Abstract
Background
Secondhand smoke causes cardiovascular and respiratory disease. Smokefree legislation is associated with a lower risk of hospitalization and death from these diseases.
Methods and Results
Random effects meta-analysis was conducted by law comprehensiveness to determine the relationship between smokefree legislation and hospital admission or death from cardiac, cerebrovascular, and respiratory diseases. Studies were identified using a systematic search for studies published before November 30, 2011 using Science Citation Index, Google Scholar, PubMed, and Embase and references in identified papers. Change in hospital admissions (or deaths) in the presence of a smokefree law, duration of follow-up, and law comprehensiveness (workplaces only; workplaces and restaurants; or workplaces, restaurants, and bars) were recorded. Forty-five studies of 33 smokefree laws with median follow-up of 24 months (range 2–57 months) were included. Comprehensive smokefree legislation was associated with significantly lower rates of hospital admissions (or deaths) for all 4 diagnostic groups: coronary events (RR .848, 95% CI .816–.881), other heart disease (RR .610, 95% CI .440–.847), cerebrovascular accidents (RR .840, 95% CI .753–.936), and respiratory disease (RR .760, 95% CI .682–.846). The difference in risk following comprehensive smokefree laws does not change with longer follow-up. More comprehensive laws were associated with larger changes in risk.
Conclusions
Smokefree legislation was associated with a lower risk of smoking-related cardiac, cerebrovascular, and respiratory diseases, with more comprehensive laws associated with greater changes in risk.
Keywords: myocardial infarction, stroke, lung, health care policy, tobacco
Secondhand smoke causes cardiovascular, respiratory, and neoplastic disease in adults, adverse reproductive outcomes in women, and delayed growth and respiratory and infectious disease in children.1–3 Smokefree legislation, which prohibits smoking in certain settings, reduces exposure of nonsmokers to secondhand smoke and creates an environment that helps smokers cut down or quit smoking.4, 5 Because of the large and rapid effects of secondhand smoke on the cardiovascular system,3, 6 these laws would be expected to lead to reductions in acute myocardial infarctions (AMI) and other cardiac events. Because it is impossible to do a randomized controlled trial of a large scale public policy interventions such as a smokefree law, these laws are studied using interrupted time series analysis, in which one estimates changes following the law, typically after accounting for pre-existing time trends (often including seasonal variation) and other factors.7 Three prior meta-analyses of the literature confirmed that smokefree laws were followed by immediate reductions in AMI8, 9 and other cardiac 10 hospitalizations and that effects grew over time. The number of studies on the effect of smokefree laws has rapidly grown since these earlier meta-analyses to include not only AMI but also non-AMI cardiac disease, cerebrovascular accidents, and respiratory disease. These new reports add extended follow-up periods, new study populations and locations, and smokefree laws with varying degrees of comprehensiveness (i.e., workplaces only; workplaces and restaurants only; or workplaces, restaurants, and bars). This paper presents a meta-analysis of these new outcomes, including assessment of a dose-response effect of the comprehensiveness of the laws.
METHODS
Study Identification
Study identification occurred from October 1, 2011 through November 30, 2011. Because there was already an identified literature in this area, we began our search for new studies by using Science Citation Index, Google Scholar and PubMed to identify publications that cited the paper that first reported a drop in AMI after implementation of a smokefree law in Helena, Montana,11 three recent meta-analyses of AMI or other cardiac outcomes,8–10 and the first paper identifying a reduction in respiratory (asthma) emergency admissions after a smokefree law.12 We also searched PubMed and Embase using search terms “smoking ban,” or “smoke-free” or “smokefree” with “legislation” or “law” or “ordinance” with “acute myocardial infarction,” “heart attack,” “asthma,” “respiratory,” “pulmonary,” and “stroke.” Reference lists were reviewed for all papers located as well as for the Institute of Medicine report Secondhand Smoke Exposure and Cardiovascular Effects,3 and the Cochrane review, “Legislative smoking bans for reducing secondhand smoke exposure, smoking prevalence and tobacco consumption.”4 Finally, we identified relevant reports written by state public health departments and independent researchers through contacts in the tobacco control network. One non-English study13 was translated from French using Google Translate.
We identified 47 studies: 36 peer-reviewed publications,11, 12, 14–47 7 abstracts,48–54 1 presentation,13 and 3 reports by state health departments.55–57 These studies cover 37 different smokefree laws (10 national, 12 state, and 15 local).
We included studies examining the association between smokefree laws and cardiovascular or respiratory hospitalizations or deaths with sufficient data to calculate the relative risk and confidence interval before and after or, in two studies,27, 34 localities with and without a law. Two of the 47 studies were excluded because they did not meet these inclusion criteria. One tobacco industry-supported paper41 comparing trends in AMI death rates in six US states that passed state laws was conducted using nonstandard methodology that did not report or present data that permitted estimating relative risk and confidence intervals. In addition, the analysis was based on a very small number of data points, had very low power to detect changes, and did not account for the presence of a large number of comprehensive local laws in two states (California and New York), all of which bias the results to the null. An abstract53 based a Malta study was excluded because of discrepancies between the results reported in text and the figure that could not be resolved; we contacted the authors who reported they had not completed a manuscript based on the abstract.
