Abstract
Background
Phosphorylation of the H2AX histone is an early indicator of DNA double-strand breaks and of the resulting DNA damage response. In the present study, we assessed the expression and prognostic significance of γ-H2AX in a cohort of 96 patients with operable non-small cell lung carcinoma.
Methods
Ninety-six paraffin-embedded specimens of non-small cell lung cancer patients were examined. All patients underwent radical thoracic surgery of primary tumor (lobectomy or pneumonectomy) and regional lymph node dissection. γ-H2AX expression was assessed by standard immunohistochemistry. Follow-up was available for all patients; mean duration of follow-up was 27.50 ± 14.07 months (range 0.2–57 months, median 24 months).
Results
Sixty-three patients (65.2%) died during the follow-up period. The mean survival time was 32.2 ± 1.9 months (95% confidence interval [CI]: 28.5–35.8 months; median 30.0 months); 1-, 2- and 3-year survival rates were 86.5% ± 3.5%, 57.3% ± 5.1%, and 37.1% ± 5.4%, respectively. Low γ-H2AX expression was associated with a significantly better survival as compared with those having high γ-H2AX expression (35.3 months for low γ-H2AX expression versus 23.2 months for high γ-H2AX expression, P = 0.009; hazard ratio [HR] 1.95, 95% CI: 1.15–3.30). Further investigation with multivariate Cox proportional hazards regression analysis revealed that high expression of γ-H2AX remained an independent prognostic factor of shorter overall survival (HR 2.15, 95% CI: 1.22–3.79, P = 0.026). A combined p53/γ-H2AX analysis was performed, and we found that the p53 low/γ-H2AX low phenotype was associated with significantly better survival compared with all other phenotypes.
Conclusion
Our study is the first to demonstrate that expression of γ-H2AX detected by immunohistochemistry may represent an independent prognostic indicator of overall survival in patients with non-small cell lung cancer. Further studies are needed to confirm our results.
Keywords: H2AX, DNA damage response, non-small cell lung cancer, p53, prognosis
Introduction
Lung cancer is the most common cause of cancer mortality worldwide for both men and women, causing approximately 1.2 million deaths per year.1 In the United States, there will be an estimated 221,000 new cases of lung cancer and 157,000 deaths in 2011.2 The traditional evaluation of prognosis in non-small cell lung carcinoma (NSCLC) has relied, as in most other malignant tumors, on the stage of disease at the time of clinical presentation. Other factors currently commonly considered include performance status, weight loss, and presence or absence of symptoms at diagnosis, as well as time-honored pathologic parameters, eg, tumor size, tumor differentiation, and histologic subtype.3 However, advances in molecular biology have provided important insights into other potentially significant prognostic biomarkers during the last decade.4 Phosphorylation of the H2AX histone is an early indicator of DNA double-strand breaks (DSB) and of the resulting response (DNA damage response [DDR]).5 H2AX is a histone H2A variant that constitutes 2%–25% of mammalian histone H2A depending on the organism and cell type.5 As a result of DNA DSBs in eukaryotic cells, the serine amino acid at position 139 of the H2AX proteins is phosphorylated in response to DNA damage.5,6 Moreover, the occurrence of a DSB is generally accompanied by the formation of hundreds of histone γ-H2AX (H2AXS139PO4 in humans) molecules in the chromatin flanking the DSB site.7 Both these events can be detected by means of fluorescent-tagged specific antibodies, thus revealing the number of DSB foci within a nucleus and providing valuable information of the DNA damage-response status of the cell.8,9 Detection of γ-H2AX foci has been used as a biomarker for aging and cancer, as a biodosimeter for drug development and radiation exposure, and for clinical trials for cancer chemo- and radiotherapy.6,10 Furthermore, emerging uses for γ-H2AX include detection of toxic environmental agents and of chronic inflammation.11
The aim of the present study was to assess the expression of γ-H2AX in a cohort of 96 consecutive patients with NSCLC and correlate its expression with survival and other clinicopathological parameters. This is, to the best of our knowledge, the first study correlating γ-H2AX expression with survival in patients with NSCLC.
