Abstract
The combination of laser tweezers, fluorescent imaging, and real-time automated tracking and trapping (RATTS) can measure sperm swimming speed and swimming force simultaneously with mitochondrial membrane potential (MMP). This approach is used to study the roles of two sources of ATP in sperm motility: oxidative phosphorylation, which occurs in the mitochondria located in the sperm midpiece and glycolysis, which occurs along the length of the sperm tail (flagellum). The relationships between (a) swimming speed and MMP and (b) swimming force and MMP are studied in dog and human sperm. The effects of glucose, oxidative phosphorylation inhibitors and glycolytic inhibitors on human sperm motility are examined. The results indicate that oxidative phosphorylation does contribute some ATP for human sperm motility, but not enough to sustain high motility. The glycolytic pathway is shown to be a primary source of energy for human sperm motility.
Quantitative measurements of sperm are critical for assessing their overall quality and viability. Several systems for sperm analysis have been developed. For example, Computer Assisted Sperm Analysis (CASA) systems measure motility parameters of sperm populations (Amann and Katz, 2004). Flow cytometry systems in combination with fluorescent probes that monitor sperm mitochondrial membrane potential (MMP) have been used to demonstrate that high MMP correlates with increased motility (Marchetti et al., 2002) as well as high fertility (Kasai et al., 2002; Marchetti et al., 2004; Gallon et al., 2006). Laser tweezers have been used to measure sperm swimming force, a measurement that positively correlates with sperm swimming speed (Tadir et al., 1990; Nascimento et al., 2006, 2007).
The recent combination of laser tweezers, fluorescent imaging, and the real-time automated tracking and trapping system (RATTS) (Shi et al., 2006) has provided a system that can measure sperm motility parameters, such as curvilinear velocity (VCL) and swimming force (in terms of the laser power at which a sperm can escape the optical trap, Pesc), simultaneously with MMP (Shi et al., 2008). This system’s capabilities have been described in detail in Nascimento et al. (2008). Specifically, the system can monitor changes in MMP over time, study the effects of prolonged exposed to the laser tweezers on VCL and MMP, and quantify VCL, Pesc and MMP in real-time for hundreds of sperm. The study also found that when sperm were exposed to a mitochondrial uncoupling agent (carbonyl cyanide 3-chlorophenylhydrazone, CCCP), a significant decrease in MMP was observed yet VCL appeared to be unaffected. As a result of these findings, it was suggested that perhaps a pathway other than oxidative phosphorylation, such as glycolysis which is known to occur along the sperm tail (Turner, 2003; Mukai and Okuno, 2004; Ford, 2006), may be providing the ATP (energy) needed to support motility (Nascimento et al., 2008).
Oxidative phosphorylation is, indeed, a more efficient means of generating ATP than glycolysis (the metabolism of sugars in mitochondria can produce fifteen times more ATP than glycolysis) (Alberts et al., 2002). However, there is some doubt that ATP can sufficiently diffuse from the midpiece down to the distal segments of the tail (Turner, 2003; Ford, 2006). Recent studies suggest glycolysis is an important pathway to generate ATP in sperm cells. For example, analysis of the flagellum has shown that several glycolytic enzymes are localized to the fibrous sheath in mouse and human sperm (Krisfalusi et al., 2006; Kim et al., 2007) as well as an ADP/ATP carrier protein in human sperm (Kim et al., 2007). Having energy production and translocation mechanisms localized to the sperm tail suggests that sperm may rely upon glycolysis for the generation of ATP that is used for motility (Kim et al., 2007). Other studies have demonstrated the importance of glucose for sperm motility. Williams and Ford (2001) showed that the percent of motile human sperm as well as their smoothed path velocity was statistically greater when incubated in media containing glucose. One of the findings in Mukai and Okuno (2004), who measured the beat frequency of mouse sperm flagella, was that the beat frequency was significantly reduced for sperm in media without substrate and that exposure to an electron transport chain inhibitor (antimycin A) did not affect flagellum beat frequency so long as the media contained glucose. They also found that sperm were motile with the mitochondrial substrate, pyruvate, in the absence of glucose, and that the beat frequency was the same in this medium as in the medium containing glucose. Sperm motility was significantly reduced in this medium in the presence of the uncoupler, CCCP, as would be expected if the ATP for motility were being supplied by oxidative phosphorylation with pyruvate as substrate. Krzyzosiak et al. (1999) found that bull sperm were motile in the presence of both antimycin A and rotenone (another electron transport chain inhibitor) only when suspended in media that contained glucose. Miki et al. (2004) found that motility (measured by CASA) was reduced in sperm from mice lacking a sperm-specific glycolytic enzyme (glyceraldehyde 3-phosphate dehydrogenase-S, GAPDS) compared to the wild-type with glucose as substrate.
