Abstract
Despite differences in the crystal structures of ice and nucleosides, antifreeze polypeptides (AFPs) have been demonstrated to inhibit nucleation of 5-methyluridine, cytidine, and inosine and modify the crystal growth of the nucleosides efficiently. The molecular recognition repertoire of AFPs has been expanded to non-ice-like crystalline solids.
Crystal growth control is essential in various fields in science and technology (e.g., chemistry, materials science, pharmaceutical development).1–3 The concept of molecular recognition has been successfully used to elucidate the effects of additives (foreign ions or molecules) on crystal growth.4 Peptides and proteins are often used in vivo and in vitro to control the growth of minerals and produce new forms of solids with different physicochemical properties.5–8 Size and shape control of organic crystals, however, is more difficult due to their anisotropic properties (different atomic arrangements in three dimensions).1,9,10
Antifreeze polypeptides (AFPs) are a structurally diverse group of proteins found in many cold-adapted organisms (e.g., fish, insect) to protect them from freeze damage through a noncolligative manner, providing an intriguing example of ice crystal growth control. AFPs bind to specific faces of ice crystals and modify the habit of the ice crystals.11 Their affinity to ice depends on hydrogen bonding and hydrophobic interactions, unlike most protein-mineral interactions where ionic interactions often play a dominant role.4 Ice and clathrate hydrates (ice-like crystalline solids)12 are known to be inhibited and modified by AFPs. Nevertheless, we speculate that the recognition ability of AFPs is beyond ice and ice-like crystalline solids based on the facts: (1) ice surfaces are not well ordered at the molecular level compared to other inorganic or organic crystal surfaces13 and (2) AFPs coexist with many other solutes in vivo (e.g., various low molecular solutes present with AFPs in the hemolymph of Dendroides canadensis)14 diminishing the chance of the growth of single ice crystals.
In this study, we for the first time demonstrate that AFPs can efficiently inhibit the nucleation and modify the single crystal growth of 5-methyluridine (m5U), cytidine (C), and inosine (I). m5U, C, and I are widely used nucleosides in pharmaceutical industry, but little is known about their size and shape control using additives.15–18 This study also presents a distinct example of effective control of nucleoside crystal growths by additives.
We obtained the crystals of m5U, C, and I, respectively, by evaporation of their aqueous solutions at room temperature (ESI†). The resulting crystals of the three nucleosides appear as orthorhombic needles (Figs. 1a, S2, and S4).19 The effect of a beetle AFP from D. canadensis (DAFP-1) on m5U crystal growth was first investigated. DAFP-1 is a β-helical repeat protein with a size of 9 kDa containing 8 disulfide bonds, which are important for its structure and function as antifreeze.20 DAFP-1 was studied previously in our laboratory for its antifreeze enhancement effect by small molecular enhancers and interactions between DAFP-1 and reduced nicotinamide adenine dinucleotide in solution have been demonstrated recently.21 To completely inhibit single crystal growth, a reasonable additive/nucleoside molar ratio (the critical ratio) is needed. Different amounts of DAFP-1 were added directly to m5U solution to determine the critical ratio of DAFP-1/m5U (ESI†). The direct addition of DAFP-1 at all the tested concentrations delayed the first appearances of m5U precipitates in the solutions (Table S1), while higher additive/m5U molar ratios resulted in more significant delay. The critical ratio of DAFP-1/m5U was estimated to be 3.0 × 10−5, where the ratio is at 3.0 × 10−5 or higher, no single m5U crystal was detected, but reed-like amorphous m5U precipitates (Fig. 1b).
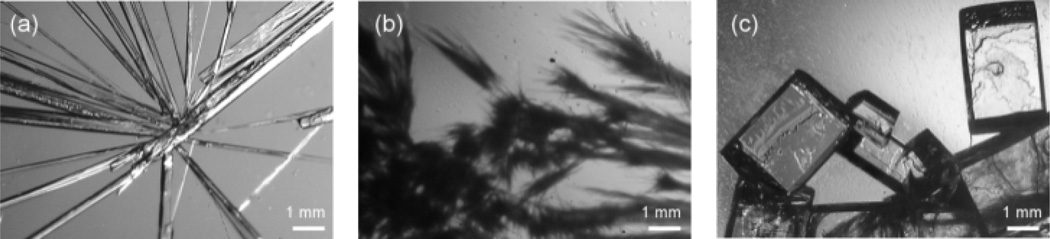
Fig. 1.