Three studies performed separate analyses of reductions in hospitalizations following state laws on localities with no prior law versus localities with existing laws.18, 32, 35 In this situation, we only used the estimates from localities without prior laws only to capture the full effect of the state law. One result for stroke from the New York State study18 was excluded because no information was available from localities without prior laws; other results from this study were included in our analysis.
Because the risk of coronary heart disease due to smoking decreases with age,58 in the seven studies that stratified results on age,14, 20, 21, 26, 32, 36, 50 we used the results for 65 years and younger (or the nearest alternative) for the primary meta-analysis.
For studies that presented estimates for diseases nested within diagnostic categories (e.g. AMI and unstable angina classified under acute coronary syndrome),14, 44, 47 we used the most disaggregated level of data.
For studies that provide multiple estimates of the change in hospitalization rates for different time periods after law implementation,15, 17, 23, 28, 38, 42 we used the estimate from the longest follow-up period to prevent double-counting in the meta-analysis. Separately, we performed a metaregression to test whether hospitalization rates changed over time following implementation of the law; in this case, we included all available estimates from various time points. For this regression, when a law was phased in13, 29, 54 (with restaurant or bar provisions typically taking effect after workplace restrictions), we used only the first implementation phase so that the post-implementation period and risk change associated with the law was measured consistently from the “no law” condition.
After screening all studies and excluding those with missing or incomplete data and those that did not meet inclusion criteria, 43 papers 11–40, 42–52, 54–57 were selected for meta-analysis (Supplementary Tables 1–5, Supplementary Figure 1). The outcomes are AMI, acute coronary syndrome (ACS), acute coronary events (ACE), ischemic heart disease (IHD), angina, coronary heart disease (CHD), sudden cardiac death (SCD), stroke, transient ischemic attack (TIA), chronic obstructive pulmonary disease (COPD), asthma, lung infections, and spontaneous pneumothorax.
Median pre-legislation time was 29.5 months (range 3–99 months); median follow-up time was 24 months (range 2–57 months) (Supplementary Table 6). Laws were categorized based on comprehensiveness: 1) laws applying only to workplaces, 2) workplaces and restaurants, and 3) workplaces, restaurants, and bars. Since many studies looked at more than one law or one disease outcome or stratified results by age or gender, our review collectively yielded 86 risk estimates for the meta-analysis.
Estimates of Risk Reductions Following Laws
Relative risks are estimated taking “no law” as the reference condition. Thirteen studies11, 13, 16, 29, 35, 37, 38, 44, 49, 51, 52, 55, 56 reported changes in absolute number or rates of disease events rather than the relative risk following implementation of a smokefree law. For these, we used the frequency data published in the paper or obtained by contacting the authors to estimate incidence rate reduction (as an estimate of relative risk) using negative binomial regressions. Models included the effect of the law and, when applicable, seasonality, or they were structured to mirror the analysis in the published study (as detailed in Supplementary Tables 1–4). Thirty-one of the 43 papers accounted for long-term secular trends, 26 by including time as a variable in the analysis and 5 by doing time-matched comparisons with control communities. Nineteen of the papers included seasonality in their models.
Analysis
All analyses were conducted using two-sided tests with a significance level of α = 0.05.
Q tests revealed statistically significant heterogeneity (p<0.001) between studies for all outcomes except for acute coronary events (2 studies20, 50 with borderline heterogeneity, p=.067). To account for this heterogeneity and to employ a more conservative approach, we performed a random effects meta-analysis for each outcome, stratified by comprehensiveness of laws using Stata 10.1 or 9.2 metan.
We performed a random effects metaregression (Stata metareg) with dummy variables for the 13 disease outcomes to determine whether they were similar enough to be grouped into diagnostic categories for further analysis. The regressions (Supplementary Table 7) showed no significant differences between hospital admissions or deaths for:
Coronary events: AMI, ACS, ACE, IHD
Other heart disease: Angina, CHD, and out-of-hospital SCD
Cerebrovascular accident: Stroke and TIA
Respiratory disease: COPD, asthma, lung infection, and spontaneous pneumothorax
We performed analyses for these 4 diagnostic groups as well as the 13 individual outcomes.
We conducted a random effects metaregression to test whether the risk reduction following smokefree laws increased over time, as previously reported,8–10 for each outcome and each diagnostic group. For each study, the duration of follow-up post-legislation was used as the time measure.
To test whether the comprehensiveness of a law was associated with greater reductions in hospital admissions (or deaths in 6 cases14, 20, 24, 32, 50, 54), we performed a random effects metaregression with comprehensiveness of law as an ordinal variable (0 for workplaces only; 1 for workplaces and restaurants; 2 for workplaces, restaurants and bars) including dummy variables for different outcomes.