In this article, laser tweezers, fluorescent imaging, and RATTS (Nascimento et al., 2008; Shi et al., 2008) are combined to study the roles of two sources of ATP: oxidative phosphorylation, which occurs in the mitochondria located in the sperm midpiece, and glycolysis, which occurs along the sperm tail (flagellum). Since previous studies showed that there is a relationship between VCL and Pesc (Nascimento et al., 2006) and that high MMP correlated with increased motility (Marchetti et al., 2002), the custom system is first used to analyze the relationships between MMP (i.e., oxidative phosphorylation) and sperm swimming force and swimming speed in domestic dog and human species. Second, the effects of different media, the presence/absence of glucose in the media, and both oxidative phosphorylation and glycolytic inhibitors on human sperm motility (VCL, Pesc) and MMP are studied. The results provide quantitative evidence supporting the theory that glycolysis is a critical pathway for the generation of energy (ATP) for human sperm motility.
Materials and Methods
Specimen
Domestic dog
Semen samples collected from several domestic dogs are cryogenically frozen according to a standard protocol (Harper et al., 1998; Durrant et al., 2000). For each experiment, a sperm sample is thawed in a water bath (37°C) for approximately 1 min and its contents are transferred to an Eppendorf centrifuge tube. The sample is centrifuged at 2,000 rpm for 10 min (centrifuge tip radius is 8.23 cm). The supernatant is removed and the remaining sperm pellet is suspended in 1 milliliter (ml) of pre-warmed media (1 mg of bovine serum albumin (BSA) per 1 ml of Biggers, Whitten, and Whittingham (BWW), osmolality of 270–300 mmol/kg water, pH of 7.2–7.4) (Biggers et al., 1971). A table listing the components of the BWW media can be found in the Online Supplemental Material.
Human
Semen samples from several humans are frozen according to published protocol (Ethics Committee of the American Fertility Society, 1986; Serfini and Marrs, 1986; DiMarzo et al., 1990) and prepared for analysis using a twice wash protocol (Toffle et al., 1985; DiMarzo and Rakoff, 1986). To study the relationships between MMP and both Pesc and VCL, the human sperm are suspended in modified Human Tubal Fluid (HTF) HEPES buffered (osmolarity 272–288 mOsm/kg water, pH of 7.3–7.5) with 5% Serum Substitute Supplement (SSS) filtered through 0.2 µm syringe filter, first five drops are disposed (Irvine Scientific, Santa Ana, CA). (A different preparation method, described in detail below, is used to study of the effects of glucose and inhibitors on sperm motility.) A table listing the components of the HTF media can be found in the Online Supplemental Material.
Final dilutions of ~30,000 sperm (dog and/or human) per ml of media are used in all of the experiments. The diluted sperm suspension is loaded into a 3 ml Rose tissue culture chamber and mounted into a microscope stage holder according to previously described methods (Liaw and Berns, 1981). The sample is kept at 37°C using an air curtain incubator (NEVTEK, ASI 400 Air Stream Incubator, Burnsville, VA). A thermocouple is attached to the Rose chamber to ensure temperature stability.
Mitochondrial membrane potential (MMP) assessment
To monitor the potential across the inner membrane of the mitochondria, sperm are labeled with the cationic cyanine dye, DiOC2(3) (3,3′-dithyloxacarbocyanine, 30 nM final dye concentration, Molecular Probes, Invitrogen Corp., Carlsbad, CA). At such low concentrations, the dye, DiOC2(3), primarily accumulates in the mitochondria of a cell in response to the electrochemical proton gradient (Novo et al., 1999). The probe emits both a red and green fluorescence (red/green intensity is a size-independent ratiometric measure of MMP, as green fluorescence varies with size and red fluorescence is dependent on both size and MMP) (Novo et al., 2000). After the dye is added, the cells are incubated for 20 min in a 37°C water bath and then centrifuged for 10 min (2,000 rpm). The pellet is suspended in the media by “flicking” the tube according to the protocol for the MitoProbe assay kit (Invitrogen Corp.) for flow cytometry. A figure in Shi et al. (2008) demonstrates how the fluorescent signal is coming only from the midpiece of a swimming sperm. In addition, in the Online Supplemental Material, a set of images of a non-motile sperm in both phase contrast and fluorescence verify that the signal is coming from the sperm midpiece.