Optical micrographs of the finally achieved m5U solids. (a) Needle-like orthorhombic m5U crystals, (b) reed-like amorphous m5U precipitates obtained in the presence of DAFP-1, (c) normal orthorhombic m5U crystals obtained in the presence of DAFP-1 and m5U seed crystals.
Additives usually affect crystal growths through (1) affecting nucleation (i.e., prevent, delay, or promote nucleation); (2) modifying crystal habit (i.e., adsorbing onto specific crystal growing face(s) and changing its growth rate); or (3) both of (1) and (2).1,22 To further investigate how AFPs affect m5U crystal growth, DAFP-1 was added to the saturated m5U solutions in the presence of m5U seed crystals at the additive/m5U molar ratio of 1.2 × 10−5. The growth of m5U crystals was able to continue, but with an apparent change in the crystal habit, from needle-shaped to normal orthorhombic (Fig. 1c).
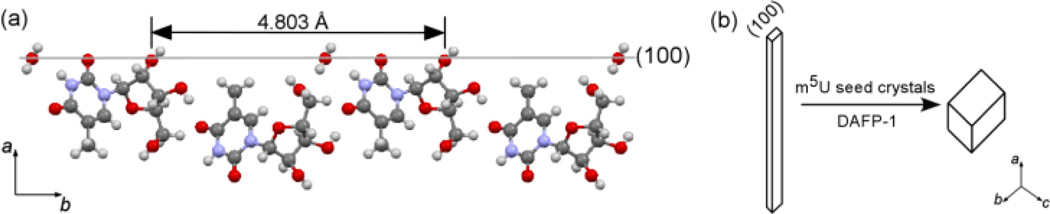
Examining by x-ray crystallography, the crystalline m5U samples in figs. 1a and 1c have the same orthorhombic crystal structure, while no samples in fig. 1b are suitable for single crystal x-ray diffraction (ESI†). In the absence of DAFP-1, the propagation of the needle-like m5U crystals is along the a-axis. The crystal habit is determined by the relative growth rates of different crystal faces, suggesting that the altered crystal habit in the presence of DAFP-1 was caused by selective interaction of DAFP-1 with the (100) faces of m5U crystals (Fig. 2). The distance between the repeated oxygen atoms in the ribose moieties on the (100) face of m5U crystal, 4.80 Å (Fig. 2a), matches well to 4.74 Å, the average distance of the hydroxyl oxygen atoms in conserved repeated threonine residues in adjacent loops of DAFP-1,20 suggesting hydrogen bonding interactions between the hydroxyl groups of the ribose moieties in m5U crystal and conserved threonines of DAFP-1. Due to such interactions, m5U crystal growth rate along the a-axis decreased to be less than those along the b- or c-axis, resulting in normal orthorhombic m5U crystals (Fig. 2b).
Fig. 2.
(a) The structure of the (100) face of m5U crystals (carbon, grey; oxygen, red; hydrogen, white; nitrogen, blue). The c-axis points into the plane of the paper. (b) Schematic representation: the growth rate along the a-axis is much faster than that along the b- or c-axis in a typical m5U crystal habit, while the growth along the a-axis is significantly inhibited in the presence of DAFP-1 becoming less than that along the b- or c-axis.