We conducted a separate random effects meta-analysis for older people that were excluded from the primary meta-analysis, using results from six studies14, 20, 26, 32, 36, 50 that reported the risk of coronary events in older populations (median cutoff age 70, range 60–75, Supplementary Tables 1–4).
For 10 studies21, 23–26, 30, 32, 36, 42, 43 that presented results from gender-stratified analyses, we also conducted meta-analyses for females and males.
Finally, to test for the possibility of publication bias in the meta-analysis, we performed Egger’s test, examined a funnel plot (using Stata metafunnel), and conducted a Duval and Tweedie59 nonparametric trim and fill to estimate the effects of any publication bias (using Stata metatrim for a random effects meta-analysis).
RESULTS
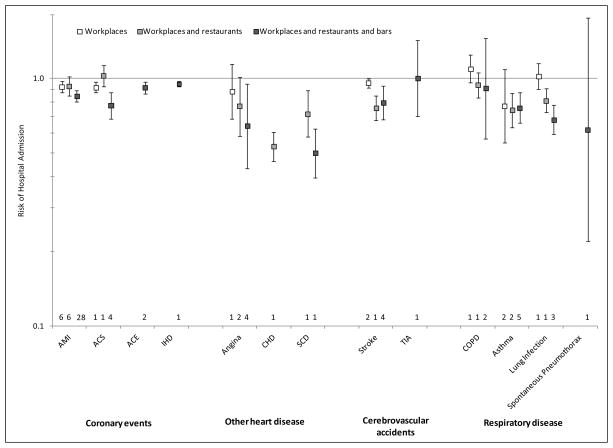
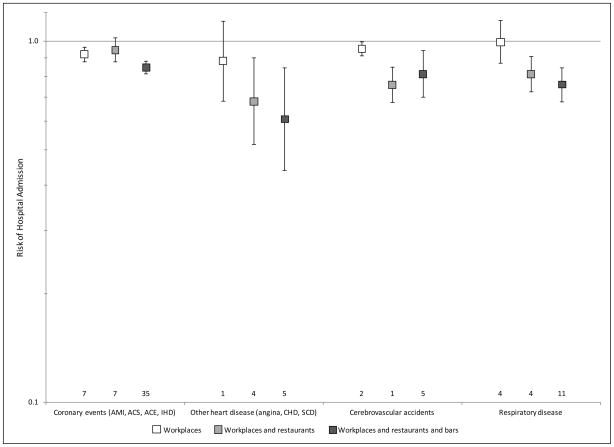
Comprehensive smokefree laws were followed by significant reductions in hospital admissions for AMI, ACS, ACE, IHD, angina, CHD, SCD, stroke, asthma, and lung infection but not TIA, COPD, or spontaneous pneumothorax (Figure 1). Because there were only a few studies for some of these specific outcomes, we also pooled specific outcomes into 4 diagnostic groups as described in Methods in order to increase the number of studies in each group; comprehensive smokefree laws were followed by significant reductions in hospital admissions for all four diagnostic groups (Figure 2 and Supplementary Figures 2–5).
Figure 1.
Relative risk of hospital admissions for various conditions (except sudden cardiac death [SCD], which is defined as out-of-hospital deaths) after implementation of a smokefree law compared to before the law was implemented. Error bars indicate 95% confidence intervals, and numbers above the horizontal axis indicate number of studies used to compute the estimate.
Figure 2.
Relative risk of hospital admissions for various disease categories after implementation of a smokefree law compared to before the law was implemented. Error bars indicate 95% confidence intervals, and numbers above the horizontal axis indicate number of studies used to compute the estimates.
ers above the horizontal axis indicate number of studies used to compute the estimates.
There was an overall pattern of more comprehensive laws being associated with greater reductions in hospital admissions (p=.001 for individual outcomes, Figure 1, and p=.002 for disease groups, Figure 2).
Contrary to previous findings,8–10 we did not find that the AMI risk reduction associated with smokefree laws increased with time (p=.537, Supplementary Figure 6) or other disease outcomes and diagnostic groups for which there was sufficient data to conduct this analysis (p>.318 for all of them).
Consistent with the fact that the relative risk of coronary heart disease due to smoking declines with age, 58 there was no significant change in risk of AMI or coronary events among older patients14, 20, 26, 32, 36, 50 following a comprehensive smokefree law (RR .973, 95% CI .918–1.032 and RR .980, 95% CI .953–1.008, respectively).
Reductions in AMI hospitalizations were similar for females (RR .897; 95% CI .847–.950) and males (RR .912, 95% CI .872–.955) following smokefree laws of all degrees of comprehensiveness).
While Egger’s test was statistically significant for publication bias (p=.007) and the funnel plot suggested possible publication bias among the papers selected for the meta-analysis (Supplementary Figure 7), the nonparametric trim and fill estimate of the effects of publication bias59 produced essentially the same results as the meta-analysis of the published studies: RR .839 (95% CI .818–.861) for actual studies vs. RR .829 (95% CI .808–.851) from the fill and trim analysis for all outcomes and RR .846 (95% CI .803–.890) vs. RR .803 (95% CI .764–.84.) for studies of AMI following comprehensive laws, suggesting that publication bias is not likely to explain our findings.