Hardware, software, and optical design
The optical system is described in great detail in Nascimento et al. (2008). Briefly, a single point gradient trap is generated using an Nd:YVO4 continuous wave 1,064 nm wavelength laser (Spectra Physics, BL-106C, Mountain View, CA), coupled into an inverted microscope equipped with a phase III, 40×, NA 1.3, oil immersion objective (Zeiss Axiovert S100, Zeiss, Thornwood, NY). Laser power in the specimen plane is attenuated by rotating a polarizer in the optical path, which is mounted in a stepper-motor-controlled rotating mount (Newport Corporation, Model PR50PP, Irvine, CA). Two dual video adapters are used to incorporate the laser into the microscope and simultaneously image the sperm in both phase contrast and fluorescence. A Dual-View system (Optical-Insights, Tucson, AZ) coupled to a digital camera (Quantix 57, Roper Scientific, Inc., Tucson, AZ) is used to split the red and green fluorescent light.
The hardware and software used in these experiments are described in greater detail elsewhere (Shi et al., 2008). Briefly, two computers are networked together. An upper-level computer that acquires and displays the phase images is responsible for tracking and trapping, under decaying laser power, the sperm of interest (measuring the sperm’s VCL and Pesc). Sperm are tracked for 5 sec both prior to and after being trapped. The lower-level computer is prompted by the upper-level computer to acquire the fluorescent images of the sperm’s mitochondria at a given interval (measuring the MMP). During the 5-sec tracking phases, fluorescent images are acquired once every second. When the sperm is in the trap, fluorescent images are acquired continuously.
Relationships between (a) VCL and MMP and (b) Pesc and MMP
The swimming speed value measured prior to trapping the sperm is used as the VCL parameter. Swimming force is measured in terms of escape laser power, Pesc (minimum laser power needed to hold a sperm in the optical trap is directly proportional to the sperm’s swimming force: F = Q × P/c where F is the swimming force in the pN range, P the laser power in mW, c the speed of light in the medium with a given index of refraction, and Q the geometrically determined trapping efficiency parameter) (Konig et al., 1996). The average of the five fluorescent ratio values measured prior to trapping the sperm is used as the MMP measurement. The relationships between VCL and MMP as well as Pesc and MMP are investigated for both dog and human species.
Effects of glucose, a glycolytic inhibitor and oxidative phosphorylation inhibitors on VCL, Pesc and MMP for human sperm
For the next series of experiments, human sperm are prepared in the following manner. After thawing, human sperm are centrifuged and washed with BWW media lacking glucose. The suspension is divided into aliquots of equal size and centrifuged a second time. Each aliquot is then washed with either BWW media lacking glucose, BWW media containing glucose at a 5.55 mM concentration, or HTF media containing glucose at a 2.78 mM concentration. Sperm from each aliquot are labeled with DiOC2(3) (see labeling method above). For the inhibitor experiments, certain aliquots of sperm are exposed to the inhibitor simultaneously with the dye. Sperm are then further diluted prior to analysis in the appropriate media (that with which it was washed the second time). Table 1 lists which aliquots are washed with which media for each experiment. In addition, the table lists which aliquots are exposed to a given inhibitor. The sperm are then analyzed. The distributions of the three measured parameters (VCL, Pesc and MMP) are statistically compared.
TABLE 1.
Summary of human sperm preparation for experiments that test the effects of glucose, a glycolytic inhibitor and oxidative phosphorylation inhibitors on sperm motility and MMP
| Experiment to test effects of … |
# Aliquots made |
Media used for second wash and subsequent dilution for aliquot # … |
Aliquots that are exposed to respective inhibitor |
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Glucose | 3 | BWW lacking glucose | BWW containing glucose | HTF | — | — |
| DOG | 3 | BWW lacking glucose | BWW lacking glucose | BWW containing glucose | — | 2, 3 |
| Antimycin A | 4 | BWW lacking glucose | BWW lacking glucose | BWW containing glucose | HTF | 2, 3, 4 |
| Rotenone | 4 | BWW lacking glucose | BWW lacking glucose | BWW containing glucose | HTF | 2, 3, 4 |
First, the effects of different concentrations of glucose (0 mM in BWW media lacking glucose, 5.55 mM in BWW media containing glucose, and 2.78 mM in HTF media) on sperm motility and MMP are assessed. Then, the effects of three different inhibitors on sperm motility and MMP are assessed. 2-deoxy-D-glucose (DOG, 6 mM), an anti-metabolite of glucose, is used to inhibit the glycolytic pathway (Mukai and Okuno, 2004). The DOG experiment is repeated three times to verify the results. Data from the three experiments are pooled together. Antimycin A (40 µM) is used to inhibit the oxidative phosphorylation pathway via the electron transport chain (inhibits the oxidation of ubiquinol in complex III) (Krzyzosiak et al., 1999; Mukai and Okuno, 2004). The high concentration of antimycin A was chosen as preliminary studies working with human sperm suspended in HTF media showed no decrease in MMP or motility measurements when exposed to 5, 10, 20, or 40 µM (data not shown). The experiment is repeated three times to verify the results. Data from the three experiments are pooled together. Rotenone (40 µM) is used to inhibit the oxidative phosphorylation pathway via the electron transport chain (inhibits the transfer of electrons from the iron-sulfur centers to ubiquinone in complex I) (Krzyzosiak et al., 1999). The high concentration of rotenone was chosen as preliminary studies working with human sperm suspended in HTF media showed no decrease in MMP or motility measurements when exposed to 20, 40, or 50 µM (data not shown). The experiment is repeated three times to verify the results. Data from the three experiments are pooled together.
