55 [1, 4]. Moreover, advanced age is associated with adverse outcomes and delayed recovery after the initial acute cerebrovascular accident [5].
Brain damage in stroke is caused by ischemia resulting in a cascade of events including energy and sodium-potassium pump failure, increase in intracellular calcium, depolarization, generation of free radicals, blood-brain barrier disruption, and apoptosis [6]. As a result of these mechanisms, there is cellular and architectural damage of the brain parenchyma. In addition to a primary hemorrhagic stroke, there can also be secondary hemorrhagic transformation of ischemic strokes. The reported incidence of hemorrhagic transformation varies between 10 and 43% and occurs because of increased permeability of blood vessels in the infarcted area, another consequence of the ischemic/inflammatory cascade described above [6].
Imaging Tools to evaluate Cerebrovascular Disease
Many diagnostic medical tests exist to evaluate and monitor cerebrovascular disease (Table 1). In recent decades, with the development and widespread availability of imaging tests, neuroradiologic evaluation has become a vital component in the diagnosis, monitoring, and treatment of patients with cerebrovascular disease. Computed tomographic (CT) and magnetic resonance imaging (MRI) techniques are tools now available to evaluate cerebrovascular disease and can document structural abnormalities and functional impairments that occur in the brain and cerebral vasculature with aging.
Table 1.
Role of different imaging tests in the work-up of cerebrovascular disease in the aging
| Imaging modality | Applications in cerebrovascular disease |
|---|---|
| Computed tomography (CT) |
|
| Magnetic resonance imaging (MRI) |
|
| CT angiography (CTA) |
|
| MR angiography (MRA) |
|
| Catheter digital subtraction angiography (DSA) |
|
| CT perfusion (CTP) |
|
| MR perfusion (MRP) |
|
CT uses X-rays to generate tomographic images or slices of the body. CT based techniques include non-contrast CT, CT angiography (CTA), and CT perfusion (CTP). Non-contrast CT is very sensitive in the detection of hemorrhage, the presence of which changes the management of stroke drastically (Figure 1). Immediate non-contrast computed tomography (CT) scanning of all patients suspected of having a stroke has been found to be cost-effective [7] and is the most common imaging modality used in patients with suspected acute ischemia in acute stroke management algorithms due to widespread availability and short acquisition time [8]. Though less sensitive to detect brain parenchymal pathology than MRI, CT can still assess the degree of chronic white matter disease (leukoaraiosis) frequently present in aging individuals and can assess the presence of chronic infarctions as well (Figure 2).
Figure 1.

Hemorrhage on noncontrast head CT. Axial CT image (A) demonstrates hypodensity and loss of gray-white differentiation in the left middle cerebral artery territory, consistent with acute-to-subacute infarction, with a focus of internal hyperdensity (arrow), representing hemorrhagic transformation. Axial CT image in another patient (B) demonstrates a left temporooccipital intraparenchymal hematoma (arrow) and a left hemispheric subdural hematoma (arrowheads).
Figure 2.

Leukoaraiosis and Chronic Infarcts on CT and MRI. Non-contrast axial head CT (A) and axial T2 FLAIR MR (B) images demonstrating advanced chronic white matter ischemia (arrowheads) and left frontotemporal (short arrows), right frontal (long arrows), and right parietal (angled arrows) chronic infarctions.
CT angiography (CTA) involves thin section arterial phase CT images acquired after the administration of intravenous iodinated contrast. In the context of cerebrovascular disease, CTA is a highly valuable technique for assessing structural abnormalities of the intracranial and extracranial cerebrovasculature. Common uses in clinical practice included the assessment of vessel stenosis, occlusion, or dissection (Figure 3) and the characterization of other structural abnormalities such as aneurysms or arteriovenous malformations. Unlike catheter angiography techniques, CTA also allows visualization of the vessel walls and adjacent soft tissues.
Figure 3.
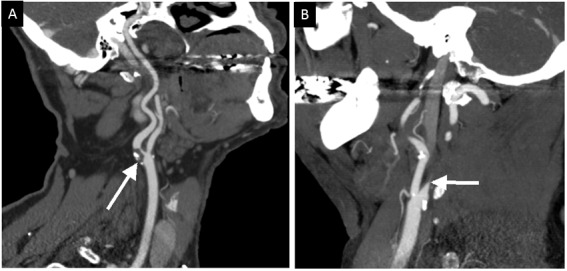
CT angiogram (CTA) showing stenosis and occlusion. (A) Curved multiplanar reconstruction (MPR) of a CTA of the neck demonstrates a large partially calcified atherosclerotic plaque resulting in an 80% stenosis of the proximal left internal carotid artery (arrow). (B) Sagittal maximal intensity projection (MIP) of a CTA of the neck shows complete occlusion of the proximal right internal carotid artery. The abrupt cutoff of contrast at this level is consistent with vascular dissection.
CT perfusion (CTP) allows qualitative and quantitative evaluation of cerebral perfusion bygenerating parametric maps of cerebral blood volume (CBV), cerebral blood flow (CBF), and mean transit time (MTT)[7].This can be used to assist in estimating the region of core infarction andthe adjacent or surrounding potentially salvageable areas of hypoperfusion, considered as ischemic penumbra [9].