Materials and methods
Study subjects
Paraffin-embedded specimens from 96 consecutive patients with NSCLC were examined. All patients underwent radical thoracic surgery of primary tumor (lobectomy or pneumonectomy), together with regional lymph-node dissection between January 2002 and December 2005 at the Cardiac Surgery Department of Evaggelismos Hospital. Histology reports were issued according to World Health Organization criteria.12 Staging was performed according to the seventh edition of TNM in Lung Cancer.13 Institutional review board approval was obtained to use archived material for research purposes. Patient survival was calculated from the day of surgery until death, in months.
Tissue microarrays
Representative areas from each tumor were chosen in hematoxylin and eosin-stained sections; these areas were subsequently punched out from donor paraff in blocks, in order to construct tissue microar rays (TMAs), using a manual tissue arrayer from Chemicon International Inc (Temecula, CA) (model ATA-100). One to five 2 mm-wide tissue cores from each tumor were chosen. All cases were distributed over eight TMA recipient paraffin blocks, which were incubated at 56°C for 5 minutes, in order for recipient and donor paraffin to stick together.
Immunohistochemistry
Immunostaining of TMAs was performed on 3 μm-thick sections, deparaffinized in xylene and hydrated in a series of graded alcohol dilutions. Slides were boiled in a microwave at 650 W for 20 minutes, immersed in a high-pH target-retrieval solution, (K8004; Dako, Glostrup, Denmark) and subsequently cooled at room temperature for 20 minutes. Endogenous peroxidase activity was blocked by means of the peroxidase-blocking reagent S2001 (K5007; Dako). Immunohistochemistry was performed using the Dako Autostainer Plus device. Sections were incubated with primary antibodies against Ki67 (mouse monoclonal, clone MIB-1; DAKO, 1:100 dilution), cleaved caspase-3 (Asp175) (rabbit monoclonal, clone 5A1 [cell signaling], 1:100 dilution), p53 (mouse monoclonal, clone DO-7; Dako, 1:50 dilution) and phosphohistone H2AX (Ser139) (γ-H2AX) (rabbit monoclonal, clone 20E3 [cell signaling], 1:100 dilution). Slides were then incubated with Envision/HRP for 30 minutes, following which the antigen–antibody complex was visualized using DAB as chromogen for 10 minutes. All sections were lightly counterstained with hematoxylin prior to mounting. All series included both positive (ie, tissues known to express the relevant antigen) and negative (ie, duplicate sections processed as above, apart from omitting incubation with the primary antibody solution) controls.
Immunostained slides were evaluated by estimating the percentage of positive cells per TMA punch. If more than one punch was used from a case, the average percentage was calculated.
For the evaluation of immunoreactivity in tumor cells, a dichotomised scoring system was used as follows: high p53 mutant-protein expression if >20% of tumor cells demonstrated nuclear staining,14 high Ki-67 labeling index if >30% tumor cells were stained,15 high caspase-3 expression if nuclear or cytoplasmic staining was present in >3.22% of tumor cells according to the 75th percentile; high γ-H2AX expression if >21.85% of tumor cells demonstrated nuclear staining according to the 75th percentile.
Statistical analysis
Statistical analysis of the data was performed using SPSS version 19.0 (IBM, Armonk, NY). The Chi-square test was used to evaluate any potential association between the molecular expression and the clinicopathological parameters; odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated by means of simple logistic regression analysis as the measure of the above associations. Multivariate stepwise logistic regression analysis was performed to explore the independent effect of the clinicopathological parameters on the molecular expression. Patients’ overall survival (OS) was measured in months from the day of surgery until death. Survival rates were calculated with the Kaplan–Meier method, and the statistical difference between survival curves was determined with the log-rank test. Multivariate Cox proportional hazards regression analysis, using a backward-selection approach, was performed to explore the independent effect of variables on survival. All tests were two-tailed, and statistical significance was considered to be P < 0.05.