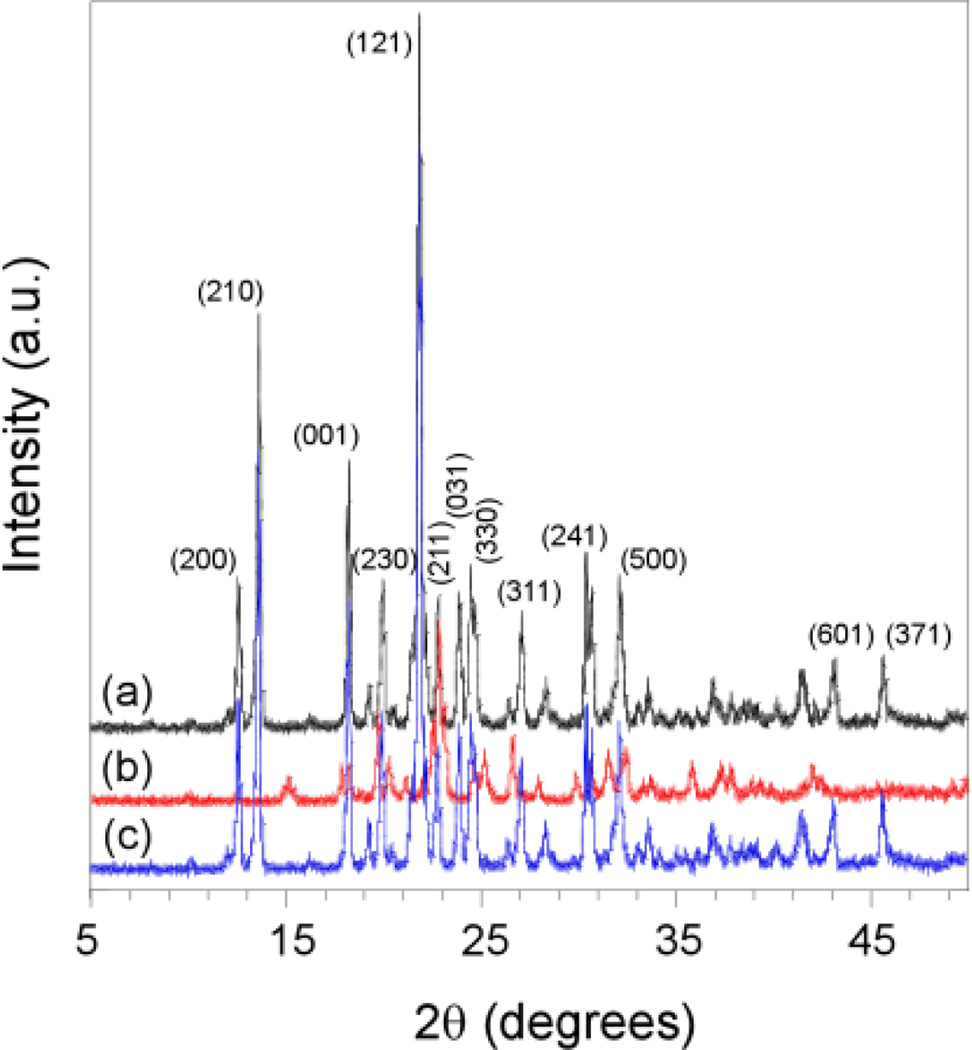
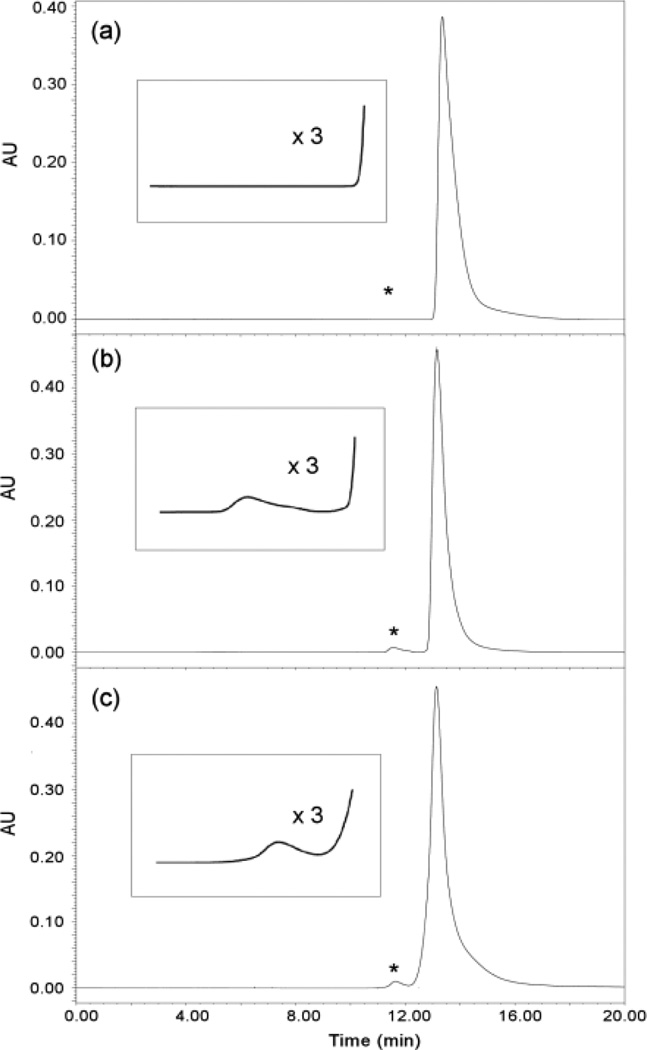
The powder X-ray diffraction (PXRD) patterns (Fig. 3a and 3c, ESI†) of the single crystalline m5U samples in figs. 1a and 1c show the same characteristic peaks assigned to the same unit cell. In contrast, few peaks of very low intensity were observed in the PXRD pattern (Fig. 3b) of the reed-like amorphous m5U precipitates in fig. 1b, suggesting that these solids lack well-ordered structures, and the few weak peaks that cannot be assigned to any known crystalline phase of m5U might result from a local order in these m5U samples.23 The PXRD results are in good agreement with the single crystal X-ray diffraction data. The finally obtained solids were also analyzed using high performance liquid chromatography (HPLC) to identify whether DAFP-1 presents in the samples (ESI†). The HPLC analysis (Figs. 4b and 4c) indicated the presence of both m5U and DAFP-1 in the samples in figs. 1b and 1c, suggesting that DAFP-1 is occluded in the reed-like amorphous m5U precipitates and the normal orthorhombic m5U crystals, which supports interactions between DAFP-1 and m5U.
Fig. 3.
Overlay of representative powder XRD profiles of the samples from (a) needle-like crystalline m5U, (b) reed-like amorphous m5U precipitates obtained in the presence DAFP-1, and (c) normal orthorhombic crystalline m5U modified by DAFP-1. Major crystalline peaks in (a) are labeled with miller indexes (hkl).
Fig. 4.
Gel filtration HPLC analysis of the crystals of m5U monitored at 280 nm. Typical chromatograms of (a) crystalline m5U obtained in the presence of denatured DAFP-1, (b) reed-like amorphous m5U precipitates obtained in the presence of DAFP-1, (c) normal orthorhombic crystalline m5U modified using DAFP-1 in the presence of m5U seed crystals. The retention times for m5U and DAFP-1 are 13.1 min and 11.5 min, respectively. Insets: enlarged portions (denoted by asterisks).