As non-contrast CT, CTA, and CTP can all be acquired in less than 5 minutes during a suspected episode of acute ischemia, the information derived from these techniques can be considered complementary in nature. In fact, a comparison of the utility of non-contrast CT, CTA, and CTP techniques showed that a combination of CTA and CTP yielded the most accurate assessment of the occlusion site, infarct core, salvageable brain tissue, and collateral circulation [10].
MR imaging techniques are useful in further selecting patients for treatment and in determining prognosis in acute stroke, and are useful in assessing the degree of chronic brain parenchymal and vascular changes in patients with long-standing cerebrovascular disease. MRI is a technique based on signal emittedby hydrogen protons in an external magnetic field during alteration of proton spins by various applied radio-frequency wave excitations.MR sequences commonly used in the evaluation of the brain parenchyma in the context of acutestroke include T1-weighted, T2-weighted, T2-weighted fluid attenuated inversion recovery (FLAIR), gradient echo (GRE), susceptibility weighted (SWI), diffusion-weighted imaging (DWI), and perfusion-weighted imaging (PWI). MR angiography (MRA) techniques are also valuable methods of evaluating the intracranial and extracranial vasculature for stenosis or occlusion, hallmarks of atherosclerotic or thromboembolic disease.
T2 weighted FLAIR images are highly sensitive in the detection of chronic ischemic white matter changes and can characterize the extent of abnormal brain parenchyma significantly more accurately than non contrast CT techniques (Figures 4 and 5). DWI identifies cytotoxic edema and has been shown to be significantly more accurate than non-contrast CT (Figure 6) in the diagnosis of acute ischemic stroke within 12 hours of symptom onset [11], and even as soon as 5 to 10 minutes after an acute stroke has occurred [12]. With non-contrast head CT, on the other hand, the earliest and most subtle imaging signs of cerebral infarction, such as blurring of the gray-white differentiation, are rarely apparent before three hours after symptom onset in an ischemic stroke.
Figure 4.
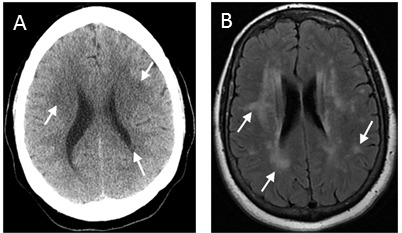
Leukoaraiosis on CT versus MRI. Leukoaraiosis in the same patient on CT (A), seen as multifocal patchy hypodensities in the periventricular and subcortical white matter (arrows), and MR (B), much more clearly demonstrated as foci and patches of hyperintensity on T2 FLAIRimage (arrows). The differences in the shape of the skull and lateral ventricles are due to the difference in scan angle; the CT gantry is tilted to reduce radiation dose to the orbit.
Figure 5.

Degree of leukoaraiosis on MR. Axial T2 FLAIR images demonstrate normal white matter (a), as well as minimal (b), mild (c), moderate (d), and severe (e) leukoaraiosis (representative areas of abnormal signal intensity depicted by arrows).
Figure 6.
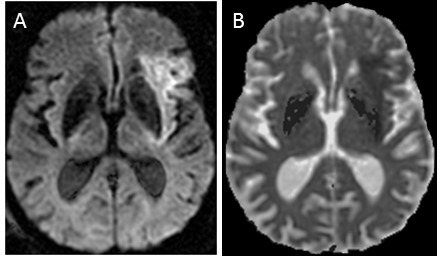
Hyperacute stroke on MR. Axial diffusion-weighted image (DWI) and apparent diffusion coefficient (ADC) map (A and B, respectively) show DWI hyperintensity and ADC hypointensity, consistent with restricted diffusion caused by acute infarction involving the left middle cerebral artery territory.
Aging and incidence of cerebrovascular disease
Though there are many risk factors for cerebrovascular disease, the strongest independent risk is advanced age [13, 14]. Aging has been found to be associated with poorer outcomes in patients suffering from stroke. Older stroke patients perform more poorly at activities of daily living compared to younger patients thereby more likely necessitating long-term rehabilitation services rather than discharge to home [15]. Moreover, advanced age has been associated with increased rates of subsequent dementia among surviving stroke patients [16]. As stroke and cerebrovascular disease in general are associated with aging, the elevated risk associated with advanced age may be directly related to the biological aging of the vascular system.
Aging and Blood Vessels
Though a detailed discussion of the complex and multifactorial biochemical mechanisms underlying age-related vascular changes are beyond the scope of this paper, some of these proposed mechanisms included medial degeneration, endothelial dysfunction due to deposition of advanced glycation end products, calcium deposition, oxidative stress, and the repetitive trauma of longstanding pulsation. These factors can together contribute to senescence of endothelial cells and smooth muscle cell hypertrophy.This in turn may result in increased stiffness and reduced flow mediated dilation, particularly of the large arteries with resulting increased transmitted systolic blood pressure, pulse pressure, and pulse wave velocity [17].