Statistical analysis
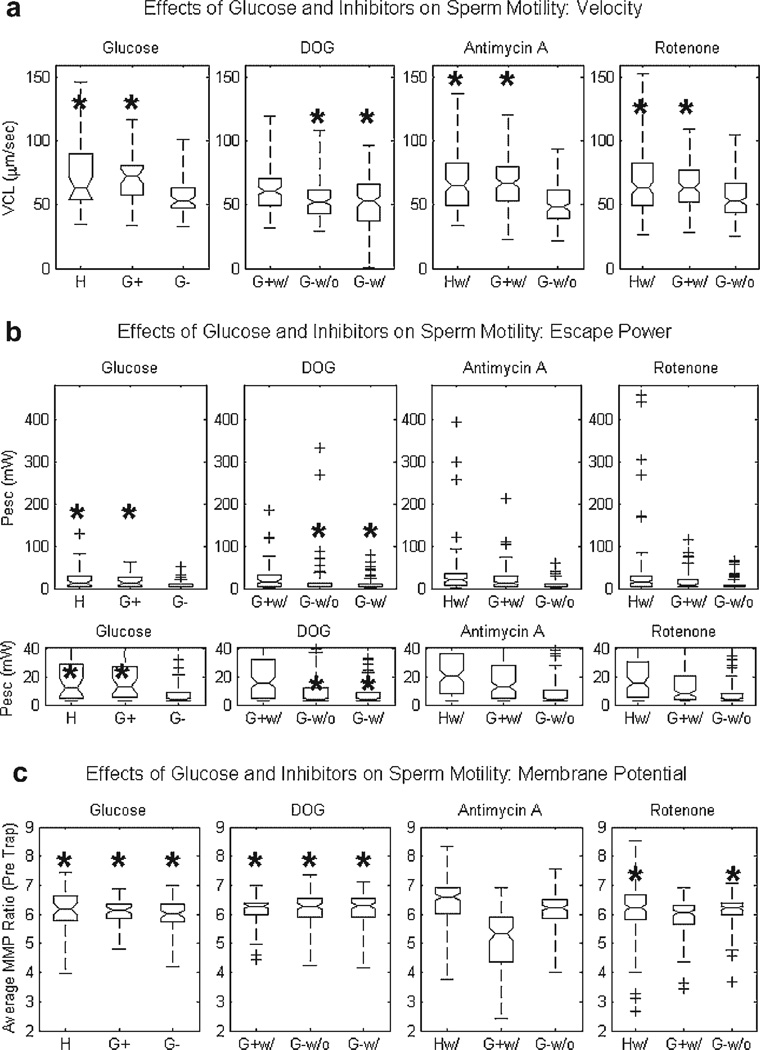
The VCL, Pesc and MMP distributions resulting from the experiments testing the effects of glucose and the three inhibitors are statistically compared using the non-parametric Wilcoxon rank sum test (based on 5% significance). (The Wilcoxon test is a two-sided rank sum test of the hypothesis that two independent samples come from distributions with equal medians. Since the distributions are not Gaussian, statistical comparison requires a non-parametric test.) The distributions are displayed graphically in box plot form (see Fig. 3). Each box plot displays the following parameters for a given distribution: (a) median (center line of box), (b) lower and upper quartile values (bottom and top line of box, respectively), (c) the range of the data (dashed lines extending from the top and bottom of box), and (d) the data points lying outside three times the interquartile range (labeled as ‘+’ marks). Notches in the box represent an estimate of the uncertainty about the median value. If notches on the box plots of two groups do not overlap, it can be concluded with 95% confidence that the two medians differ.