DISCUSSION
Given that secondhand smoke has been established to cause cardiovascular and respiratory disease,1–3 one would expect that hospitalization for these disease would drop when exposure to secondhand smoke is substantially reduced or eliminated. Consistent with three prior meta-analyses8–10 that concluded that smokefree laws are associated with significant decreases in AMI and other cardiac hospital admissions, we found that comprehensive smokefree laws (covering workplaces, restaurants, and bars) were associated with a 15% decrease in AMI hospitalizations. In addition, we found that the laws were followed by decreases in hospitalizations for ACS, ACE, IHD, angina, CHD, SCD, stroke, asthma, and lung infection (Figure 1), as well as decreased risk of hospitalizations for coronary events, other heart disease, cerebrovascular accident, and respiratory disease (Figure 2). For TIA, COPD, and spontaneous pneumothorax, which demonstrated no statistically significant association, negative findings should be interpreted cautiously because of the small numbers of studies that examined these outcomes.
Based on a much larger evidence base than prior meta-analyses,8–10 we did not find that the reduction in risk associated with these laws increased with longer follow-up.
We also found evidence of a dose-response, with more comprehensive laws being associated with larger effects (Figures 1 and 2).
Our results are consistent with an earlier meta-analysis of stroke associated with secondhand smoke exposure quantified in individuals, which showed an overall risk of 1.25 (95% CI 1.12–1.38) and a nonlinear dose response.60 This overall risk is consistent with the reductions in hospital admissions for stroke that we observed following smokefree laws (RR .795; 95% CI .680, .930 [Figure 1], corresponding to risk increases associated with secondhand smoke of RR 1.26; 95% CI 1.08–1.47).
Several studies included in the meta-analysis documented reductions in health care costs associated with fewer hospitalizations for cardiovascular or respiratory diseases. Health care savings were reported at the city, state, and national levels, ranging from $302,000 in AMI expenses after 35 months in Starkville, Mississippi55 to €2.6 million ($3.3 million, 9.6% decrease from baseline) in angina-related hospitalization costs and €5.3 million ($6.9 million, 20.1% decrease from baseline) AMI-related hospitalization costs during the first year after smokefree law implementation in Germany.46 (See Supplementary Tables 1–4 for more details.)
Evidence on the association between smokefree legislation and other health effects is emerging. A study in Ireland61 found a drop in pre-term births (OR 0.75; 95% CI .59–.96) but an increase in low birthweight (OR 1.43; 1.10, 1.85) one year after the smokefree law. Another study from Scotland62 found a significant decreases in babies small for gestational age (by 4.5%), preterm delivery (11.7%), and spontaneous preterm labor (11.4%).
Smokefree legislation per se does not produce the effects that we observed, which are due to the associated reductions in secondhand smoke exposure and increases in smoking cessation that accompany these laws. As more places adopt smokefree policies (whether by law in subordinate jurisdictions or voluntarily), the marginal effects of subsequent laws will be smaller, as was observed in New York and Massachusetts when those states passed comprehensives law after many localities had.18, 32 The passage of these laws reflects changes in social norms that also affect smoking behavior; the laws both formalize and accelerate this social change and the associated health benefits.
Limitations
The interrupted time series observational studies that form the foundation for this meta-analysis alone do not establish causation. At the same time, a randomized controlled trial of the effects of enacting legislation is impractical or impossible. The studies included in our meta-analysis consistently meet standards for high quality interrupted time series studies;7 in particular, all used objective measures of outcomes, and most considered secular trends and seasonality. The observed reductions in hospitalizations are, however, consistent with the known biological pathways by which tobacco smoke exposure causes disease and triggers acute events. The observation that AMI admissions in Helena, Montana11 rebounded after enforcement of its smokefree law was suspended due to a lawsuit also supports a causal link.
While compliance with smokefree laws is generally high and many studies have documented drops in secondhand smoke exposure after law implementation (Supplementary Tables 1–4), we could not assume any one individual’s level of exposure has decreased and subsequently reduced their risk of hospitalization. Few studies included in the meta-analysis measured tobacco smoke exposure or smoking status in individual cases.16, 22, 38, 39 Because a randomized control trial is impossible, an analysis measuring individual smoking and secondhand smoke exposure would offer the most valid evidence regarding the effectiveness of smokefree laws.