The increase in arterial stiffness with aging can compromise the cushioning effect that large arteries normally have in absorbing the pulsatile energy transmitted from the heart.When this pulsatile energy is not appropriately absorbed, there is resulting injury to microcirculation of end-organs such as the brain[18]. Furthermore, aortic pulse wave velocity, a measure of arterial stiffness, has been found to be an independent predictor of stroke [19]. Increased systolic hypertension, another indicator of arterial stiffness, is associated with a 3-fold increase in risk of stroke [17]. Therefore, aging of blood vessels is intimately associated with cerebrovascular disease.
In addition to arterial stiffening with aging, there can also be focal areas of narrowing as a result of atherosclerosis. Carotid artery stenosis is narrowing of the major arteries supplying blood to the brain with approximately 25–30% of ischemic strokes attributable to atherosclerosis of the carotid arteries [20]. The prevalence of carotid artery stenosis 50% or greater increases with age, with prevalence data demonstrating an increase from 1% in people aged 50–59 to as high as 10% in people older than 70[21].
Imaging modalities used to evaluate carotid stenosis include gray-scale and color Doppler ultrasound, MRA, CTA, and catheter angiography (digital subtraction angiography or DSA). Gray-scale and color Doppler ultrasound together allow the evaluation of both structural and physiologic manifestations of vessel disease byimaging plaque in the vessel wall causing stenosis and detecting areas of abnormal blood flowsecondary to the hemodynamic alterations caused by vessel stenosis or occlusion [22]. Limitations of ultrasound include acoustic shadowing from calcified plaque and inability to evaluate the carotid arteries beyond the angle of the jaw due to poor acoustic windows that cannot provide visualization of the vessel [23].
MRA of the neck can be performed with or without contrast (Figure 7). The advantages of both types of MRA include lack of ionizing radiation and lack of exposure to iodinated contrast that occur in CT techniques [23]. Contrast-enhanced and non-contrast MRA neck techniques both have similar sensitivities and specificities in detecting carotid stenosis compared to DSA as a gold standard (average sensitivities 90% and 88% and specificities 77% and 75% respectively) [24]. Noncontrast MRA is limited by its susceptibility to image degradation by turbulence and slow flow. Contrast MRA, which is less likely to be limited by turbulent or slow flow, has been reported to slightly overestimate the degree of stenosis compared to DSA [23].
Figure 7.

Carotid Stenosis on Contrast Enhanced MRA. MR angiography with contrast demonstrates moderate stenosis of the proximal left internal carotid artery due to an ulcerated atherosclerotic plaque (arrow).
CTA is also noninvasive like MRA. It exposes the patient to ionizing radiation and iodinated contrast but has better spatial resolution and is less susceptible to flow-related artifacts than MRA [23]. One study found that the sensitivity and specificity for detection of a 70% to 99% carotid stenosis with CTA were 85% and 93%, respectively [24], compared to DSA. DSA, as mentioned before, is the reference standard in assessing the vasculature and is rarely used in detecting carotid stenosis except when noninvasive methods are not sufficient given its higher risks associated with arterial puncture and catheterization as well as higher patient radiation dose exposures [23].
Besides carotid stenosis in the neck, intracranial atherosclerosis also plays a major role in ischemic stroke. In a study of 4157 patients with stroke or transient ischemic attacks (TIA), 6.5 % of patients had stenosis in their intracranial vessels [25]. Intracranial atherosclerosis is associated with increasing age, with an overall prevalence of over 80% in patients with mean age of 70 years in one study [26]. As is the case of carotid stenosis in the neck, MRA, CTA, DSA, and, in this case, transcranial Doppler (TCD), can also be used to detect and evaluate intracranial atherosclerosis [27]. CTA in particular has been described as a highly accurate test in characterizing intracranial stenosis, with a recent study demonstrating that when compared to the gold standard of DSA, CTA detected large arterial occlusion with 100% sensitivity and specificity, and in cases of greater than 50% arterial stenosis, detected the vascular stenosis with a 97.1% sensitivity and 99.5% specificity [28].
Historically, vascular imaging has focused on the degree of structural luminal narrowing, often used as a surrogate for plaque size. Recent advances in neuroimaging have focused on evaluation of the propensity for plaque rupture, which is a pathophysiologic hallmark of atherosclerotic disease and one of the critical events in vascular thromboembolic disease.For example, recent advances in contrast MRA can reliably evaluate the thickness of the fibrous cap of the plaque, which enhances, and if thinned, can indicate higher probability of rupture. MRA can also identify intra-plaque hemorrhage, another marker that has been associated with development of unstable plaque and resultant symptomatic cerebrovascular disease [29]. It has been recently hypothesized that plaque stability decreases with age, as patients undergoing carotid stenting for atheromatous carotid disease have increased risk of periprocedural plaque rupture and subsequent cerebrovascular events [30]. Pathologic evaluation of atherosclerotic plaques from patients undergoing carotid endarterectomy demonstrated that the histologic features of unstable plaques were more commonly seen in samples taken from elderly patients; plaques harvested from older patients with carotid stenosis demonstrated low smooth muscle content, high numbers of large lipid cores, and larger amounts of calcium deposition [30]. Thus, MR characterization of plaque morphology is an emerging area in the imaging of age-related cerebrovascular disease.