Under the same conditions, m5U crystal growth was also investigated in the presence of two controls, bovine serum albumin (BSA) and denatured DAFP-1 with complete reduction of its disulfide bonds, respectively (ESI†). Neither BSA nor the denatured DAFP-1 inhibited or delayed the appearance of m5U precipitates at the control/m5U molar ratio of 1.8 × 10−4, a much higher ratio than those used for DAFP-1 (Figs. S1a–b, Table S1). The crystals were characterized to be m5U by X-ray crystallography and the results were confirmed by PXRD (ESI†). HPLC analysis of these crystals revealed that only pure m5U presents, suggesting that the controls are simply excluded from the m5U crystals as impurities (ESI†). These results indicate that the controls have no effect on the crystal growth of m5U.
To test the generality of the findings, the effect of DAFP-1 and the controls on the crystal growth of two other nucleosides, C and I, were examined. The space groups of m5U, C and I are all orthorhombic and repeated oxygen atoms in the hydroxyl groups in their ribose moieties are identified on their growing crystal faces, but the dimensions of the unit cells of these nucleosides are quite different.24–26 The two controls, BSA and the denatured DAFP-1, had no effects on the crystal growth of C and I. Interestingly, the effects of growth inhibition and habit modification were also observed for DAFP-1 on the crystal growth of C and I (Figs. S2–S5, Table S1), suggesting that DAFP-1 can also interact with C and I crystals and such recognition can be flexible. The critical ratios of DAFP-1/C and /I were estimated to be 3.0 × 10−5 and 0.5 × 10−5 (ESI†). To determine whether a different type of AFP can have such effects, all the above experiments were carried out with a globular fish AFP, type III AFP.27 Similarly, direct additions of type III AFPs in the m5U, C, and I solutions effectively inhibited the crystal nucleation of m5U, C, and I, respectively (Figs. S1c, S2f, S4f, Table S1). Additions of type III AFPs in the presence of the seed crystals of m5U, C, and I, altered the crystal habits of these nucleosides, respectively, and the resulting habits (Figs. S1d, S2g, S4g) are similar to those caused by DAFP-1. However, we did not observe such inhibitory or crystal habit change effect of the AFPs on the crystal growth of thymine, the component base of m5U, under similar conditions (Fig. S6, ESI†), suggesting that the hydroxyl containing ribose plays an important role in the recognition of m5U by AFPs.
