Abstract
An efficient synthesis of the C13–C29 fragment of amphidinolide N is described. The synthesis relies on a new strategy involving palladium-catalyzed asymmetric allylic alkylation to generate diastereoselectively either the cis-or trans-THF unit simply by varying the enantiomer of the ligand. The C19 hydroxyl-bearing stereocenter was introduced using a chelation-controlled allylation which led exclusively to a single diastereoisomer.
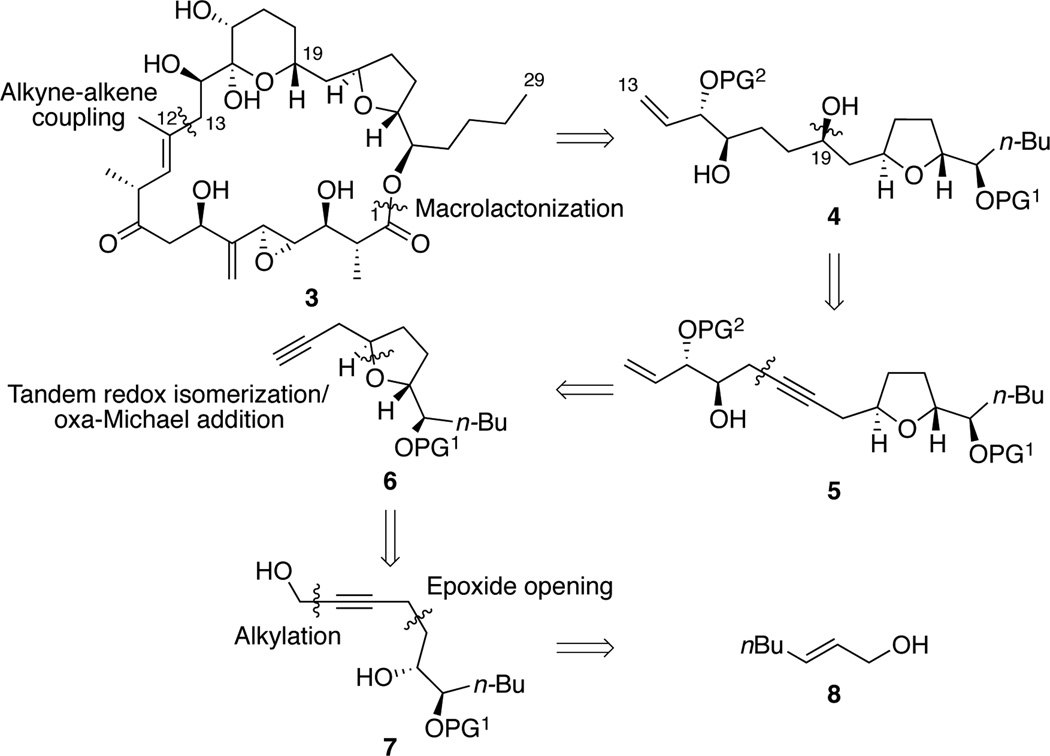
Amphidinolide N was isolated in 1994 from the marine microorganism Amphidinium sp. and displayed highly promising preliminary biological activity with high cytotoxicity in vitro against the murine lymphoma L1210 (IC50 = 0.08 nM) and the human epidermoid carcinoma KB cell-lines (IC50 = 0.09 nM).1 Kobayashi initially reported a partial structure of amphidinolide N (1) consisting of a 26-membered lactone, an allylic epoxy alcohol and a 6-membered hemiacetal ring; the relative configuration (C14, C15, C16 and C19) was assigned by nOe experiment (Scheme 1). Soon after, Shimizu reported the isolation of caribenolide I (2), a natural product whose structure shared many structural similarities with 1, the only difference being the hydration of the C21–C24 tetrahydrofuran (THF) ring.2 Considering the chemical structures of the amphidinolide natural product family,3 the possibility that 1 and 2 are isomeric compounds has been suggested and a revised structure of amphidinolide N (3), including a complete relative stereochemical assignment is postulated (Scheme 1).4
Scheme 1.