Small vessel cerebrovascular disease
Small vessel infarctions, also referred to as “lacunar” infarctions, are focal areas of ischemia measuring less than 15 mm in the territory supplied by a small penetrating artery [31]. White matter CT or MRI ischemic abnormalities, also known as leukoaraiosis, are thought to be a marker for the presence of small vessel infarctions [32, 33]. The distinction between very small lacunar infarctions and chronic small vessel ischemic change on imaging studies can be challenging with both representing related but distinct entities along a continuous spectrum of microvascular disease. Commonly identified white matter abnormalities are thought to represent the end result of cerebral small vessel disease and have been found to be increased in prevalence with age in large population based studies [34]. Reported prevalence of leukoaraiosis in the elderly ranges up to 90% [35], with the prevalence and severity of leukoaraiosis significantly correlated with age [36]. Interestingly, the presence of leukoaraiosis tends to be associated with the presence of fatty plaques, and not with calcified or mixed plaques [36]. While age is the strongest risk factor for leukoaraiosis, common vascular risk factors of systemic hypertension and smoking are also strongly associated with leukoaraiosis in age-controlled analyses [34]. Thus, biochemical aging of the vascular system in both vasculopathic and chronologically aged individuals is a probable pathophysiologic mediator of white matter disease.
Leukoaraiosis can occur in different forms ranging from punctate lesions to patchy areas to confluent white matter abnormality. Leukoaraiosis has previously been classified by location into two classes: periventricular or adjacent to the ventricles, and deep, located away from the ventricle in the subcortical white matter [37]. Given the confluence of signal abnormality in advanced leukoaraiosis, the distinction between these regions of signal abnormality is somewhat arbitrary [37]. Other schemes have been used to try to classify white matter abnormalities. For example, one study distinguished periventricular and deep lesions based on distance from the ventricles with 1 cm being the cutoff [38]. Another study’s classification scheme takes into account the size of the lesion and the location in order to distinguish the two types of lesions [39]. However, despite multiple attempts at systematic characterization of age-related small vessel ischemic change on neuroimaging studies, no real consensus exists with regards to its classification.
As mentioned, risk factors for small vessel infarctions and leukoaraiosis identified in most epidemiologic studies are similar to those for cerebrovascular disease in general and include aging, hypertension, diabetes, hypercholesterolemia and cigarette smoking [40]. However, given the varying prevalence reported in numerous studies, it has been hypothesized that at least a subset of small vessel infarctions may represent the variable phenotypic manifestation of a unique genetic disorder or set of disorders with variable penetrance or expressivity, and, subsequently, variable functional severity[31]. For example, the presence of small vessel infarctions was associated with lower scores on tests of cognitive function and subjective mental decline in one study [19]. Another study found that in addition to being associated with declining episodic memory and executive function, white matter abnormalities significantly increased over time implying progression of white matter abnormalities detectable on neuroimaging studies may in part parallel the cognitive decline associated with aging [41].
Interestingly, diffusion weighted imaging (DWI), an MR based imaging technique commonly used in the setting of acute ischemia to detect regions of restricted Brownian motion of water molecules in ischemic brain parenchyma, has been used to characterize white matter lesions in leukoaraiosis. In a study comparing patients with leukoaraiosis with normal controls as well as patients with infarctions of varying ages, diffusion coefficients in white matter lesions were similar to those of early chronic ischemia (approximately one month old); moreover, the degree of diffusion restriction reflected the severity of leukoaraiosis [42]. In fact, patients with restricted diffusion consistent with acute infarction had higher levels of leukoaraiosis than those with transient ischemia without radiographically detectable infarction, suggesting that leukoaraiosis may be reflective of a deficiency of cerebrovascular “fitness.” [43].
Diffusion tensor imaging (DTI), a spin-echo-echo-planar imaging sequence commonly used to evaluate white matter tract integrity through the principle of anisotropic movement of water molecules along intact tracts, has also been employed to evaluate changes to white matter tracts as a result of ischemic leukoaraiosis. Areas of leukoaraiosis demonstrated decreased anisotropy in comparison to healthy brain parenchyma in age matched controls [44]. A study evaluating white matter tracts across a wide age range in phenotypically normal individuals determined that particular white matter tracts are prone to age-related decline, particularly in the frontal white matter, corpus callosum, and posterior limb of the internal capsule, suggesting that leukoaraiosis may be a more severe manifestation of age related decline [45]. Moreover, DTI may have potential utility in identifying individuals at risk of functional decline from age, genetic, or lifestyle-associated cerebrovascular senescence.