We entered the ordinal variable for comprehensiveness of a law (0 for workplaces only; 1 for workplaces and restaurants; 2 for workplaces, restaurants and bars) in the metaregression to test whether more comprehensive laws were followed by greater reductions in hospital admissions (or deaths). We treated comprehensiveness of law as an ordinal, not an interval (continuous) variable, which is why we only reported the P value for law comprehensiveness and not an effect size. While this is a standard approach for integrating ordinal variables into regression analyses, we investigated use of this procedure to ensure that our conclusions were not sensitive to this technique by treating law comprehensiveness as a categorical variable (together with dummy variables for the different outcome groups, as we do in the analysis in the paper that treats law comprehensiveness as an interval variable) and tried recoding the law comprehensiveness using alternative codings (0, 1, 3) and (0, 1, 4). As described in detail in the Supplemental Text, these analyses gave essentially the same results as the main analysis, indicating that the approach we use in the paper produces robust evidence for a dose-response effect of the law, treating law comprehensiveness as an ordinal variable.
Although it is not usual in epidemiological studies, we did not consider multiple testing. Readers should take into account potential inflation from multiple testing when interpreting significance levels (alpha) and confidence intervals.
In one study,47 authors expressed concern about misclassification between different outcomes.
Publication bias is always a concern in meta-analysis (Supplementary Figure 7). The nonparametric trim and fill analysis, however, indicated that adjusting for publication bias had little effect on the results.
Conclusion
This study provides evidence that smokefree laws are followed by fewer hospitalizations and lower health care expenditures for a wide range of diseases and that comprehensive laws ending smoking in workplaces, restaurants, and bars are associated with greater effects. The general public, public health professionals, and policy makers should consider these positive associations as they develop smokefree legislation and decide whether or not to include exceptions to these laws.
Supplementary Material
CLINICAL COMMENTARY.
Secondhand smoke causes cardiovascular and respiratory disease, and implementation of smokefree legislation is followed by drops hospitalizations and deaths from these diseases. This meta-analysis of 45 studies of 33 smokefree laws found that smokefree legislation was associated with significantly lower rates of hospital admissions (or deaths) for coronary events, other heart disease, cerebrovascular accidents, and respiratory disease. There was a dose-response relationship between the strength of the law, with more comprehensive laws (including workplaces, restaurants, and bars) having the largest health benefits. This study provides strong evidence not only of the health benefits of smokefree laws but also of the need to enact comprehensive laws without exceptions.
Acknowledgments
FUNDING
This work was supported by National Cancer Institute Grants CA-61021 and CA-87472. The funding agency played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
Both authors had full access to all the data in the study, worked together on the analysis, and take responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST
Neither author has any conflict of interest.
References
- 1.California Environmental Protection Agency. Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant. 2005 Available from: http://www.arb.ca.gov/regact/ets2006/ets2006.htm.
- 2.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Presention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 3.IOM (Institute of Medicine) Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 4.Callinan JE, Clarke A, Doherty K, Kelleher C. Legislative Smoking Bans for Reducing Secondhand Smoke Exposure, Smoking Prevalence and Tobacco Consumption. Cochrane Database Syst Rev. 2010:CD005992. doi: 10.1002/14651858.CD005992.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Naiman AB, Glazier RH, Moineddin R. Is There an Impact of Public Smoking Bans on Self-Reported Smoking Status and Exposure to Secondhand Smoke? BMC Public Health. 2011;11:146. doi: 10.1186/1471-2458-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnoya J, Glantz SA. Cardiovascular Effects of Secondhand Smoke: Nearly as Large as Smoking. Circulation. 2005;111:2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted Time Series Designs in Health Technology Assessment: Lessons from Two Systematic Reviews of Behavior Change Strategies. Int J Technol Assess Health Care. 2003;19:613–23. doi: 10.1017/s0266462303000576. [DOI] [PubMed] [Google Scholar]
- 8.Lightwood JM, Glantz SA. Declines in Acute Myocardial Infarction after Smoke-Free Laws and Individual Risk Attributable to Secondhand Smoke. Circulation. 2009;120:1373–9. doi: 10.1161/CIRCULATIONAHA.109.870691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers DG, Neuberger JS, He J. Cardiovascular Effect of Bans on Smoking in Public Places: A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2009;54:1249–55. doi: 10.1016/j.jacc.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Mackay DF, Irfan MO, Haw S, Pell JP. Meta-Analysis of the Effect of Comprehensive Smoke-Free Legislation on Acute Coronary Events. Heart. 2010;96:1525–30. doi: 10.1136/hrt.2010.199026. [DOI] [PubMed] [Google Scholar]
- 11.Sargent RP, Shepard RM, Glantz SA. Reduced Incidence of Admissions for Myocardial Infarction Associated with Public Smoking Ban: Before and after Study. BMJ. 2004;328:977–80. doi: 10.1136/bmj.38055.715683.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayens MK, Burkhart PV, Zhang M, Lee S, Moser DK, Mannino D, Hahn EJ. Reduction in Asthma-Related Emergency Department Visits after Implementation of a Smoke-Free Law. J Allergy Clin Immunol. 2008;122:537–41.e3. doi: 10.1016/j.jaci.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Dautzenberg B. Indicateurs Mensuels Du Tabagisme Passif: Mesure Des Bénéfices De L’interdiction Totale De Fumer. 2008 Available from: http://www.la-croix.com/content/download/686570/20854176/2008_2_24_tabac.pdf.