Revised postulated structure of amphidinolide N (3)
To date, no total synthesis of amphidinolide N has been reported in the literature.5 In view of its biological activity and unprecedented carbon framework, as well as its limited availability from natural sources, and in order to prove the structural proposition, we recently decided to undertake a concise and convergent total synthesis of 3. A common structural feature of some members of the amphidinolide family is the presence of a α-hydroxy-THF motif (amphidinolides C, E, F, M and U).3 As part of our ongoing projects involving the development of new synthetic methods using asymmetric metal-catalysis, we initially focused on the synthesis of the C13–C29 fragment of 3 with a particular interest for the diastereoselective synthesis of the challenging α-hydroxy trans-THF unit. From a retrosynthetic point of view, it was proposed that 3 could be disconnected into two fragments of equal complexity, C1–C12 and C13–C29, which might be assembled by means of our ruthenium catalyzed alkene/alkyne coupling6 and subsequent α-oxidation of the newly formed ketone followed by macrolactonization (Scheme 2).
Scheme 2.
Retrosynthetic analysis of amphidinolide N (3)
We report herein the highly diastereoselective synthesis of the C13–C29 subunit 4 of amphidinolide N (3) (Scheme 2). It was initially envisaged that the C19 hydroxy group could be introduced by hydrosilylation of homopropargylic alcohol 5, followed by Fleming-Tamao oxidation of the resulting vinylsiloxane and diastereoselective ketone reduction. The homopropargylic alcohol 5 could be generated by epoxide opening of terminal alkyne 6. The key step to install the challenging trans-THF 6 would rely on a tandem redox isomerization/oxa-michael addition reaction. Finally, the precursor propargyl alcohol 7 could be synthesized from allylic alcohol 8.
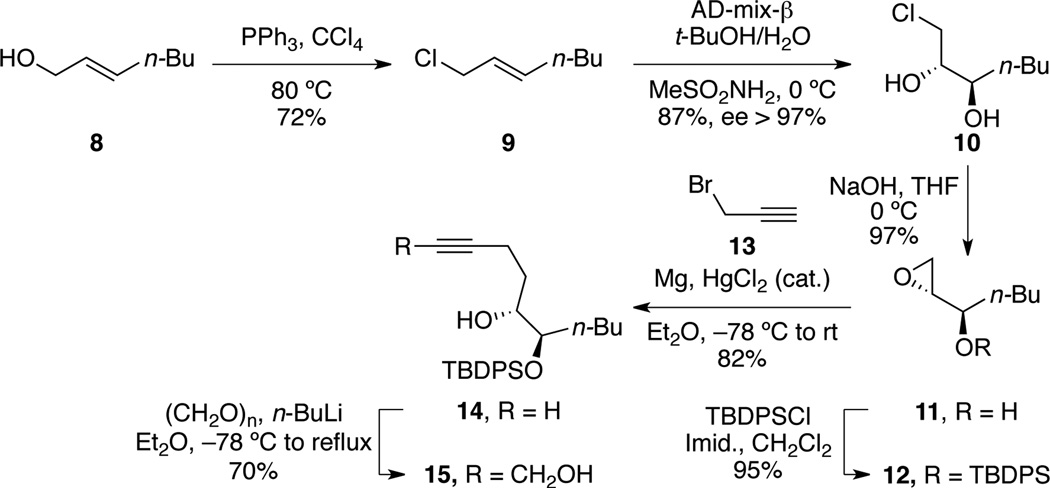
The synthesis started with the conversion of primary allylic alcohol 8 to the corresponding allylic chloride 9 (Scheme 3).7 The trans-olefin was then subjected to Sharpless dihydroxylation conditions, leading to the known diol 108 in excellent yield and enantioselectivity.9 The latter was then treated with sodium hydroxide to form epoxide 11 and converted to silyl ether 12. Our choice to introduce a TBDPS ether was driven by our need to use protecting groups stable under the redox isomerization reaction conditions.10 Epoxide 12 was then opened using allenylmagnesium bromide, generated in situ from propargyl bromide (13) to form terminal alkyne 14.11 Finally, primary propargyl alcohol 15 was obtained by reaction of 14 with paraformaldehyde.
Scheme 3.
Synthesis of redox-isomerization precursor 15
The redox isomerization of propargyl alcohol 15 was then investigated (Table 1). When 15 was treated with 5 mol % of IndRu(PPh3)2Cl, 5 mol % of indium triflate and 30 mol % of CSA in THF at reflux, the desired cyclized product was formed in excellent yield (Table 1, entry 1).12 It was initially thought that the chiral diol might induce some degree of diastereoselectivity during the oxa-Michael addition but diastereomeric THF’s 17 and 18 were isolated as an inseparable 1:1 mixture. Reducing the amount of acid to 10 mol % or lowering the temperature of the reaction to decrease the rate of cyclization led either to formation of the cyclized product with no diastereocontrol or no reactivity at room temperature (Table 1, entries 2–4). In all cases, no trace of the enal intermediate 16 was detected by crude NMR of the reaction mixture. Addition of L-proline to the reaction mixture in order to generate an iminium species in situ and catalyze the diastereoselective formation of the cis or trans THF only led to unreacted starting material (probably because of catalyst poisoning by the secondary amine) (Table 1, entry 5).