Functional and Physiologic Neuroimaging
Physiologic or functional neuroimaging is an emerging field within the imaging sciences which attempts to combine spatial and morphologic assessments with real-time evaluation of physiologic function. Perfusion imaging and functional MRI (fMRI) are two such imaging modalities, with particular utility in the determination of local cerebrovascular hemodynamics and metabolic activity, respectively. Perfusion imaging techniques are an active area of investigation, as studies using this imaging modality have generated numerous insights in the diagnosis and staging of cerebrovascular disease. For example, the age-dependent association of stroke and leukoaraiosis may in part be related to decreased cerebral blood flow (CBF) associated with chronologic aging. CTP-derived CBF is independently associated with white matter disease severity [46]. Similar advanced MRI studies confirm that quantification of brain abnormality with advanced techniques may lend insights into small vessel disease which are not detectable on conventional, structural MRI [47].
Perfusion MRI assessments in healthy cognitively intact individuals across the age span have demonstrated decreases in both total and regional blood flow [48, 49]. Interestingly, the regional blood flow deficits identified on arterial spin labeling perfusion MRI were independent of cortical thickness in the associated vascular territory, suggesting that the age-related perfusion deficits may either precede parenchymal atrophy, or may represent a distinct aging hallmark [49]. While there is no consensus as to the specific sites of regional cerebral blood flow derangements that occur with aging, the same study demonstrated that the majority of age-related perfusion deficits were cortical, and that the sub-cortical parenchyma was relatively spared.
Epidemiologic evidence associating dementia and cognitive decline with cardiovascular risk factors has led to further inquiry into the role of age-related perfusion deficits in patients with dementia. A study comparing regional cerebral perfusion in symptomatic Alzheimer’s disease (AD) and sub-cortical ischemic vascular dementia (SIVD) to age-matched cognitively intact patients found that patients with AD and SIVD had marked CBF reductions in the frontal and parietal cortices, independent of parenchymal volume [50]. Interestingly SIVD patients with severe leukoaraiosis showed reduced frontal CBF as well as cortical atrophy in the frontal and parietal lobes.
Thus, the advent of perfusion imaging has led to a further understanding of cerebrovascular physiology in the progression of neurologic disease, as functional perfusion deficits may often precede structural abnormalities currently associated with disease. The variable severity of cerebrovascular disease manifestation in elderly patients has generated interest in understanding predictors of resilience of the vascular tree, or cerebrovascular reserve (CVR) as often described in the literature. CVR is a term used to describe the capacity of the brain to maintain adequate cerebral blood flow in the setting of decreased perfusion pressure or decreased incoming blood volume. CVR can be evaluated by a number of functional imaging techniques [51], with the general principle requiring measurements of cerebral blood flow at baseline and after a vasodilatory challenge such as increased CO2 inhalation or pharmacologic challenge with acetazolamide. Reduced or no increase in CBF after such a vasodilatory challenge implies exhaustion of the cerebrovascular reserve and has been associated with an increased risk of stroke or TIA in a number of prospective studies [52–55]. TCD has been long used as surrogate marker of CVR, with changes in MCA velocity after vasodilatory stimulus (proportional to a patient’s CVR) [56]. More recent techniques have utilized tissue level perfusion measures such as nuclear medicine flow studies [57, 58] as well as CT and MRI perfusion before and after a vasodilatory stimulus (Figure 8).
Figure 8.

Impairment of cerebrovascular reserve. Acetazolamide challenged CT perfusion study. Pre-acetazolamide CBF image is shown in (A) and post-acetazolamide CBF image in (B). There is a severe left middle cerebral artery (LMCA) stenosis. As a consequence there is reduced CBF in the LMCA territory on the baseline image (arrows in panel A). After acetazolamide challenge, there is a normal response, increase in CBF, to acetazolamide on the right side (solid arrows in panel B). The LMCA territory (dashed arrows in in panel B) demonstrates “misery perfusion” in which the CBF further reduces because of impaired CVR.
A variable decline in CVR with respect to CBF could be an important mediator in the development of symptomatic cognitive impairment and dementia with age. A study evaluating the role of cardiovascular risk factors in the progression from mild cognitive impairment (MCI) to AD determined that both carotid intima-media thickness (CIMT) and impaired CVR to hypercapnia (Breath-Holding Test-BHT) were associated with greater odds of developing Alzheimer’s disease at 12 months by neuropsychological testing criteria[59]. Interestingly, an impaired BHT was associated with greater odds of developing dementia than CIMT. When both were present, there was a four-fold increased incidence of dementia in comparison to normal controls. This suggests that decreased CBF associated with proximal vessel stenosis and impaired CVR as measured by hypercapneic reactivity may be two independent risk factors in the development of cerebrovascular disease.