Fig. 3.
Effects of glucose, DOG, Antimycin A and Rotenone on human sperm (a) swimming speed (VCL), (b) swimming force (Pesc) and (c) mitochondrial membrane potential (MMP): The four sub-plots demonstrate the effects of glucose, DOG, Antimycin A and Rotenone (from left to right) for parts a–c. Asterisks (*) indicate statistically equal distributions within a given category. The Pesc distributions in (b) are shown twice: the top set of plots displays the full escape power range, whereas the bottom set of plots scales they-axis to emphasize differences in median values. ‘H’ stands for Human Tubal Fluid media with glucose; ‘G+’ stands for BWW media with glucose; ‘G−’ stands for BWW media without glucose; ‘w/’and ‘w/o’ stand for with and without a given inhibitor, respectively. Sperm suspended in BWW media without glucose are found to be immotile when exposed to either antimycin A or rotenone and thus not plotted.
Results
Relationships between (a) VCL and MMP and (b) Pesc and MMP
A total of 309 dog sperm are analyzed. VCL and Pesc are both plotted against MMP (Fig. 1a,b respectively). No relationship between either set of parameters is found. A total of 255 human sperm are analyzed. VCL and Pesc are both plotted against MMP (Fig. 2a,b respectively). Again, no relationship between either set of parameters is found. The results for both the dog and human demonstrate a lack of relationship between sperm motility and mitochondrial respiration.
Fig. 1.
Dog—swimming speed and escape power versus fluorescence ratio pre-trap: (a) VCL and (b) Pesc are plotted against the average ratio value prior to being trapped for dog sperm. Each data point represents an individual sperm(N=309). No relationship found between sperm motility and mitochondrial respiration.
Fig. 2.
Human—swimming speed and escape power versus fluorescence ratio pre-trap: (a) VCL and (b) Pesc are plotted against the average ratio value prior to being trapped for human sperm. Each data point represents an individual sperm (N=255). No relationship found between sperm motility and mitochondrial respiration.
Effects of glucose, a glycolytic inhibitor and oxidative phosphorylation inhibitors in the media on VCL, Pesc and MMP
Glucose
VCL, Pesc and MMP are measured for human sperm suspended in three different media with varying concentrations of glucose. The box plot distributions of the measurements shown in Figure 3a–c (the data set is the first sub-plot, from the left, and labeled “Glucose”) are labeled as follows: sperm suspended in HTF media are labeled ‘H’ (N=40), in BWW media containing glucose are labeled ‘G+’ (N=40), in BWW media lacking glucose are labeled ‘G−’ (N=40). For VCL and Pesc (Fig. 3a,b respectively), sperm in both media containing glucose(BWW and HTF) are found to be statistically equal (P>0.05; equality indicated by the asterisks above the ‘H’ and ‘G+’ distributions in Fig. 3a,b). The sperm in the BWW media lacking glucose are found to be statistically slower and weaker. However, the MMP distributions of sperm from all three groups are found to be statistically equal (P>0.05; equality indicated by the asterisks above all three distributions in Fig. 3c). Therefore, neither sperm motility nor MMP are affected by the differences in the two media containing glucose (BWW and HTF). The results demonstrate that the absence of glucose does significantly affect sperm motility but not MMP.
DOG
VCL, Pesc and MMP are measured for human sperm exposed to the glycolytic inhibitor, DOG. The distributions shown in Figure 3a–c (the data set is the second sub-plot, from the left, and labeled “DOG”) are labeled as follows: sperm suspended in BWW media containing both glucose and DOG are labeled ‘G+w/’ (N=120), in BWW media lacking both glucose and DOG are labeled ‘G-w/o’ (N=120), in BWW media lacking glucose but containing DOG are labeled ‘G-w/’ (N=120). Sperm in BWW media lacking both glucose and DOG are found to be statistically equal in terms of VCL and Pesc to sperm in BWW media lacking glucose but containing DOG (P>0.05; equality indicated by the asterisks above the ‘G-w/o’ and ‘G-w/’ distributions in Fig. 3a,b). Sperm in BWW media containing both glucose and DOG are found to be statistically faster and stronger than sperm in either of the other two media (P<0.05). All three groups are found to have equal MMP distributions (P>0.05; equality indicated by the asterisks above all three distributions in Fig. 3c). Therefore, the presence of DOG does not affect sperm mitochondrial MMP. These results are consistent with the findings in a previous study that showed that DOG had no effect on mitochondrial respiration (as assessed by fluorescence from the probe JC-1) and failed to suppress flagellar movement in mice sperm when glucose was present in the media (Mukai and Okuno, 2004).