In conclusion, the inhibitory and habit-modifying effects of AFPs on the stable nuclei formations and on the crystals of nucleosides have been first demonstrated. Such effects of AFPs are analogous to their effects on ice28 despite the large structural differences among ice Ih and these nucleoside crystals,24,26,29 suggesting flexibility in AFP molecular recognition. The crystal-recognition repertoire of AFPs has been expanded beyond ice and ice-like crystals. Moreover, the effects of AFPs on the nucleoside crystal growth are highly efficient, comparing with other additives on crystal growth control.1,22 The results encourage further studies on the roles of AFPs in controlling the crystal growths of polycrystalline ice and other important hydroxyl compounds (e.g., nucleoside analogues, glycosides, sugars) and the use of AFPs, particularly DAFP-1 (which can withstand a wide range of pHs and temperatures)30 as scaffolds to design novel, effective crystal growth inhibitors and modifiers in a number of fields including chemistry, materials science, and pharmaceutical industry.
Supplementary Material
Acknowledgments
The authors thank Prof. John Duman at University of Notre Dame for cDNA of DAFP-1; Dr. Michael Day and Mr. Larry Henling for X-ray crystallographic structural determination and Prof. Mark Davis and Mr. Mark Deimund for PXRD instrument at California Institute of Technology. XW acknowledges support by NIH (GM086249) and Research Corporation (CC10492).
Footnotes
Electronic Supplementary Information (ESI) available: CCDC 835399, Figs. S1–S6, Table S1, and experimental section. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b000000x/
Crystal data for m5U: C10H14N2O6 • 0.5(H2O) were collected on a Bruker SMART 1000 diffractometer at 100(2) K using 0.71073 Å MoKα. q range for 9892 reflections used in lattice determination: 2.77 to 30.46°; Mr = 267.24; a = 13.9973(5) Å, b = 17.2440(6) Å, c = 4.8028(2) Å, α = β = γ 90°; V = 1159.25(8) Å3; Z = 4; Orthorhombic; P21212; F(000): 564; Total data 30650, independent data 3535 [Rint = 0.0432]; Goodness-of-fit on F2: 2.877; R1 [I>2σ(I)] = 0.0318, wR2 = 0.0554.
Contributor Information
Sen Wang, Email: swang02@stanford.edu.
Xin Wen, Email: xwen3@calstatela.edu.