It has been hypothesized that symptomatic cognitive impairment in diseases such as AD may be related to a sequential course of events that begins with chronically reduced CBF that results in mitochondrial dysfunction and oxidative stress, particularly within the cerebrovascular endothelium. The resulting impairment in vasodilatory function often precedes the onset of symptomatic functional deficits, which may represent the manifestation of neuronal and vascular senescence and apoptosis [60]. There is some suggestion that the hypoperfusion-induced microvascular damage may actually initiate the mitochondrial derangements identified in patients with Alzheimer’s disease [60]. A study investigating relative cerebral blood flow (rCBF), CVR, leukoaraiosis, and dementia in hypertensive patients found that the rCBF in hypertensive patients without dementia did not decrease when compared with the normotensive controls, but the rCBF in hypertensive patients with dementia markedly decreased in the cerebral cortices and white matter. On the other hand, CVR in response to hypercapnia declined with the severity of leukoaraiosis, and it decreased most severely in patients with severe leukoaraiosis and dementia. These findings suggest a relationship between cerebrovascular endothelial function measured by CVR and structural manifestations measured by leukoaraiosis in hypertensive patients. Additionally, the findings demonstrate that reduced CVR was associated with dementia in hypertensive patients despite the presence of leukoaraiosis, suggesting that the processes are related but distinct manifestations of cerebrovascular disease, with CVR ultimately related to symptomatic disease [61]. In fact, among patients with known AD, impaired CVR at baseline was associated with the greatest cognitive decline at 12 months along with age and diabetes; however, in multivariate analysis, impaired CVR emerged as the lone significant predictor of severe cognitive decline [62].
Thus, while the CVR impairment in Alzheimer’s disease has significant overlap with cardiovascular disease and age-associated endothelial senescence, there is evidence to suggest that it may represent a pathologic entity unique to the disease. A study comparing vascular profiles of AD patients and age-matched controls demonstrated widespread reduction inCVR in the rostral brain including prefrontal, anterior cingulate, and insular cortices that could not be explained by cardiovascular risk factors [60]. Most notably, the spatial distribution of the CVR deficits differed drastically fromthe regions of CBF deficits, which were found in temporaland parietal cortices. Consistent with prior studies, individuals with greater CVR deficit tended to have a significantly greater volume of leukoaraiosis. These data demonstrated that early AD subjects have evidence of significant forebrain CVR deficits that, while differing from CBF findings, appear to spatially mimic the sites of pathologic and radiographic amyloid deposition [63]. It should be noted however, that further investigation is necessary to characterize the complex interaction between chronic cerebrovascular disease and cognitive decline, as other studies have shown that brain structural changes such as caudate atrophy may have a greater association with cognitive decline relative to other imaging-based metrics assessing cerebrovascular disease such as periventricular white matter signal [64]. Future neuroimaging research should continue to elucidate the complex interplay between chronic cerebrovascular disease and its role in the progression of cognitive decline and dementia.
Conclusion
Recent advances in medical imaging allow detailed and sophisticated evaluation of the brain and intracranial and extracranial vasculature. These tools continue to contribute to our understanding of the multifactorial processes that occur in the aging brain. Continued investigation, especially with newer functional and physiologic imaging techniques, will continue to aid in elucidating the pathophysiologic basis of age-related changes in the brain.
Acknowledgments
This research was made possible by a GE-Radiology Research Academic Fellowship award sponsored by General Electric Medical Systems and the Association of University Radiologists (AUR).
References
- [1].Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- [3].Bousser MG. Stroke prevention: An update. Front Med. 2012;6:22–34. doi: 10.1007/s11684-012-0178-6. [DOI] [PubMed] [Google Scholar]
- [4].Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. American Heart Association prevention conference. IV Prevention and rehabilitation of stroke risk factors. Stroke. 1997;28:1507–17. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- [5].Black-Schaffer RM, Winston C. Age and functional outcome after stroke. Top Stroke Rehabil. 2004;11:23–32. doi: 10.1310/DNJU-9VUH-BXU2-DJYU. [DOI] [PubMed] [Google Scholar]
- [6].Kanekar SG, Zacharia T, Roller R. Imaging of stroke: Part 2, pathophysiology at the molecular and cellular levels and corresponding imaging changes. AJR Am J Roentgenol. 2012;198:63–74. doi: 10.2214/AJR.10.7312. [DOI] [PubMed] [Google Scholar]
- [7].Wardlaw JM, Seymour J, Cairns J, Keir S, Lewis S, Sandercock P. Immediate computed tomography scanning of acute stroke is cost-effective and improves quality of life. Stroke. 2004;35:2477–83. doi: 10.1161/01.STR.0000143453.78005.44. [DOI] [PubMed] [Google Scholar]