Antimycin A
VCL, Pesc and MMP are measured for human sperm exposed to the inhibitor, antimycin A. The distributions shown in Figure 3a–c (the data set is the third sub-plot, from the left, and labeled “Antimycin A”) are labeled as follows: sperm suspended in HTF media containing antimycin A are labeled ‘Hw/’ (N=120), in BWW media containing both glucose and antimycin A are labeled ‘G+w/’ (N=120), and in BWW media lacking both glucose and antimycin A are labeled ‘G-w/o’ (N=120). Sperm suspended in media lacking glucose exposed to antimycin A are found to be immotile and therefore not measured. The velocity distributions of sperm in the two media containing both glucose and antimycin A are found to be statistically equal (P>0.05; equality indicated by the asterisks above the ‘G+w/’ and ‘Hw/’ distributions in Fig. 3a). In addition, they were found to be statistically faster than sperm in BWW media lacking both glucose and antimycin A (P<0.05). This result is consistent with that found in a previous study (antimycin A was not able to suppress flagellar movement in mice sperm when glucose was present in the media but did when glucose was absent) (Mukai and Okuno, 2004). Sperm in either of the two media containing both glucose and antimycin A are found to have statistically greater swimming forces (Pesc) than sperm in media lacking both glucose and the inhibitor (P<0.05). Sperm in the HTF media containing both glucose and antimycin A are found to have statistically greater median Pesc and MMP values than sperm in BWW media containing both glucose and antimycin A (P<0.05). In addition, sperm in BWW media lacking both glucose and antimycin A have a greater median MMP value than the sperm in BWW media containing both glucose and antimycin A. Thus, HTF media appears to be a better buffer against the effects of antimycin A than BWW media.
Rotenone
VCL, Pesc and MMP are measured for human sperm exposed to the inhibitor, rotenone. The distributions shown in Figure 3a–c (the data set is the fourth sub-plot, from the left, and labeled “Rotenone”) are labeled as follows: sperm suspended in HTF media containing rotenone are labeled ‘Hw/’ (N=120), in BWW media containing both glucose and rotenone are labeled ‘G+w/’ (N=120), and in BWW media lacking both glucose and rotenone are labeled ‘G-w/o’ (N=120). Sperm in media lacking glucose exposed to rotenone are found to be immotile and therefore not measured. The velocity distributions of sperm in the two media containing both glucose and rotenone are found to be statistically equal (P>0.05; equality indicated by the asterisks above the ‘G+w/’ and ‘Hw/’ distributions in Fig. 3a). In addition, they were found to be statistically faster than sperm in the BWW media lacking both glucose and rotenone (P<0.05). This result is consistent with that found in a previous study (rotenone did not significantly reduce bull sperm VCL when glucose was present in the media but eliminated motility when glucose was absent) (Krzyzosiak et al., 1999). Sperm in either of the two media containing both glucose and rotenone are found to have statistically greater swimming forces (Pesc) than sperm in media lacking both glucose and inhibitor (P<0.05). Sperm in the HTF media containing both glucose and rotenone are found to have statistically greater median Pesc and MMP values than sperm in BWW media containing both glucose and rotenone (P<0.05). Thus, again, HTF media appears to be a better buffer against the effects of rotenone than BWW media containing glucose. The MMP of sperm in the HTF media with rotenone is found to be statistically the same as that of the sperm in BWW media lacking both glucose and rotenone (P>0.05; equality indicated by the asterisks above the ‘Hw/’ and ‘G-w/o’ distributions in Fig. 3c).
Discussion
In this article, a combination of laser tweezers, fluorescent imaging and robotics was used to study the roles of oxidative phosphorylation and glycolysis in sperm motility. First, the relationships between (a) VCL and MMP and (b) Pesc and MMP were analyzed for both dog and human sperm. Interestingly, neither species showed any relationship between either set of parameters. This suggests that sperm motility is independent of mitochondrial respiration as measured by MMP. Furthermore, this suggests that sperm rely on alternative pathway(s) for the energy needed to sustain motility.
Second, the article examines the role of glucose on human sperm motility. No statistical difference in motility (VCL or Pesc) or MMP was found for sperm incubated in either of the two media containing glucose even though the concentration of the substrate for each media was different. The mitochondria of sperm suspended in media lacking glucose were found to be operating at a level equal to that of sperm in either media containing glucose (MMP distributions of the sperm in HTF, BWW with and without glucose were all statistically equal; P>0.05, see Fig. 3c). Although the sperm suspended in media lacking glucose were motile, they showed a significant decrease in VCL and Pesc. The addition of the glycolytic inhibitor, DOG, to the sperm in media lacking glucose did not further suppress motility. However, the addition of either oxidative phosphorylation inhibitor, antimycin A or rotenone, did fully suppress motility. These results demonstrate that, with respect to sperm motility, oxidative phosphorylation does not contribute as much energy in human sperm as compared to that supplied by glycolysis.