Table 1.
Tandem redox isomerization/oxa-Michael addition
 | |||
|---|---|---|---|
| entry | conditions | yield (conversion) |
comments |
| 1 | 30% CSA, 66 °C | 74% (100%) | 1:1 dr |
| 2 | 10% CSA, 66 °C | 70% (100%) | 1:1 dr |
| 3 | 30% CSA, rt | (0%) | SM recovered |
| 4 | 30% CSA, 40 °C | (< 10%) | 1:1 dr |
| 5 | 30% CSA, 20% L-proline, 66 °C | Traces | Catalyst poisoning |
In view of these results, a revised strategy was clearly needed to generate diastereoselectively the α-hydroxy trans-THF unit. Accordingly, a new approach for the diastereoselective formation of either 2,5-cis- or trans-disubstituted THF rings was envisaged using chiral catalysts in an unprecedented palladium-catalyzed asymmetric allylic alkylation (Pd-AAA) reaction. Thus, differenciating the gem-diacetates of 19 (Scheme 4) and directing the regioselectivity of the nucleophilic attack to the distal carbon was required.13
Scheme 4.
Revised strategy to access the trans-THF 6
Opening the previously synthesized epoxide 12 with allylmagnesium bromide (21) led to terminal alkene 22 (Scheme 5).14 The latter was then engaged in a cross-metathesis reaction with allyl gem-diacetate (20) to yield the corresponding (E)-olefin 19 in good yield.15
Scheme 5.
Synthesis of the Pd-AAA precursor 19
With olefin 19 in hand, the Pd-AAA cyclization was then investigated (Table 2). When olefin 19 was subjected to 5 mol % of Pd2(dba)3.CHCl3 and 15 mol % of (S,S)–Trost standard ligand L1 at room temperature and under oxygen-free conditions, only trace amount of the cyclized product was observed. At 50 °C, regioselective attack of the secondary alcohol on the π-allyl intermediate led to the desired cyclized products 23 and 24 as a 1:4 mixture of diastereoisomers in 75% yield (Table 2, entry 2). To determine their spatial relationship, both isomers were successfully separated by flash column chromatography and subjected to nOe experiments. Even though a matched/mismatched case could have been anticipated when using the (R,R)–L1 ligand, treatment of 19 with this ligand pleasingly afforded the desired trans-THF 23 as a 4:1 mixture of diastereoisomers, suggesting that the observed selectivity was due to catalyst control (Table 2, entry 3). In view of the observed stereochemistry, the diastereodetermining step appears to be the intramolecular nucleophilic attack on the π-allyl intermediate rather than the ionization step.16 Using other ligands developed within the Trost group led to either a drop of reactivity and/or gave poor selectivity.17 We then envisioned activating the free hydroxy group to see whether a faster attack of the π-allyl to prevent equilibration of the two diastereomeric π-allyl systems would influence the diastereoselectivity of the reaction. Additives such as Et3B18 or Et2Zn19 failed to improve the selectivity of the reaction (Table 2, entries 5–6).
Table 2.
THF cyclization via Pd-AAA approach
 | |||
|---|---|---|---|
| entry | conditions | yielda (conversion) |
drb (23:24) |
| 1 | L1 (S,S), rt, 0.15 M | traces | - |
| 2 | L1 (S,S), 50 °C, 0.15 M | 75% (100%) | 1:4 |
| 3 | L1 (R,R), 50 °C, 0.15 M | 77% (100%) | 4:1 |
| 4 | L1 (R,R), 35 °C, 0.15 M | (58%) | 3.3:1 |
| 5 | L1 (R,R), 50 °C, Et3B, 0.15 M | (100%) | 1.2:1 |
| 6 | L1 (R,R), 50 °C, Et2Zn, 0.15 M | (90%) | 1:2 |
| 7c | L1 (R,R), 50 °C, Et3N, 0.15 M | 82% (100%) | 4.6:1 |
| 8 | L1 (R,R), 66 °C, Et3N, 0.15 M | (100%) | 3.5:1 |
| 9 | L1 (R,R), 50 °C, EtN(i-Pr)2, 0.15 M | (< 10%) | - |
| 10d | L1 (R,R), 50 °C, AcOH, 0.15 M | (100%) | 3.6:1 |
| 11 | L1 (R,R), 50 °C, Et3N, 0.4 M | (100%) | 3.7:1 |
| 12 | L1 (R,R), 50 °C, Et3N, 0.05 M | (100%) | 4:1 |
Isolated yield.
dr determined from the 1H NMR of the crude mixture.