- [8].Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet. 2007;369:293–8. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mukherjee S, Raghavan P, Phillips CD. Computed tomography perfusion: Acute stroke and beyond. SeminRoentgenol. 2010;45(2):116–25. doi: 10.1053/j.ro.2009.09.011. [DOI] [PubMed] [Google Scholar]
- [10].Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61:533–43. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- [11].Schellinger PD, Bryan RN, Caplan LR, Detre JA, Edelman RR, Jaigobin C, et al. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2010;75:177–85. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gerriets T, Stolz E, Walberer M, Muller C, Kluge A, Kaps M, et al. Middle cerebral artery occlusion during MR-imaging: Investigation of the hyperacute phase of stroke using a new in-bore occlusion model in rats. Brain Res Brain Res Protoc. 2004;12:137–43. doi: 10.1016/j.brainresprot.2003.08.006. [DOI] [PubMed] [Google Scholar]
- [13].Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: Secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–80. [PubMed] [Google Scholar]
- [14].Wolf PA, D’Agostino RB, O’Neal MA, Sytkowski P, Kase CS, Belanger AJ, et al. Secular trends in stroke incidence and mortality.theframingham study. Stroke. 1992;23:1551–5. doi: 10.1161/01.str.23.11.1551. [DOI] [PubMed] [Google Scholar]
- [15].Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen stroke study. Stroke. 1994;25:808–13. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- [16].Gorelick PB. Status of risk factors for dementia associated with stroke. Stroke. 1997;28:459–63. doi: 10.1161/01.str.28.2.459. [DOI] [PubMed] [Google Scholar]
- [17].Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–62. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- [18].O’Rourke MF. Arterial aging: Pathophysiological principles. Vasc Med. 2007;12:329–41. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- [19].Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam study. Neurology. 1994;44:1246–52. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- [20].Gleason S, Furie KL, Lev MH, O’Donnell J, McMahon PM, Beinfeld MT, et al. Potential influence of acute CT on inpatient costs in patients with ischemic stroke. Acad Radiol. 2001;8:955–64. doi: 10.1016/S1076-6332(03)80639-6. [DOI] [PubMed] [Google Scholar]
- [21].Touze E. Natural history of asymptomatic carotid artery stenosis. Rev Neurol (Paris) 2008;164:793–800. doi: 10.1016/j.neurol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- [22].Tahmasebpour HR, Buckley AR, Cooperberg PL, Fix CH. Sonographic examination of the carotid arteries. Radiographics. 2005;25:1561–75. doi: 10.1148/rg.256045013. [DOI] [PubMed] [Google Scholar]
- [23].Jaff MR, Goldmakher GV, Lev MH, Romero JM. Imaging of the carotid arteries: The role of duplex ultrasonography, magnetic resonance arteriography, and computerized tomographic arteriography. Vasc Med. 2008;13:281–92. doi: 10.1177/1358863X08091971. [DOI] [PubMed] [Google Scholar]
- [24].Nederkoorn PJ, Elgersma OE, van der Graaf Y, Eikelboom BC, Kappelle LJ, Mali WP. Carotid artery stenosis: Accuracy of contrast-enhanced MR angiography for diagnosis. Radiology. 2003;228:677–82. doi: 10.1148/radiol.2283020824. [DOI] [PubMed] [Google Scholar]
- [25].Weimar C, Goertler M, Harms L, Diener HC. Distribution and outcome of symptomatic stenoses and occlusions in patients with acute cerebral ischemia. Arch Neurol. 2006;63:1287–91. doi: 10.1001/archneur.63.9.1287. [DOI] [PubMed] [Google Scholar]
- [26].Bos D, van der Rijk MJ, Geeraedts TE, Hofman A, Krestin GP, Witteman JC, et al. Intracranial carotid artery atherosclerosis: Prevalence and risk factors in the general population. Stroke. 2012;43:1878–84. doi: 10.1161/STROKEAHA.111.648667. [DOI] [PubMed] [Google Scholar]
- [27].McTaggart RA, Jayaraman MV, Haas RA, Feldmann E. Intracranial atherosclerotic disease: Epidemiology, imaging and treatment. Med Health R I. 2009;92:412–4. [PubMed] [Google Scholar]
- [28].Nguyen-Huynh MN, Wintermark M, English J, Lam J, Vittinghoff E, Smith WS, Johnston SC. How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke. 2008;39:1184–8. doi: 10.1161/STROKEAHA.107.502906. [DOI] [PubMed] [Google Scholar]
- [29].Wasserman BA. Advanced contrast-enhanced MRI for looking beyond the lumen to predict stroke: Building a risk profile for carotid plaque. Stroke. 2010;41:S12–6. doi: 10.1161/STROKEAHA.110.596288. [DOI] [PubMed] [Google Scholar]
- [30].van Lammeren GW, Reichmann BL, Moll FL, Bots ML, de Kleijn DP, de Vries JP, et al. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke. 2011;42:2550–5. doi: 10.1161/STROKEAHA.110.607382. [DOI] [PubMed] [Google Scholar]
- [31].Behrouz R, Malek AR, Torbey MT. Small vessel cerebrovascular disease: The past, present, and future. Stroke Res Treat. 2012;2012:839151. doi: 10.1155/2012/839151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].O’Sullivan M. Leukoaraiosis. Pract Neurol. 2008;8:26–38. doi: 10.1136/jnnp.2007.139428. [DOI] [PubMed] [Google Scholar]
- [33].Rost NS, Rahman RM, Biffi A, Smith EE, Kanakis A, Fitzpatrick K, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology. 2010;75:1670–7. doi: 10.1212/WNL.0b013e3181fc279a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: The ARIC study. Neuroepidemiology. 1997;16:149–62. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- [35].de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam scan study. J NeurolNeurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saba L, Sanfilippo R, Pascalis L, Montisci R, Mallarini G. Carotid artery abnormalities and leukoaraiosis in elderly patients: Evaluation with MDCT. AJR Am J Roentgenol. 2009;192:W63–70. doi: 10.2214/AJR.07.3566. [DOI] [PubMed] [Google Scholar]