The inhibition of the electron transport chain by antimycin A and rotenone is demonstrated by the decrease in MMP of sperm suspended in BWW media containing glucose with respect to the MMP of sperm suspended in BWW media lacking both glucose and the respective inhibitor (see Fig. 3c). This inhibition did not decrease sperm swimming speed (see VCL distribution in Fig. 3a) but did decrease their swimming force (see Pesc distribution in Fig. 3b), as compared to the distributions of sperm in HTF media with the respective inhibitor. The subtle differences in concentrations of substrates in HTF appear to make it a better buffer against the effects of either drug. Nonetheless, the sperm in media lacking both glucose and the inhibitor were still slower and weaker than sperm in media containing both glucose and inhibitor. These results suggest that the energy supplied by oxidative phosphorylation alone is unable to sustain high motility and can be considered negligible when glucose is present.
The results reported here explain the lack of decrease in motility when dog sperm were exposed to CCCP even though MMP was significantly reduced (Nascimento et al., 2008). In addition, the results of the effects of electron transport chain and glycolytic inhibitors for human sperm, namely that the human sperm were found to be motile in media containing glucose and either antimycin A or rotenone, are consistent with those reported for mice (Mukai and Okuno, 2004) and bull sperm (Krzyzosiak et al., 1999). In conclusion, both oxidative phosphorylation and glycolysis are shown to generate energy for human sperm motility. However, unlike sperm from other mammalian species such as mice and bulls (Krzyzosiak et al., 1999; Galantino-Homer et al., 2004; Mukai and Okuno, 2004), it appears that the glycolytic pathway plays a more significant role in supplying energy for human sperm motility. The fact that no relationship exists between motility (VCL and Pesc) and MMP as seen in Figure 2, therefore, is not surprising, but rather is to be expected since glucose is present in the media.
Acknowledgments
This work was supported by funds from the Beckman Laser Institute, Inc., Foundation and a grant from the Air Force Office of Scientific Research (AFOSR # F9620-00-1-0371) awarded to MWB.
Footnotes
Additional supporting information may be found in the online version of this article.
Literature Cited
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Energy conversion: Mitochondria and chloroplasts. In: Gibbs S, editor. The molecular biology of the cell. 4th edition. New York: Garland Science; 2002. p. 769. [Google Scholar]
- Amann RP, Katz DF. Reflections on CASA after 25 years. J Androl. 2004;25:317–325. doi: 10.1002/j.1939-4640.2004.tb02793.x. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whitten WD, Whittingham DG. The culture of mouse embryos in vitro. In: Daniel JC, editor. Methods of mammalian embryology. San Francisco, California: Freeman; 1971. pp. 86–116. [Google Scholar]
- DiMarzo SJ, Rakoff JS. Intrauterine insemination with husband’s washed sperm. Fertil Steril. 1986;46:470–475. doi: 10.1016/s0015-0282(16)49588-9. [DOI] [PubMed] [Google Scholar]
- DiMarzo SJ, Huang J, Kennedy JF, Villanueva B, Hebert SA, Young PE. Pregnancy rates with fresh versus computer-controlled cryopreserved semen for artificial insemination by donor in a private practice setting. Am J Obstet Gynecol. 1990;162:1483–1490. doi: 10.1016/0002-9378(90)90910-y. [DOI] [PubMed] [Google Scholar]
- Durrant BS, Harper D, Amodeo A, Anderson A. Effects of freeze rate on cryosurvival of domestic dog epididymal sperm. J Androl. 2000;59:21. [Google Scholar]
- Ethics Committee of the American Fertility Society. New Guidelines for the use of Semen for Donor Insemination. Fertil Steril. 1986;6:85. [Google Scholar]
- Ford WCL. Glycolysis and sperm motility: Does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12:269–274. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS. Bovine sperm capacitation: Assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev. 2004;67:487–500. doi: 10.1002/mrd.20034. [DOI] [PubMed] [Google Scholar]
- Gallon F, Marchetti C, Jouy N, Marchetti P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril. 2006;86:1526–1530. doi: 10.1016/j.fertnstert.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Harper SA, Durrant BS, Russ KD, Bolamba D. Cryopreservation of domestic dog epididymal sperm: A model for the preservation of genetic diversity. J Androl. 1998;50:19. [Google Scholar]