Results
The study included tumors from 96 patients (77 males and 19 females), aged between 36 and 80 years (median age, 66 years). In this cohort, 81 (84.4%) patients were smokers, and 17 (17.7%) patients had FEV1 < 70%. Regarding histology, 42 (43.8%) tumors were adenocarcinomas and 42 (43.8%) were squamous cell carcinomas, while eight (8%) were large-cell carcinomas and four (4%) were undifferentiated carcinomas. Clinicopathological data are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of patients included in the study
| Variables | n | % |
|---|---|---|
| Total number of patients | 96 | |
| Age (years) | ||
| Mean ± standard deviation | 65.64 ± 7.23 | |
| <65 | 36 | 37.5 |
| ≥65 | 60 | 62.5 |
| Sex | ||
| Male | 77 | 80.2 |
| Female | 19 | 19.8 |
| Histology | ||
| Adenocarcinoma | 42 | 43.8 |
| Squamous cell carcinoma | 42 | 43.8 |
| Large-cell carcinoma | 8 | 8.3 |
| Undifferentiated carcinoma | 4 | 4.1 |
| Grade | ||
| Low | 25 | 26 |
| Medium/high | 71 | 74 |
| T status | ||
| T1 | 13 | 13.5 |
| T2 | 65 | 67.7 |
| T3 | 18 | 18.8 |
| Regional lymph-node status | ||
| N0 | 50 | 52.1 |
| N1 | 27 | 28.1 |
| N2 | 19 | 19.8 |
| Stage | ||
| I | 41 | 42.7 |
| II | 22 | 22.9 |
| III | 31 | 32.3 |
| IV | 2 | 2.1 |
| Ploidy | ||
| Diploidy | 78 | 81.3 |
| Aneuploidy | 18 | 18.7 |
| Smoking history | ||
| Yes | 81 | 84.4 |
| No | 15 | 15.6 |
| Adjuvant treatment | ||
| Chemotherapy-adjuvant | 72 | 75.0 |
| Radiotherapy-adjuvant | 41 | 42.7 |
| Blood-vessel infiltration | 41 | 42.7 |
| Lymphatic infiltration | 21 | 21.9 |
| High expression of p53 | 55 | 57.3 |
| High expression of caspase-3 | 24 | 25.0 |
| High expression of γ-H2AX | 25 | 26.0 |
| High Ki67 labeling index | 29 | 30.2 |
High expression of all antigens assessed was found as follows: γ-H2AX in 25 (26.0%) cases, p53 in 55 (57.3%) cases, caspase-3 in 24 (25.0%) cases, and Ki67 in 29 (30.2%) cases.
Correlation between immunohistochemical profile of tumors and clinical data
High levels of γ-H2AX were significantly correlated with T3 tumors (44.4% vs 21.8%, P = 0.048; OR 2.87, 95% CI: 1.00–8.40), disease stage III/IV (42.4% vs 17.5%, P = 0.008; OR 3.48, 95% CI: 1.35–8.99), and lymphatic infiltration (42.9% vs 21.3%, P = 0.047; OR 2.77, 95% CI: 1.00–7.71). No significant association was found between γ-H2AX expression patterns and caspase 3 (P = 0.081), p53 (P = 0.208), N stage (P = 0.075), and Ki67 percentage (P = 0.081).