< 5% formation of the undesired byproduct.
30% formation of the undesired byproduct.
However, addition of 1.1 equivalent of Et3N led to the formation of an improved 4.6:1 mixture in favor of the trans-THF in an excellent 82% yield (Table 2, entry 7). It is believed that the improved yield and selectivity for this transformation is due to the quenching of AcOH formed in the reaction by the added base. In fact, when no base was added (see entry 3), formation of around 10–15% of an unidentified byproduct was observed in the crude NMR,20 while less than 5% was detected with addition of Et3N. Formation of this byproduct is probably acid-catalyzed. To validate this hypothesis, 1.1 equivalent of AcOH was added to the reaction mixture leading to both a drop of diastereoselectivity and an increase of the byproduct formation (30% by the crude NMR) (Table 2, entry 10). The use of the Hünig base surprisingly shut down the reaction suggesting that the Et3N also plays a role in activating the alcohol by hydrogen bonding (Table 2, entry 9). Working on more diluted or concentrated conditions did not increase the diastereoselectivity of the reaction (Table 2, 11–12).
Conversion of vinyl acetate 23 to the corresponding aldehyde 17 to access the desired terminal alkyne was then required (Scheme 6). Using K2CO3 in MeOH only led to epimerization of the substrate (dr = 1:1). Under these conditions, the rate of protonation of the in situ generated enolate was slower than the rate of ring opening leading to epimerization. Fortunately, when Et3N was used, complete conversion to the desired aldehyde 17 was observed. Using the Corey-Fuchs protocol,21 aldehyde 17 was then converted to terminal alkyne 25 in excellent yield over two steps.22 Alkyne 25 was reacted with the known epoxide 2623 to afford homopropargyl alcohol 27 in good yield.
Scheme 6.
Hydrosilylation of homopropargyl alcohol 27
Using our previously reported method for the hydrosilylation of homopropargyl alcohols,24 27 was regioselectively converted to the intermediate 6-membered vinylsiloxane 28 via a two-step sequence (Scheme 6). Unfortunately, despite many attempts to oxidize 28 using Fleming-Tamao conditions, we were unable to successfully form the desired ketone 29 and either no reaction (with KHF2 and KF) or decomposition was observed (with n-Bu4NF).25
At that point, we decided to revise our synthetic strategy and introduce the C19 tertiary stereocenter earlier in the synthesis. Keck showed that β-hydroxy aldehydes could be converted to anti-1,3-diols using chelation control with various Lewis acids.26 Considering the fact that hydroxylbearing stereocenters C19 and C21 of 3 are anti-1,3-diols, we were intrigued to see how β-alkoxy aldehyde 17 would react under Keck conditions. Pleasingly, when 17 was treated with MgBr2 and allyltributyltin (30), alcohol 32 was obtained as a single diastereoisomer (Scheme 7). It seems likely from the transition state 31 that the nucleophile will attack the “re” face of the aldehyde to avoid any axial interactions with the THF unit (a similar transition state has been proposed by Keck26).
Scheme 7.
Access to C13–C29 fragment via chelation control
TIPS protection of the resulting secondary alcohol followed by a hydroboration/oxidation sequence led to aldehyde 34 in excellent yield (Scheme 7). Finally, Brown’s alkoxyallylation27 provided the desired C13–C29 fragment 36 in good yield and diastereoselectivity. It is worth noting that the stereocenter C15 will be destroyed during the alkene/alkyne coupling reaction to generate the corresponding ketone.
In conclusion, we have reported the synthesis of the C13–C29 fragment of amphidinolide N. Our initial strategy based on our redox isomerization protocol did not show any diastereocontrol during the oxa-Michael addition step. The implementation of a new strategy based upon the use of a Pd-AAA approach allowed access to either the required trans-THF or to the cis-isomer diastereoselectively in a catalyst controlled reaction. A chelation-controlled allylation allowed us to set efficiently the C19 stereogenic center. Efforts are now underway to complete the total synthesis of amphidinolide N.