- Kasai T, Ogawa K, Mizuno K, Nagai S, Uchida Y, Ohta S, Fujie M, Suzuki K, Hirata S, Hoshi K. Relationship between spermmitochondrial membrane potential, sperm motility, and fertility potential. Asian J Androl. 2002;4:97–103. [PubMed] [Google Scholar]
- Kim YH, Haidl G, Schaefer M, Egner U, Mandal A, Herr JC. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Falgellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol. 2007;302:463–476. doi: 10.1016/j.ydbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig K, Svaasand L, Liu Y, Sonek G, Patrizio P, Tadir Y, Berns MW, Tromberg BJ. Determination of motility forces of human spermatozoa using an 800 nm optical trap. Cell Mol Biol (Noisy-le-grand) 1996;42:501–509. [PubMed] [Google Scholar]
- Krisfalusi M, Miki K, Magyar PL, O’Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75:270–278. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- Krzyzosiak J, Molan P, Vishwanath R. Measurements of bovine sperm velocities under true anaerobic and aerobic conditions. Anim Reprod Sci. 1999;55:163–173. doi: 10.1016/s0378-4320(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Liaw LH, Berns MW. Electron microscope autoradiography on serial sections of preselected single living cells. J Ultrastruct. 1981;75:187–194. doi: 10.1016/s0022-5320(81)80134-7. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species,DNAfragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Jouy N, Leroy-Martin B, Deffosez A, Formstecher P, Marchetti P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod. 2004;19:2267–2276. doi: 10.1093/humrep/deh416. [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- Nascimento JM, Botvinick EL, Shi LZ, Durrant B, Berns MW. Analysis of sperm motility using optical tweezers. J Biomed Opt. 2006;11:044001. doi: 10.1117/1.2337559. [DOI] [PubMed] [Google Scholar]
- Nascimento JM, Shi LZ, Meyers S, Gagneux P, Loskutoff NM, Botvinick EL, Berns MW. The use of optical tweezers to study sperm competition and motility in primates. J R Soc Interface. 2007;5:297–302. doi: 10.1098/rsif.2007.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento JM, Shi LZ, Chandsawangbhuwana C, Tam J, Durrant B, Botvinick EL, Berns MW. Use of laser tweezers to analyze sperm motility and mitochondrial membrane potential. J Biomed Opt. 2008;13:014002. doi: 10.1117/1.2839051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo D, Perlmutter NG, Hunt RH, Shapiro HM. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry. 1999;35:55–63. doi: 10.1002/(sici)1097-0320(19990101)35:1<55::aid-cyto8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Novo D, Perlmutter NG, Hunt RH, Shapiro HM. Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob Agents Chemother. 2000;44:827–834. doi: 10.1128/aac.44.4.827-834.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfini P, Marrs RP. Computerized staged-freezing technique improves sperm survival and preserves penetration of zona-free hamster ova. Fertil Steril. 1986;45:854–858. doi: 10.1016/s0015-0282(16)49406-9. [DOI] [PubMed] [Google Scholar]
- Shi LZ, Nascimento JM, Chandsawangbhuwana C, Berns MW, Botvinick E. Real-time automated tracking and trapping system (RATTS) Microsc Res Tech. 2006;69:894–902. doi: 10.1002/jemt.20359. [DOI] [PubMed] [Google Scholar]
- Shi LZ, Nascimento JM, Chandsawangbhuwana C, Botvinick EL, Berns MW. An automatic system to study sperm motility and energetics. J Biomed Microdev. 2008;10:573–583. doi: 10.1007/s10544-008-9169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadir Y,Wright WH, Vafa O, Ord T, Asch RH, Berns MW. Force generated by human sperm correlated to velocity and determined using a laser generated optical trap. Fertil Steril. 1990;53:944–947. doi: 10.1016/s0015-0282(16)53539-0. [DOI] [PubMed] [Google Scholar]
- Toffle RC, Nagel TC, Tagatz GE, Phansey SA, Okagaki T, Wavrin CA. Intrauterine insemination: The University of Minnesota Experience. Fertil Steril. 1985;43:743–747. doi: 10.1016/s0015-0282(16)48558-4. [DOI] [PubMed] [Google Scholar]
- Turner RM. Tales from the tail: What dowe really know about sperm motility? J Androl. 2003;24:790–803. doi: 10.1002/j.1939-4640.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Williams AC, Ford WCL. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001;22:680–695. [PubMed] [Google Scholar]