- 14.Barone-Adesi F, Vizzini L, Merletti F, Richiardi L. Short-Term Effects of Italian Smoking Regulation on Rates of Hospital Admission for Acute Myocardial Infarction. Eur Heart J. 2006;27:2468–72. doi: 10.1093/eurheartj/ehl201. [DOI] [PubMed] [Google Scholar]
- 15.Bartecchi C, Alsever RN, Nevin-Woods C, Thomas WM, Estacio RO, Bartelson BB, Krantz MJ. Reduction in the Incidence of Acute Myocardial Infarction Associated with a Citywide Smoking Ordinance. Circulation. 2006;114:1490–6. doi: 10.1161/CIRCULATIONAHA.106.615245. [DOI] [PubMed] [Google Scholar]
- 16.Seo DC, Torabi MR. Reduced Admissions for Acute Myocardial Infarction Associated with a Public Smoking Ban: Matched Controlled Study. J Drug Educ. 2007;37:217–26. doi: 10.2190/DE.37.3.a. [DOI] [PubMed] [Google Scholar]
- 17.Khuder SA, Milz S, Jordan T, Price J, Silvestri K, Butler P. The Impact of a Smoking Ban on Hospital Admissions for Coronary Heart Disease. Prev Med. 2007;45:3–8. doi: 10.1016/j.ypmed.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Juster HR, Loomis BR, Hinman TM, Farrelly MC, Hyland A, Bauer UE, Birkhead GS. Declines in Hospital Admissions for Acute Myocardial Infarction in New York State after Implementation of a Comprehensive Smoking Ban. Am J Public Health. 2007;97:2035–9. doi: 10.2105/AJPH.2006.099994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemstra M, Neudorf C, Opondo J. Implications of a Public Smoking Ban. Can J Public Health. 2008;99:62–5. doi: 10.1007/BF03403743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cesaroni G, Forastiere F, Agabiti N, Valente P, Zuccaro P, Perucci CA. Effect of the Italian Smoking Ban on Population Rates of Acute Coronary Events. Circulation. 2008;117:1183–8. doi: 10.1161/CIRCULATIONAHA.107.729889. [DOI] [PubMed] [Google Scholar]
- 21.Vasselli S, Papini P, Gaelone D, Spizzichino L, De Campora E, Gnavi R, Saitto C, Binkin N, Laurendi G. Reduction Incidence of Myocardial Infarction Associated with a National Legislative Ban on Smoking. Minerva Cardioangiol. 2008;56:197–203. [PubMed] [Google Scholar]
- 22.Pell JP, Haw S, Cobbe S, Newby DE, Pell AC, Fischbacher C, McConnachie A, Pringle S, Murdoch D, Dunn F, Oldroyd K, Macintyre P, O’Rourke B, Borland W. Smoke-Free Legislation and Hospitalizations for Acute Coronary Syndrome. N Engl J Med. 2008;359:482–91. doi: 10.1056/NEJMsa0706740. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease C Prevention. Reduced Hospitalizations for Acute Myocardial Infarction after Implementation of a Smoke-Free Ordinance--City of Pueblo, Colorado, 2002–2006. MMWR Morb Mortal Wkly Rep. 2009;57:1373–7. [PubMed] [Google Scholar]
- 24.Gasparrini A, Gorini G, Barchielli A. On the Relationship between Smoking Bans and Incidence of Acute Myocardial Infarction. Eur J Epidemiol. 2009;24:597–602. doi: 10.1007/s10654-009-9377-0. [DOI] [PubMed] [Google Scholar]
- 25.Villalbí JR, Castillo A, Cleries M, Salto E, Sanchez E, Martinez R, Tresserras R, Vela E, Barcelona G. Acute Myocardial Infarction Hospitalization Statistics: Apparent Decline Accompanying an Increase in Smoke-Free Areas. Rev Esp Cardiol. 2009;62:812–5. doi: 10.1016/s1885-5857(09)72362-x. [DOI] [PubMed] [Google Scholar]
- 26.Barnett R, Pearce J, Moon G, Elliott J, Barnett P. Assessing the Effects of the Introduction of the New Zealand Smokefree Environment Act 2003 on Acute Myocardial Infarction Hospital Admissions in Christchurch, New Zealand. Aust N Z J Public Health. 2009;33:515–20. doi: 10.1111/j.1753-6405.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- 27.Shetty KD, DeLeire T, White C, Bhattacharya J. Changes in U.S. Hospitalization and Mortality Rates Following Smoking Bans. J Policy Anal Manage. 2010;30:6–28. doi: 10.1002/pam.20548. [DOI] [PubMed] [Google Scholar]
- 28.Trachsel LD, Kuhn MU, Reinhart WH, Schulzki T, Bonetti PO. Reduced Incidence of Acute Myocardial Infarction in the First Year after Implementation of a Public Smoking Ban in Graubuenden, Switzerland. Swiss Med Wkly. 2010;140:133–8. doi: 10.4414/smw.2010.12955. [DOI] [PubMed] [Google Scholar]