- [37].Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry. 2008;64:273–80. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–5. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- [40].Fisher CM. Lacunar strokes and infarcts: A review. Neurology. 1982;32:871–6. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- [41].Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, Decarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79:442–8. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Helenius J, Soinne L, Salonen O, Kaste M, Tatlisumak T. Leukoaraiosis, ischemic stroke, and normal white matter on diffusion-weighted MRI. Stroke. 2002;33:45–50. doi: 10.1161/hs0102.101228. [DOI] [PubMed] [Google Scholar]
- [43].Arsava EM, Bayrlee A, Vangel M, Rost NS, Rosand J, Furie KL, et al. Severity of leukoaraiosis determines clinical phenotype after brain infarction. Neurology. 2011;77:55–61. doi: 10.1212/WNL.0b013e318221ad02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jones DK, Lythgoe D, Horsfield MA, Simmons A, Williams SC, Markus HS. Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke. 1999;30:393–7. doi: 10.1161/01.str.30.2.393. [DOI] [PubMed] [Google Scholar]
- [45].Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- [46].Huynh TJ, Murphy B, Pettersen JA, Tu H, Sahlas DJ, Zhang L, et al. CT perfusion quantification of small-vessel ischemic severity. AJNR Am J Neuroradiol. 2008;29:1831–6. doi: 10.3174/ajnr.A1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: A systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–35. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- [48].Stoquart-ElSankari S, Baledent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27:1563–72. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- [49].Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–78. doi: 10.1016/j.neuroimage.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5:454–62. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Eskey CJ, Sanelli PC. Perfusion imaging of cerebrovascular reserve. Neuroimaging Clin N Am. 2005;15:367, 81, xi. doi: 10.1016/j.nic.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [52].Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124:457–67. doi: 10.1093/brain/124.3.457. [DOI] [PubMed] [Google Scholar]
- [53].Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–7. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- [54].Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke. 1999;30:593–8. doi: 10.1161/01.str.30.3.593. [DOI] [PubMed] [Google Scholar]
- [55].Reinhard M, Gerds TA, Grabiak D, Zimmermann PR, Roth M, Guschlbauer B, et al. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol. 2008;255:1182–9. doi: 10.1007/s00415-008-0865-z. [DOI] [PubMed] [Google Scholar]
- [56].Webster MW, Makaroun MS, Steed DL, Smith HA, Johnson DW, Yonas H. Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid artery occlusive disease. J Vasc Surg. 1995;21:338–44. doi: 10.1016/s0741-5214(95)70274-1. [DOI] [PubMed] [Google Scholar]
- [57].Yamamoto KK, Miyata T, Momose T, Nagayoshi M, Akagi D, Hosaka A, et al. Reduced vascular reserve measured by stressed single photon emission computed tomography carries a high risk for stroke in patients with carotid stenosis. Int Angiol. 2006;25:385–8. [PubMed] [Google Scholar]
- [58].Ogasawara K, Ogawa A, Terasaki K, Shimizu H, Tominaga T, Yoshimoto T. Use of cerebrovascular reactivity in patients with symptomatic major cerebral artery occlusion to predict 5-year outcome: Comparison of xenon-133 and iodine-123-IMP single-photon emission computed tomography. J Cereb Blood Flow Metab. 2002;22:1142–8. doi: 10.1097/00004647-200209000-00012. [DOI] [PubMed] [Google Scholar]
- [59].Viticchi G, Falsetti L, Vernieri F, Altamura C, Bartolini M, Luzzi S, et al. Vascular predictors of cognitive decline in patients with mild cognitive impairment. Neurobiol Aging. 2012;33:1127.e1–9. doi: 10.1016/j.neurobiolaging.2011.11.027. [DOI] [PubMed] [Google Scholar]
- [60].Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of azheimer disease. Neurotox Res. 2003;5:491–504. doi: 10.1007/BF03033159. [DOI] [PubMed] [Google Scholar]
- [61].Kuwabara Y, Ichiya Y, Sasaki M, Yoshida T, Fukumura T, Masuda K, et al. Cerebral blood flow and vascular response to hypercapnia in hypertensive patients with leukoaraiosis. Ann Nucl Med. 1996;10:293–8. doi: 10.1007/BF03164735. [DOI] [PubMed] [Google Scholar]
- [62].Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, et al. Cerebrovascular reactivity and cognitive decline in patients with alzheimer disease. Stroke. 2006;37:1010–5. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- [63].Yezhuvath US, Uh J, Cheng Y, Martin-Cook K, Weiner M, Diaz-Arrastia R, et al. Forebrain-dominant deficit in cerebrovascular reactivity in alzheimer’s disease. Neurobiol Aging. 2012;33:75–82. doi: 10.1016/j.neurobiolaging.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Samton JB, Ferrando SJ, Sanelli P, Karimi S, Raiteri V, Barnhill JW. The clock drawing test: Diagnostic, functional, and neuroimaging correlates in older medically ill adults. J Neuropsychiatry ClinNeurosci. 2005;17:533–40. doi: 10.1176/jnp.17.4.533. [DOI] [PubMed] [Google Scholar]