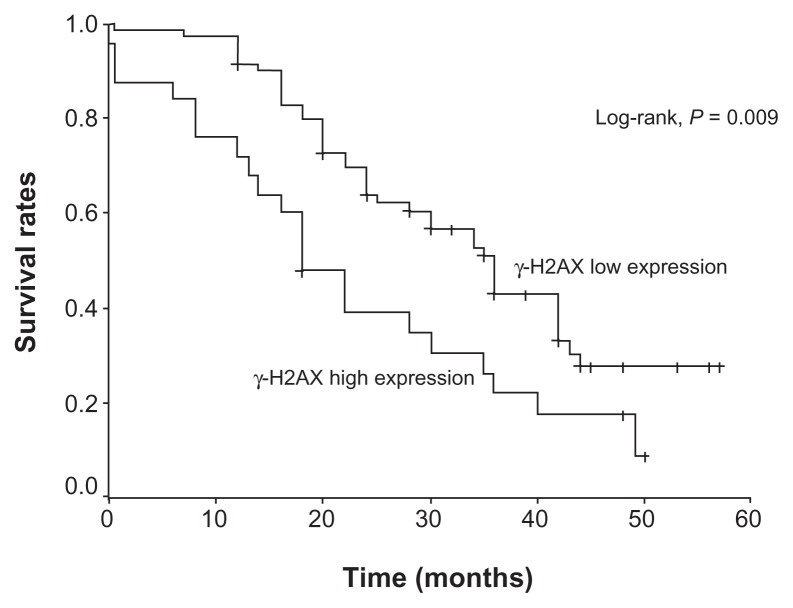
Follow-up was available for all patients; mean duration of follow-up was 27.50 ± 14.07 months (range 0.2–57 months, median 24 months). Sixty-three patients (65.2%) died during follow-up. The mean survival time was 32.2 ± 1.9 months (95% CI: 28.5–35.8 months, median 30.0 months); 1-, 2-, 3-, and 4-year survival rates were 86.5% ± 3.5%, 57.3% ± 5.1%, 37.1% ± 5.4%, and 25.4% ± 5.2%, respectively. A significant association between high γ-H2AX levels and a shorter survival was detected (23.2 months vs 35.3 months, P = 0.009; hazard ratio [HR] 1.95, 95% CI: 1.15–3.30) (Figure 1). Other factors significantly associated with overall survival are listed in Table 2.
Figure 1.
Overall survival of patients with non-small cell lung carcinoma in relation to γ-H2AX expression.
Table 2.
Survival analysis according to clinicopathologic and histologic parameters
| Variables | Mean survival time (95% CI), months | Survival rate (%) | P-value | |
|---|---|---|---|---|
|
|
||||
| 2-year | 3-year | |||
| Age | 0.377 | |||
| <65 years | 30.2 (24.4–36.0) | 50.0 | 33.9 | |
| ≥65 years | 33.3 (28.6–37.9) | 62.1 | 39.1 | |
| Sex | 0.019 | |||
| Male | 30.0 (26.0–33.9) | 52.0 | 30.0 | |
| Female | 41.5 (33.7–49.3) | 79.0 | 65.1 | |
| Histology | ||||
| Adenocarcinoma | 37.2 (31.8–42.5) | 73.1 | 46.0 | 0.008 |
| Squamous cell carcinoma | 28.1 (23.8–32.4) | 49.0 | 32.1 | |
| Large-cell and undifferentiated carcinomas | 24.6 (13.3–35.9) | 33.3 | 25.0 | |
| T | 0.146 | |||
| T1–T2 | 33.6 (29.6–37.6) | 63.5 | 40.0 | |
| T3 | 20.0 (16.0–24.0) | 29.6 | 23.7 | |
| N | <0.001 | |||
| N0–N1 | 35.6 (31.5–39.7) | 66.7 | 46.5 | |
| N2 | 18.4 (14.4–22.4) | 18.4 | 0.0 | |
| Stage | <0.001 | |||
| I–II | 37.9 (33.7–42.1) | 74.1 | 51.0 | |
| III–IV | 19.9 (15.8–24.0) | 23.8 | 7.9 | |
| Grade | 0.005 | |||
| Low | 23.3 (17.6–28.9) | 36.0 | 20.6 | |
| Medium/high | 35.2 (31.0–39.4) | 65.0 | 43.0 | |
| p53 | 0.003 | |||
| High | 38.7 (33.2–44.2) | 74.8 | 51.2 | |
| Low | 27.1 (22.7–31.6) | 44.1 | 26.4 | |
| Caspase 3 | 0.650 | |||
| Low | 32.5 (28.2–36.8) | 58.7 | 39.3 | |
| High | 30.7 (23.8–37.6) | 52.9 | 29.8 | |
| γ-H2AX | 0.009 | |||
| Low | 35.3 (31.2–39.4) | 63.8 | 42.8 | |
| High | 23.2 (16.8–29.6) | 39.3 | 21.8 | |
| Ki67 labeling index | 0.018 | |||
| Low | 35.0 (30.5–39.4) | 64.9 | 44.9 | |
| High | 26.4 (20.5–32.3) | 39.7 | 19.8 | |
| Blood-vessel infiltration | 0.104 | |||
| No | 34.7 (29.9–39.6) | 62.6 | 41.1 | |
| Yes | 27.5 (22.9–32.1) | 50.3 | 31.4 | |
| Lymphatic infiltration | 0.009 | |||
| No | 34.7 (30.6–38.7) | 62.7 | 40.7 | |
| Yes | 22.5 (16.0–29.0) | 38.1 | 23.8 | |
| Chemotherapy-adjuvant | 0.440 | |||
| No | 34.0 (25.1–42.9) | 57.6 | 46.2 | |
| Yes | 31.8 (27.9–35.7) | 57.1 | 34.4 | |
| Radiotherapy-adjuvant | <0.001 | |||
| No | 37.5 (32.9–42.2) | 74.3 | 45.7 | |
| Yes | 23.5 (19.2–27.9) | 33.1 | 24.0 | |
Abbreviation: CI, confidence interval.