- 29.Naiman A, Glazier RH, Moineddin R. Association of Anti-Smoking Legislation with Rates of Hospital Admission for Cardiovascular and Respiratory Conditions. CMAJ. 2010;182:761–7. doi: 10.1503/cmaj.091130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sims M, Maxwell R, Bauld L, Gilmore A. Short Term Impact of Smoke-Free Legislation in England: Retrospective Analysis of Hospital Admissions for Myocardial Infarction. BMJ. 2010;340:c2161. doi: 10.1136/bmj.c2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay D, Haw S, Ayres JG, Fischbacher C, Pell JP. Smoke-Free Legislation and Hospitalizations for Childhood Asthma. N Engl J Med. 2010;363:1139–45. doi: 10.1056/NEJMoa1002861. [DOI] [PubMed] [Google Scholar]
- 32.Dove MS, Dockery DW, Mittleman MA, Schwartz J, Sullivan EM, Keithly L, Land T. The Impact of Massachusetts’ Smoke-Free Workplace Laws on Acute Myocardial Infarction Deaths. Am J Public Health. 2010;100:2206–12. doi: 10.2105/AJPH.2009.189662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moraros J, Bird Y, Chen S, Buckingham R, Meltzer RS, Prapasiri S, Solis LH. The Impact of the 2002 Delaware Smoking Ordinance on Heart Attack and Asthma. Int J Environ Res Public Health. 2010;7:4169–78. doi: 10.3390/ijerph7124169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dove MS, Dockery DW, Connolly GN. Smoke-Free Air Laws and Asthma Prevalence, Symptoms, and Severity among Nonsmoking Youth. Pediatrics. 2011;127:102–9. doi: 10.1542/peds.2010-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman PM, Walsh ME. Hospital Admissions for Acute Myocardial Infarction, Angina, Stroke, and Asthma after Implementation of Arizona’s Comprehensive Statewide Smoking Ban. Am J Public Health. 2011;101:491–6. doi: 10.2105/AJPH.2009.179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barone-Adesi F, Gasparrini A, Vizzini L, Merletti F, Richiardi L. Effects of Italian Smoking Regulation on Rates of Hospital Admission for Acute Coronary Events: A Country-Wide Study. PLoS One. 2011;6:e17419. doi: 10.1371/journal.pone.0017419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrante D, Linetzky B, Virgolini M, Schoj V, Apelberg B. Reduction in Hospital Admissions for Acute Coronary Syndrome after the Successful Implementation of 100% Smoke-Free Legislation in Argentina: A Comparison with Partial Smoking Restrictions. Tob Control. 2011;21:402–6. doi: 10.1136/tc.2010.042325. [DOI] [PubMed] [Google Scholar]
- 38.Bonetti PO, Trachsel LD, Kuhn MU, Schulzki T, Erne P, Radovanovic D, Reinhart WH. Incidence of Acute Myocardial Infarction after Implementation of a Public Smoking Ban in Graubunden, Switzerland: Two Year Follow-Up. Swiss Med Wkly. 2011;141:w13206. doi: 10.4414/smw.2011.13206. [DOI] [PubMed] [Google Scholar]
- 39.Gupta R, Luo J, Anderson RH, Ray A. Clean Indoor Air Regulation and Incidence of Hospital Admissions for Acute Coronary Syndrome in Kanawha County, West Virginia. Prev Chronic Dis. 2011;8:A77. [PMC free article] [PubMed] [Google Scholar]
- 40.Bruintjes G, Bartelson BB, Hurst P, Levinson AH, Hokanson JE, Krantz MJ. Reduction in Acute Myocardial Infarction Hospitalization after Implementation of a Smoking Ordinance. Am J Med. 2011;124:647–54. doi: 10.1016/j.amjmed.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Rodu B, Peiper N, Cole P. Acute Myocardial Infarction Mortality before and after State-Wide Smoking Bans. J Community Health. 2012;37:468–72. doi: 10.1007/s10900-011-9464-5. [DOI] [PubMed] [Google Scholar]
- 42.Villalbí JR, Sanchez E, Benet J, Cabezas C, Castillo A, Guarga A, Salto E, Tresserras R Barcelona Group for Smoking Regulation Policies E. The Extension of Smoke-Free Areas and Acute Myocardial Infarction Mortality: Before and after Study. BMJ Open. 2011;1:e000067. doi: 10.1136/bmjopen-2011-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn EJ, Rayens MK, Burkhart PV, Moser DK. Smoke-Free Laws, Gender, and Reduction in Hospitalizations for Acute Myocardial Infarction. Public Health Rep. 2011;126:826–33. doi: 10.1177/003335491112600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cronin EM, Kearney PM, Kearney PP, Sullivan P, Perry IJ on behalf of the Coronary Heart Attack Ireland Registry Working G. Impact of a National Smoking Ban on Hospital Admission for Acute Coronary Syndromes: A Longitudinal Study. Clin Cardiol. 2012;35:205–9. doi: 10.1002/clc.