Supplementary Material
Acknowledgement
We thank the National Institutes of Health for their generous support of our program (NIH R01 GM-033049).
Footnotes
Supporting Information Available: Experimental procedures and 1H and 13C NMR data for all compounds
References
- 1.Ishibashi M, Yamaguchi N, Sasaki T, Kobayashi J. J. Chem. Soc. Chem. Commun. 1994:1455–1456. [Google Scholar]
- 2.Bauer I, Maranda L, Young KA, Shimizu Y, Fairchild C, Cornell L, MacBeth J, Huang S. J. Org. Chem. 1995;60:1084–1086. [Google Scholar]
- 3.Kobayashi J. J. Antibiot. 2008;5:271–284. doi: 10.1038/ja.2008.39. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi J. personal communication [Google Scholar]
- 5.For partial synthesis see: Nicolaou KC, Brenzovich WE, Bulger PG, Francis TM. Org. Biomol. Chem. 2006;4:2119–2157. doi: 10.1039/b602020h. Nicolaou KC, Bulger PG, Brenzovich WE. Org. Biomol. Chem. 2006;4:2158–2183. doi: 10.1039/b602021f.
- 6. Trost BM, Toste FD. Tetrahedron Lett. 1999;40:7739–7743. (b) Application in total synthesis: Trost BM, Gunzner JL. J. Am. Chem. Soc. 2001;123:9449–9450. doi: 10.1021/ja011424b.
- 7.Snyder EI. J. Org. Chem. 1972;37:1466. [Google Scholar]
- 8.Vanhessche KPM, Wang Z-M, Sharpless KB. Tetrahedron Lett. 1994;35:3469–3472. [Google Scholar]
- 9.Ee determined on intermediate 12
- 10.Trost BM, Livingston RC. J. Am. Chem. Soc. 2008;130:11970–11978. doi: 10.1021/ja804105m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama Y, Kumar BG, Kobayashi Y. J. Org. Chem. 2000;65:707–715. doi: 10.1021/jo9913199. [DOI] [PubMed] [Google Scholar]
- 12.Trost BM, Gutierrez AC, Livingston RC. Org. Lett. 2009;11:2539–2542. doi: 10.1021/ol9007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trost BM. Org. Process Res. Dev. 2012;16:185–194. doi: 10.1021/op200294r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi S, Ogawa N, Koshino H, Nakata T. Org. Lett. 2005;7:2783–2786. doi: 10.1021/ol0508126. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 16.Trost BM, Chulbom L. J. Am. Chem. Soc. 2001;123:3671–3686. doi: 10.1021/ja003774o. [DOI] [PubMed] [Google Scholar]
- 17.Details are given in the Supporting Information.
- 18.Trost BM, McEachern E, Toste FD. J. Am. Chem. Soc. 1998;120:12702–12703. [Google Scholar]
- 19.Kim H, Chulbom L. Org. Lett. 2002;4:4369–4371. doi: 10.1021/ol027104u. [DOI] [PubMed] [Google Scholar]
- 20.This byproduct could not be isolated in pure form and consequently characterized.
- 21.Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;36:3769–3772. [Google Scholar]
- 22.One-step procedures using the Bestmann-Ohira reagent led to epimerization while the Colvin rearrangement gave decomposition.
- 23.Nicolaou KC, Rodríguez RM, Mitchell HJ, Van Delft FL. Angew. Chem. Int. Ed. 1998;37:1874–1876. [Google Scholar]
- 24.Trost BM, Ball ZT, Laemmerhold KM. J. Am. Chem. Soc. 2005;127:10028–10038. doi: 10.1021/ja051578h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Fleming I, Barbero A, Walter D. Chem. Rev. 1997;97:2063–2192. doi: 10.1021/cr941074u. [DOI] [PubMed] [Google Scholar]; (b) Marshall JA, Yanik MM. Org. Lett. 2000;2:2173–2175. doi: 10.1021/ol0061182. [DOI] [PubMed] [Google Scholar]
- 26.Keck GE, Castellino S, Wiley MR. J. Org. Chem. 1986;51:5478–5480. [Google Scholar]
- 27.Brown HC, Jadhav PK, Bhat KS. J. Am. Chem. Soc. 1988;110:1535–1538. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.