Notes and references
- 1.Mullin JW. Crystallization. 4th ed. Oxford: Butterworth-Heinemann; 2001. [Google Scholar]
- 2.Cox JR, Ferris LA, Thalladi VR. Angew. Chem. Int. Ed. 2007;46:4333–4336. doi: 10.1002/anie.200605257. [DOI] [PubMed] [Google Scholar]
- 3.Cölfen H. Angew. Chem. Int. Ed. 2008;47:2351–2353. doi: 10.1002/anie.200800418. [DOI] [PubMed] [Google Scholar]
- 4.Mann S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry. 1st edition ed. USA: Oxford University Press; 2002. [Google Scholar]
- 5.Belcher M, Wu XH, Christensen RJ, Hansma PK, Stucky GD, Morse DE. Nature. 1996;381:56–58. [Google Scholar]
- 6.Falini G, Albeck S, Weiner S, Addadi L. Science. 1996;271:67–69. [Google Scholar]
- 7.DeOliveira DB, Laursen RA. J. Am. Chem. Soc. 1997;119:10627–10631. [Google Scholar]
- 8.Volkmer D, Fricke M, Huber T, Sewald N. Chem. Commun. 2004:1872–1873. doi: 10.1039/b405613b. [DOI] [PubMed] [Google Scholar]
- 9.Miller JM, Collman BM, Greene LR, Grant DJ, Blackburn AC. Pharm. Dev. Technol. 2005;10:291–297. doi: 10.1081/pdt-54467. [DOI] [PubMed] [Google Scholar]
- 10.Chemburkar SR, Bauer J, Deming K, Spiwek H, Patel K, Morris J, Henry R, Spanton S, Dziki W, Porter W, Quick J, Bauer P, Donaubauer J, Narayanan BA, Soldani M, Riley D, McFarland K. Org. Process Res. Dev. 2000;4:413–417. [Google Scholar]
- 11.Raymond JA, Wilson P, DeVries AL. Proc. Natl. Acad. Sci. U. S. A. 1989;86:881–885. doi: 10.1073/pnas.86.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng H, Wilson LD, Walker VK, Ripmeester JA. J. Am. Chem. Soc. 2006;128:2844–2850. doi: 10.1021/ja0548182. [DOI] [PubMed] [Google Scholar]
- 13.Watkins M, Pan D, Wang EG, Michaelides A, VandeVondele J, Slater B. Nat Mater. 2011;10:794–798. doi: 10.1038/nmat3096. [DOI] [PubMed] [Google Scholar]
- 14.Duman JG, Serianni AS. J. Insect Physiol. 2002;48:103–111. doi: 10.1016/s0022-1910(01)00150-0. [DOI] [PubMed] [Google Scholar]
- 15.Mansuri MM, Starrett JE, Wos JA, Tortolani DR, Brodfuehrer PR, Howell HG, Martin JC. J. Org. Chem. 1989;54:4780–4785. [Google Scholar]
- 16.Gerland, Desire J, Lepoivre M, Décout JL. Org. Lett. 2007;9:3021–3023. doi: 10.1021/ol071088p. [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, You SW, Yao LP, Liu HL, Jiao XY, Shi M, Zhao QB, Ju G. Spinal Cord. 2006;44:421–426. doi: 10.1038/sj.sc.3101878. [DOI] [PubMed] [Google Scholar]
- 19.Chiarella RA, Gillon AL, Burton RC, Davey RJ, Sadiq G, Auffret A, Cioffi M, Hunter CA. Faraday Discuss. 2007;136:179–193. doi: 10.1039/b616164m. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Amornwittawat N, Juwita V, Kao Y, Duman JG, Pascal TA, Goddard WA, Wen X. Biochemistry. 2009;48:9696–9703. doi: 10.1021/bi901283p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen X, Wang S, Amornwittawata N, Houghton EA, Sacco MA. J. Mol. Recognit. 2011;24:1024–1031. doi: 10.1002/jmr.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangwal K. Additives and Crystallization Processes: From Fundamentals to Applications. West Sussex, England: John Wiley & Sons Ltd.; 2007. [Google Scholar]
- 23.Tremayne M, Kariuki BM, Harris KDM. Angew. Chem. Int. Ed. 1997;36:770–772. [Google Scholar]
- 24.Furberg S, Peterson CS, Romming C. Acta Cryst. 1965;18:313–320. [Google Scholar]
- 25.Hunt DJ, Subramanian E. Acta Cryst. 1969;B25:2144–2152. doi: 10.1107/s0567740869005267. [DOI] [PubMed] [Google Scholar]
- 26.Munns ARI, Tollin P. Acta Cryst. B. 1970;26:1101–1113. doi: 10.1107/s0567740870003679. [DOI] [PubMed] [Google Scholar]
- 27.Jia Z, DeLuca CI, Chao H, Davies PL. Nature. 1996;384:285–288. doi: 10.1038/384285a0. [DOI] [PubMed] [Google Scholar]
- 28.Jia ZC, Davies PL. Trends Biochem. Sci. 2002;27:101–106. doi: 10.1016/s0968-0004(01)02028-x. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher NH. The Chemical Physics of Ice. 1 ed. Cambridge: Cambridge University Press; 1970. [Google Scholar]
- 30.Li N, Andorfer C, Duman J. J. Exp. Biol. 1998;201:2243–2251. doi: 10.1242/jeb.201.15.2243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.