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sebrie EM, Sandoya E, Hyland A, Bianco E, Glantz SA, Cummings KM. Hospital Admissions for Acute Myocardial Infarction before and after Implementation of a Comprehensive Smoke-Free Policy in Uruguay. Tob Control. 2012 doi: 10.1136/tobaccocontrol-2011-050134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sargent JD, Demidenko E, Malenka DJ, Li Z, Gohlke H, Hanewinkel R. Smoking Restrictions and Hospitalization for Acute Coronary Events in Germany. Clin Res Cardiol. 2012;101:227–35. doi: 10.1007/s00392-011-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kent BD, Sulaiman I, Nicholson TT, Lane SJ, Moloney ED. Acute Pulmonary Admissions Following Implementation of a National Workplace Smoking Ban. Chest. 2012 doi: 10.1378/chest.11-2757. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Heinz J, Rasmussen CM, Johnson CJ. The Effect of Smoking Bans on Myocardial Infarctions: The Boise Experience. Nicotine Tob Res. 2007;9:S301. [Google Scholar]
- 49.Gudnason T, Viktorsson T, Andersen K. A Smoking Ban in Public Places May Reduce the Incidence of Acute Coronary Syndrome among Non-Smoking Men. Eur Heart J. 2009;30:153. [Google Scholar]
- 50.Barone-Adesi F, Vizzini L, Merletti F, Richiardi L. Italian Smoking Regulation Decreased Hospital Admissions for Acute Coronary Events:Effect Modification by Age and Day of the Week. Eur Heart J. 2009;30:148. [Google Scholar]
- 51.Di Valentino M, Limoni C, Rigoli A, Gallino A, Muzzarelli S, Pedrazzini G. Reduced Hospitalization for Acute Coronary Syndrome after Introduction of Smoking Ban in Public Places in Canton Ticino, Southern Switzerland. Eur Heart J. 2010;31:680. [Google Scholar]
- 52.Di Valentino M, Rigoli A, Limoni C, Barazzoni F, Gallino A, Muzzarelli S, Pedrazzini G. Reduced Incidence of St-Elevation Myocardial Infarction in the First Two Years after Introduction of a Public Smoking Ban in Canton Ticino, Switzerland. Eur Heart J. 2011;32:502. [Google Scholar]
- 53.Xuereb R, Calleja N, Distefano A, England K, Gatt M, Grech V. Smoking Ban: The Malta Paradox. Eur Heart J. 2011;32:379. [Google Scholar]
- 54.Hurt RD, Weston SA, Ebbert JO, McNallan SM, Croghan IT, Schroeder DR, Roger VL. Myocardial Infarction and Sudden Cardiac Death in Olmsted County, Minnesota, before and after Smoke-Free Workplace Laws. Circulation. 2011;124:21. doi: 10.1001/2013.jamainternmed.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMillen R, Hill A, Valentine N, Collins R. The Starkville and Hattiesburg Heart Attack Studies: Reductions in Heart Attack Admissions Following the Implementation of Local Smoke-Free Ordinances. 2010 Available from: http://www.ttac.org/resources/pdfs/120810_Miss_Heart_Attack_Report.pdf.
- 56.Bruckman D, Bennett B. Significant Change in Statewide Rates of Hospital Discharge Data for Myocardial Infarction Due to the Enactment of Ohio’s Smoke-Free Work Place Law. Analyses of the Impact of the Ohio Smoke-Free Workplace Act [serial on the Internet] 2011 Available from: http://www.odh.ohio.gov/ASSETS/81B904A706574FFB97271C46256E53C2/Final%20Reports.pdf.
- 57.The North Carolina Smoke Free Restaurants and Bars Law and Emergency Department Admissions for Acute Myocardial Infarction: A Report to the North Carolina State Health Director. 2011 Available from: http://tobaccopreventionandcontrol.ncdhhs.gov/smokefreenc/docs/TPCB-2011SFNCReport-SHD.pdf.
- 58.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 59.Duval S, Tweedie R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. JASA. 2000;95:89–98. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 60.Oono IP, Mackay DF, Pell JP. Meta-Analysis of the Association between Secondhand Smoke Exposure and Stroke. J Public Health (Oxf) 2011;33:496–502. doi: 10.1093/pubmed/fdr025. [DOI] [PubMed] [Google Scholar]
- 61.Kabir Z, Clarke V, Conroy R, McNamee E, Daly S, Clancy L. Low Birthweight and Preterm Birth Rates 1 Year before and after the Irish Workplace Smoking Ban. BJOG. 2009;116:1782–7. doi: 10.1111/j.1471-0528.2009.02374.x. [DOI] [PubMed] [Google Scholar]
- 62.Mackay DF, Nelson SM, Haw SJ, Pell JP. Impact of Scotland’s Smoke-Free Legislation on Pregnancy Complications: Retrospective Cohort Study. PLoS Med. 2012;9:e1001175. doi: 10.1371/journal.pmed.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.