Further investigation with multivariate Cox proportional hazards regression analysis revealed that squamous cell carcinomas (HR 1.95, 95% CI: 1.22–3.79; P = 0.016), N2 (HR 3.80, 95% CI: 2.01–7.17; P < 0.001), low-grade tumors (HR 2.10, 95% CI: 1.21–3.65, P = 0.009), high expression of p53 (HR 1.74, 95% CI: 1.01–3.00, P = 0.047) and high γ-H2AX expression (HR 2.15, 95% CI: 1.22–3.79; P = 0.026) remained independent prognostic factors of a shorter OS.
Combined p53 and γ-H2AX analysis
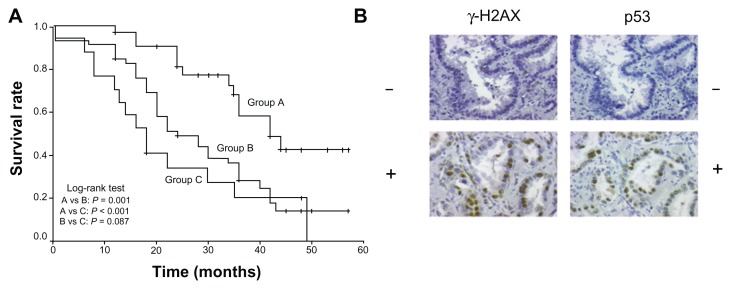
Tumors were divided into three groups according to expression of p53 and γ-H2AX. Group A (n = 33) tumors had both p53 and γ-H2AX low expression levels; group B (n = 46) tumors had either p53 high/γ-H2AX low or p53 low/γ-H2AX high expression levels; group C (n = 17) tumors had both p53 and γ-H2AX high expression levels. A significant association between different p53/γ-H2AX patterns with survival was observed with the p53 low/γ-H2AX low pattern having a statistically better outcome compared with all the other combinations (P = 0.001) (Figure 2A and B).
Figure 2.
(A) Overall survival of patients with non-small cell lung carcinoma in relation to coexpression of p53 and γ-H2AX. Group A: p53 and γ-H2AX low-expression tumors. Group B: p53 high-/γ-H2AX low- or p53 low-/γ-H2AX high-expression tumors. Group C: p53 and γ-H2AX high-expression tumors. (B) Representative serial sections of adenocarcinomas immunostained for γ-H2AX and p53.
Discussion
Before genomic instability and malignant conversion, human cells activate a DDR network in correlation with DNA damage. In later stages of DDR, the γ-H2AX foci become larger, and presumably a higher concentration of repair proteins can be measured. The mechanism that explains our results regarding H2AX and p53 should be interpreted under the light of the following mechanism that connects the DDR with p53: the response to DNA damage results in either cell-cycle arrest to allow the lesions to be repaired or apoptosis; p53 is essential in both pathways. Specifically, in cell-cycle arrest at the G1 phase, p53 enhances p21 transcription, which in turn inhibits cyclin-dependent kinase activity. This prevents pRb from depressing E2F1, inhibiting progression from G1 to S phase.16,17 Another way in which p21 leads to G1 arrest is by binding to proliferating cell nuclear antigen and inhibiting its function in replication.18 The loss of p21 results in replication defects and may lead to cell death after treatment with anticancer drugs.19,20 Progression to the S phase after p53/p21-mediated arrest is achieved by the proteosome-dependent degradation of p21;21 p21 is a known inhibitor of apoptosis, and its degradation can lead to cell death.19,22 Moreover, it has been shown to inhibit caspase-3, which is a crucial component of apoptotic cell death and therefore it was selected to be measured in our study.23 γ-H2AX levels may reflect endogenous genomic instability in tissues. Increasing staining levels of γ-H2AX in our study were associated with unfavorable risk factors like tumor stage, tumor grade, lymphatic infiltration, and shortened OS, respectively. The present study is the first to demonstrate a significant association between high γ-H2AX expression levels and decreased OS in patients with NSCLC. Additionally, tumors having both low γ-H2AX levels and low p53 expression levels were statistically associated with a better OS in comparison to patients that had both high γ-H2AX and high p53 expression levels: we cannot suggest a plausible explanation for this association. However, low γ-H2AX expression levels may be indicative of a slowly proliferating, less aggressive tumor phenotype with good prognostic features. Such tumors possibly provide enough time to the DNA repair machinery, which thus requires low H2AX levels. On the contrary, high γ-H2AX expression levels may indicate a more aggressive, highly proliferating tumor phenotype accumulating massive DNA defects and thus having a worse prognosis.
In the combined p53/γ-H2AX analysis, we found that the p53 low/γ-H2AX low phenotype was associated with a significantly better outcome compared with the other expression patterns. This finding is consistent with the previously reported roles of both proteins. Immunohistochemical detection of p53 is mostly indicative of the presence of the mutant, more stable p53 protein, associated with a rather defective DNA repair mechanism.24,25 On the contrary, absence of p53 immunohistochemical detection is – most of the time – relevant to the presence of the wild-type p53 protein; hence the association of low p53/low γ-H2AX expression pattern with a better overall survival. This theory is in accordance with the model of Halazonetis et al, who propose a barrier to tumorigenesis predominated by the apoptotic index and characterized by low levels of activation of the DDR system at normal tissues, a peak at precancerous lesions and sustained high levels at tumors.26 The link between p53 and γ-H2AX has already been proposed by Brunner et al showing that increased expression levels of γ-H2AX are associated with bad prognostic features in endometrial carcinomas.27 Nagelkerke et al also found that in triple-negative breast carcinomas, a high number of γ-H2AX foci indicated a significantly worse prognosis.28 The observed associations between high γ-H2AX expression and parameters, such as tumor size, stage, lymphatic invasion, and prognosis, in our study are in keeping with those reported in these studies.24,25 The correlation between OS and other tumor parameters such as stage, Ki67, and p53 are also in keeping with those already demonstrated by other investigators in NSCLC and are not commented upon.29,30
In conclusion, our study is the first to demonstrate that overexpression of γ-H2AX may represent an independent prognostic indicator of worse OS in patients with non-small cell lung carcinoma. Further studies in a larger cohort of patients are needed to confirm our results and establish a prognostic role for this protein, which represents a major component of the DNA repair machinery.
Acknowledgments
The authors thank Mr Konstantinos Savvatakis for his excellent technical assistance. This work has been partly supported by the program 70/3/9326 of the Special Research Fund (ELKE) of the National and Kapodistrian University of Athens. DM developed the study proposal, designed and supervised the analyses, participated in the interpretation of the results, developed the tables and figures, and wrote the manuscript. PGF participated in the design and supervision of the study. PH identified eligible patients, and provided the cohort of patients for the analysis. PGF, MK, IGP, EP, and PK issued pathological reports of the surgical specimens, assessed immunostained sections, and developed the figures. GT did the statistical analyses. DB and EC made corrections and supervised the study. SK participated in the interpretation of the results, corrected the manuscript and supervised the study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Hoang T, Xu R, Schiller JH, Bonomi P, Johnson DH. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–183. doi: 10.1200/JCO.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Felip E, Garcia-Campelo R, Balaña C. The biology of non-small-cell lung cancer: identifying new targets for rational therapy. Lung Cancer. 2004;46:135–148. doi: 10.1016/j.lungcan.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Redon C, Pilch DR, Rogakou E, Sedelnikova OA, Newrock K, Bonner WM. Histone H2A variants h2ax and h2az. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 6.Dickey JS, Redon CE, Nakamura AJ, Baird BJ, Sedelnikova OA, Bonner WM. H2AX: functional roles and potential applications. Chromosoma. 2009;118:683–692. doi: 10.1007/s00412-009-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podhorecka M, Skladanowski A, Bozko P. H2AX phosphorylation: its role in DNA damage response and cancer therapy. J Nucleic Acids. doi: 10.4061/2010/920161. Epub August 3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redon CE, Nakamura AJ, Martin OA, Parekh PR, Weyemi US, Bonner WM. Recent developments in the use of γ-H2AX as a quantitative DNA double-strand break biomarker. Aging (Albany N Y) 2011;3:168–174. doi: 10.18632/aging.100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HP, Singer B. Recursive Partitioning in the Health Sciences. New York: Springer; 1999. [Google Scholar]
- 13.Goldstraw P. The 7th edition of TNM in Lung Cancer: what now? J Thorac Oncol. 2009;4:671–673. doi: 10.1097/JTO.0b013e31819e7814. [DOI] [PubMed] [Google Scholar]
- 14.Giatromanolaki A, Koukourakis MI, Kakolyris S, et al. Vascular endothelial growth factor, wild-type p53, and angiogenesis in early operable non-small cell lung cancer. Clin Cancer Res. 1998;4:3017–3024. [PubMed] [Google Scholar]
- 15.Hommura F, Dosaka-Akita H, Mishina T, et al. Prognostic significance of p27 KIP1 protein and ki-67 growth fraction in non-small cell lung cancers. Clin Cancer Res. 2000;6:4073–4081. [PubMed] [Google Scholar]
- 16.Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol. 2001;13:738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 17.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 18.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Lozano G. p21 Stability: linking chaperones to a cell cycle checkpoint. Cancer Cell. 2005;7:113–114. doi: 10.1016/j.ccr.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Gottifredi V, McKinney K, Poyurovsky MV, Prives C. Decreased p21 levels are required for efficient restart of DNA synthesis after S phase block. J Biol Chem. 2004;279:5802–5810. doi: 10.1074/jbc.M310373200. [DOI] [PubMed] [Google Scholar]
- 22.Tian H, Wittmack EK, Jorgensen TJ. p21 WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 2000;60:679–684. [PubMed] [Google Scholar]
- 23.Suzuki A, Tsutomi Y, Miura M, Akahane K. Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP and inactivation machinery by p21. Oncogene. 1999;18:1239–1244. doi: 10.1038/sj.onc.1202409. [DOI] [PubMed] [Google Scholar]
- 24.Ishida H, Irie K, Itoh T, Furukawa T, Tokunaga O. The prognostic significance of p53 and bcl-2 expression in lung adenocarcinoma and its correlation with Ki-67 growth fraction. Cancer. 1997;15:1034–1045. [PubMed] [Google Scholar]
- 25.Gorgoulis VG, Zacharatos P, Kotsinas A, et al. Altered expression of the cell cycle regulatory molecules pRb, p53 and MDM2 exert a synergetic effect on tumor growth and chromosomal instability in non-small cell lung carcinomas (NSCLCs) Mol Med. 2000;6:208–237. [PMC free article] [PubMed] [Google Scholar]
- 26.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 27.Brunner AH, Hinterholzer S, Riss P, Heinze G, Weiss K, Brustmann H. Expression of γ-H2AX in endometrial carcinomas: an immunohistochemical study with p53. Gynecol Oncol. 2011;121:206–211. doi: 10.1016/j.ygyno.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 28.Nagelkerke A, van Kuijk SJ, Sweep FC, et al. Constitutive expression of γ-H2AX has prognostic relevance in triple negative breast cancer. Radiother Oncol. 2011;101:39–45. doi: 10.1016/j.radonc.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol. 2011;3:127–138. doi: 10.1177/1758834011401951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]